Abstract
A redox-responsive chemodynamic therapy (CDT)-based theranostic system composed of hollow mesoporous MnO2 (H-MnO2), doxorubicin (DOX), and fluorescent (FL) carbon nanodots (CDs) is reported for the diagnosis and therapy of cancer. In general, since H-MnO2 can be degraded by intracellular glutathione (GSH) to form Mn2+ with excellent Fenton-like activity to generate highly reactive ·OH, the normal antioxidant defense system can be injured via consumption of GSH. This in turn can potentiate the cytotoxicity of CDT and release DOX. The cancer cells can be eliminated effectively by the nanoplatform via the synergistic effect of chemotherapy and CDT. The FL of CDs can be restored after H-MnO2 is degraded which blocked the fluorescence resonance energy transfer process between CDs as an energy donor and H-MnO2 as an FL acceptor. The GSH can be determined by recovery of the FL and limit of detection is 1.30 μM with a linear range of 0.075–0.825 mM. This feature can be utilized to efficiently distinguish cancerous cells from normal ones based on different GSH concentrations in the two types of cells. As a kind of CDT-based theranostic system responsive to GSH, simultaneously diagnostic (normal/cancer cell differentiation) and therapeutic function (chemotherapy and CDT) in a single nanoplatform can be achieved.
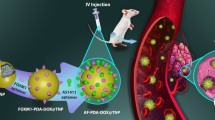
Graphical abstract

The redox-responsive chemodynamic therapy (CDT)-based theranostic system is fabricated by H-MnO2, DOX, and fluorescent CDs. The nanoplatform can realize simultaneously diagnostic (normal/cancer cell differentiation) and therapeutic function (chemotherapy and CDT) to improve the therapeutic efficiency and security.







Similar content being viewed by others
References
Zhang Y, Bo S, Feng T, Qin X, Wan Y, Jiang S, Li C, Lin J, Wang T, Zhou X, Jiang Z-X, Huang P (2019) A versatile theranostic nanoemulsion for architecture-dependent multimodal imaging and dually augmented photodynamic therapy. Adv Mater 31(21):1806444
Kim H, Kwak G, Kim K, Yoon HY, Kwon IC (2019) Theranostic designs of biomaterials for precision medicine in cancer therapy. Biomaterials 213:119207
Lim EK, Kim T, Paik S, Haam S, Huh YM, Lee K (2015) Nanomaterials for theranostics: recent advances and future challenges. Chem Rev 115(1):327–394
Xie J, Liu G, Eden HS, Ai H, Chen X (2011) Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc Chem Res 44(10):883–892
Zheng M, Liu S, Li J, Qu D, Zhao H, Guan X, Hu X, Xie Z, Jing X, Sun Z (2014) Integrating oxaliplatin with highly luminescent carbon dots: an unprecedented theranostic agent for personalized medicine. Adv Mater 26(21):3554–3560
Tang Z, Liu Y, He M, Bu W (2019) Chemodynamic therapy: tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew Chem Int Ed 58(4):946–956
Li X, Lee D, Huang J-D, Yoon J (2018) Phthalocyanine-assembled nanodots as photosensitizers for highly efficient type I photoreactions in photodynamic therapy. Angew Chem Int Ed 57(31):9885–9890
McHale A P, Callan J F, Nomikou N, Fowley C, Callan B (2016) Sonodynamic therapy: concept, mechanism and application to cancer treatment. In: Therapeutic Ultrasound. Springer, pp. 429–450
Kumar R, Shin WS, Sunwoo K, Kim WY, Koo S, Bhuniya S, Kim JS (2015) Small conjugate-based theranostic agents: an encouraging approach for cancer therapy. Chem Soc Rev 44(19):6670–6683
Kosaka PM, Pini V, Ruz JJ, Da Silva R, González M, Ramos D, Calleja M, Tamayo J (2014) Detection of cancer biomarkers in serum using a hybrid mechanical and optoplasmonic nanosensor. Nat Nanotechnol 9(12):1047–1053
Savla R, Taratula O, Garbuzenko O, Minko T (2011) Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release 153(1):16–22
Schnelldorfer T, Gansauge S, Gansauge F, Schlosser S, Beger HG, Nussler AK (2000) Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer 89(7):1440–1447
Xu J, Han W, Yang P, Jia T, Dong S, Bi H, Gulzar A, Yang D, Gai S, He F (2018) Tumor microenvironment-responsive mesoporous MnO2-coated upconversion nanoplatform for self-enhanced tumor theranostics. Adv Funct Mater 28(36):1803804
