Development and Validation of Aptasensor Based on MnO2 for the Detection of Sulfadiazine Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of MnO2
2.3. Molecular Dynamics Simulation
2.4. Optimization of Aptasensor Conditions
2.5. Standard Curves of the Aptasensor
2.6. Determination of Selectivity
2.7. Sample Preparation
2.8. Assay Validation
3. Results and Discussion
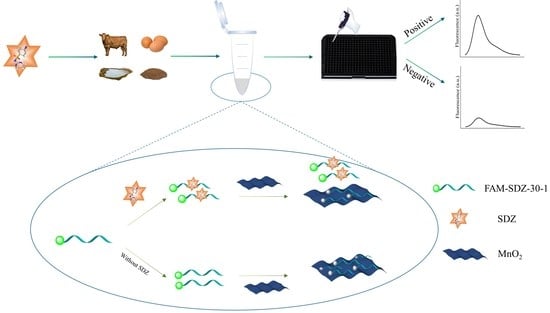
3.1. Principle of the Aptasensor
3.2. Characterization of MnO2
3.3. Molecular Simulation Analysis
3.4. Optimization of the Aptasensor
3.5. Properties of Aptasensor
3.6. Validation of the Aptasensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, T.; Tan, L.; Fu, H.; Wang, J. Application of molecular imprinting polymer anchored on CdTe quantum dots for the detection of sulfadiazine in seawater. Mar. Pollut. Bull. 2019, 146, 591–597. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Kumar, E.A.; Wang, T.J.; Cheng, I.C. Strontium tungstate-modified disposable strip for electrochemical detection of sulfadiazine in environmental samples. Ecotoxicol. Environ. Saf. 2021, 208, 111516. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, R.; Kubendhiran, S.; Chen, S.-M.; Chen, T.-W.; Al-Zaqri, N.; Alsalme, A.; Alharthi, F.A.; Abu Khanjer, M.M.; Tseng, T.-W.; Huang, C.-C. Exploring the promising potential of MoS2-RuS2 binary metal sulphide towards the electrocatalysis of antibiotic drug sulphadiazine. Anal. Chim. Acta 2019, 1086, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ms, A.; Ep, B.; Hrn, C.; Aa, D.; Fs, E. Development of a rapid efficient solid-phase microextraction: An overhead rotating flat surface sorbent based 3-D graphene oxide/ lanthanum nanoparticles @ Ni foam for separation and determination of sulfonamides in animal-based food products. Food Chem. 2021, 373 Pt A, 131421. [Google Scholar] [CrossRef]
- Dong, F.; Li, C.; Crittenden, J.; Zhang, T.; Lin, Q.; He, G.; Zhang, W.; Luo, J. Sulfadiazine destruction by chlorination in a pilot-scale water distribution system: Kinetics, pathway, and bacterial community structure. J. Hazard. Mater. 2019, 366, 88–97. [Google Scholar] [CrossRef]
- Li, Z.B.; Cui, P.L.; Liu, J.; Liu, J.X.; Wang, J.P. Production of generic monoclonal antibody and development of chemiluminescence immunoassay for determination of 32 sulfonamides in chicken muscle. Food Chem. 2020, 311, 125966. [Google Scholar] [CrossRef]
- Patyra, E.; Nebot, C.; Gavilán, R.E.; Cepeda, A.; Kwiatek, K. Development and validation of an LC-MS/MS method for the quantification of tiamulin, trimethoprim, tylosin, sulfadiazine and sulfamethazine in medicated feed. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 882–891. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Mehrabi, F.; Tayebi, L. Highly selective magnetic dual template molecularly imprinted polymer for simultaneous enrichment of sulfadiazine and sulfathiazole from milk samples based on syringe-to-syringe magnetic solid-phase microextraction. Talanta 2021, 232, 122449. [Google Scholar] [CrossRef]
- Errayess, S.A.; Lahcen, A.A.; Idrissi, L.; Marcoaldi, C.; Chiavarini, S.; Amine, A. A sensitive method for the determination of Sulfonamides in seawater samples by Solid Phase Extraction and UV-Visible spectrophotometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 181, 276–285. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, D.; Zheng, P.; Zhang, Y.; Wang, Z.; Mari, G.M.; Jiang, H. A simple and rapid immunochromatography test based on readily available filter paper modified with chitosan to screen for 13 sulfonamides in milk. J. Dairy Sci. 2021, 104, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S.; Kumar, A. Validation of HPLC Multi-residue Method for Determination of Fluoroquinolones, Tetracycline, Sulphonamides and Chloramphenicol Residues in Bovine Milk. Food Anal. Methods 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Huang, Z.; Yang, Y.; Fu, T.; Yang, Y.; Lyu, Y.; Jiang, J.; Qiu, L.; Cao, Z. Robust Covalent Aptamer Strategy Enables Sensitive Detection and Enhanced Inhibition of SARS-CoV-2 Proteins. ACS Cent. Sci. 2023, 9, 72–83. [Google Scholar] [CrossRef]
- Karadjian, G. Aptamer-Based Technologies for Parasite Detection. Sensors 2023, 23, 562. [Google Scholar] [CrossRef]
- Sun, S. The Research Advances of Aptamers in Hematologic Malignancies. Cancers 2023, 15, 300. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Xie, H.; Fu, C.; Zhou, J.; Liu, H.; Zeng, P.; Sun, Y. Double Signal Amplification Strategy for Dual-Analyte Fluorescent Aptasensors for Visualizing Cancer Biomarker Proteins. Anal. Chem. 2022, 94, 10451–10461. [Google Scholar] [CrossRef]
- Wang, X.; Rong, X.; Zhang, Y.; Luo, F.; Qiu, B.; Wang, J.; Lin, Z. Homogeneous Photoelectrochemical Aptasensors for Tetracycline Based on Sulfur-Doped g-C3N4/n-GaN Heterostructures Formed through Self-Assembly. Anal. Chem. 2022, 94, 3735–3742. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, E.J.; Park, T.K.; Bae, D.W.; Cha, S.S.; Kim, T.W.; Kim, Y.P. Gold nanoparticle-assisted SELEX as a visual monitoring platform for the development of small molecule-binding DNA aptasensors. Biosens. Bioelectron. 2021, 191, 113468. [Google Scholar] [CrossRef]
- Yang, H.; Xu, D. Highly-sensitive and simple fluorescent aptasensor for 17b-estradiol detection coupled with HCR-HRP structure. Talanta 2022, 240, 123094. [Google Scholar] [CrossRef] [PubMed]
- Sameiyan, E.; Khoshbin, Z.; Lavaee, P.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S. A bivalent binding aptamer-cDNA on MoS2 nanosheets based fluorescent aptasensor for detection of aflatoxin M1. Talanta 2021, 235, 122779. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Y.; Kou, Q.; Sun, Q.; Tang, J.; Yang, L.; Chen, X.; Xu, W.; Le, T. A novel aptasensor based on Fe3O4/Au/g-C3N4 for sensitive detection of sulfameter in food matrices. Sens. Actuators B Chem. 2021, 353, 131148. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Hao, A.; Shi, Z.; Huang, H. Silica-Coated Silver Nanoparticles Decorated with Fluorescent CdTe Quantum Dots and DNA Aptamers for Detection of Tetracycline. ACS Appl. Nano Mater. 2020, 3, 9796–9803. [Google Scholar] [CrossRef]
- Yan, X.; Song, Y.; Zhu, C.; Song, J.; Du, D.; Su, X.; Lin, Y. Graphene Quantum