Yan X, Song Y, Zhu C, Song J, Du D, Su X, Lin Y (2016) Graphene quantum dot-MnO2 nanosheet based optical sensing platform: a sensitive fluorescence turn-off-on nanosensor for glutathione detection and intracellular imaging. ACS Appl Mater Interfaces 8(34):21990–21996
Lin L-S, Song J, Song L, Ke K, Liu Y, Zhou Z, Shen Z, Li J, Yang Z, Tang W, Niu G, Yang H-H, Chen X (2018) Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew Chem Int Ed 57(18):4902–4906
Xu Z, Liu Y (2021) The behavior of carbonized polymer dots at the nano-bio interface and their luminescent mechanism: a physical chemistry perspective. Chin J Chem 39:265–273
Huang Q, Lin X, Li F, Weng W, Lin L, Hu S (2015) Synthesis and applications of carbon dots. Prog Chem 27(11):1604–1614
Sohal N, Maity B, Basu S (2020) Carbon dot─MnO2 nanosphere composite sensors for selective detection of glutathione. ACS Appl Nano Mater 3(6):5955–5964
Cai Q-Y, Li J, Ge J, Zhang L, Hu Y-L, Li Z-H, Qu L-B (2015) A rapid fluorescence “switch-on” assay for glutathione detection by using carbon dots–MnO2 nanocomposites. Biosens Bioelectron 72:31–36
Yang C, Deng W, Liu H, Ge S, Yan M (2015) Turn-on fluorescence sensor for glutathione in aqueous solutions using carbon dots─MnO2 nanocomposites. Sensor Actuat B 216:286–292
Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y, Peng R, Liu Z (2017) Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun 8(1):902
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem Int Ed 52(14):3953–3957
Xu Z-Q, Lan J-Y, Jin J-C, Dong P, Jiang F-L, Liu Y (2015) Highly photoluminescent nitrogen-doped carbon nanodots and their protective effects against oxidative stress on cells. ACS Appl Mater Interfaces 7(51):28346–28352
Niu Z, Yue T, Hu W, Sun W, Hu Y, Xu Z (2019) Covalent bonding of MnO2 onto graphene aerogel forwards: efficiently catalytic degradation of organic wastewater. Appl Surf Sci 496:143585
Wang K, Liu Y, Yi W-J, Li C, Li Y-Y, Zhuo R-X, Zhang X-Z (2013) Novel shell-cross-linked micelles with detachable PEG corona for glutathione-mediated intracellular drug delivery. Soft Matter 9(3):692–699
Jiang X, Chen J, Bajić A, Zhang C, Song X, Carroll SL, Cai Z-L, Tang M, Xue M, Cheng N (2017) Quantitative real-time imaging of glutathione. Nat Commun 8(16087):16087
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8(7):579–591
Chen Z, Wan L, Yuan Y, Kuang Y, Xu X, Liao T, Liu J, Xu Z-Q, Jiang B, Li C (2020) pH/GSH-dual-sensitive hollow mesoporous silica nanoparticle-based drug delivery system for targeted cancer therapy. ACS Biomater Sci Eng 6(6):3375–3387
Noh J, Kwon B, Han E, Park M, Yang W, Cho W, Yoo W, Khang G, Lee D (2015) Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat Commun 6(1):6907
Ponraj T, Vivek R, Paulpandi M, Rejeeth C, Nipun Babu V, Vimala K, Anand K, Sivaselvam S, Vasanthakumar A, Ponpandian N, Kannan S (2018) Mitochondrial dysfunction-induced apoptosis in breast carcinoma cells through a pH-dependent intracellular quercetin NDDS of PVPylated-TiO2NPs. J Mater Chem B 6(21):3555–3570
Panieri E, Santoro MM (2016) ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 7(6):e2253–e2253
Friedman JR, Nunnari J (2014) Mitochondrial form and function. Nature 505(7483):335–343
Xu Z, Chen X, Sun Z, Li C, Jiang B (2019) Recent progress on mitochondrial targeted cancer therapy based on inorganic nanomaterials. Mater Today Chem 12:240–260
Thangam R, Sathuvan M, Poongodi A, Suresh V, Pazhanichamy K, Sivasubramanian S, Kanipandian N, Ganesan N, Rengasamy R, Thirumurugan R, Kannan S (2014) Activation of intrinsic apoptotic signaling pathway in cancer cells by Cymbopogon citratus polysaccharide fractions. Carbohyd Polym 107:138–150
Funding
We received financial support from the National Natural Science Foundation of China (22073025, 21603067) and Hubei Nature Science Foundation of China (2019CFB748).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 4241 kb)
Rights and permissions
About this article
Cite this article
He, H., Yang, Q., Li, H. et al. Hollow mesoporous MnO2-carbon nanodot-based nanoplatform for GSH depletion enhanced chemodynamic therapy, chemotherapy, and normal/cancer cell differentiation. Microchim Acta 188, 141 (2021). https://doi.org/10.1007/s00604-021-04801-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04801-5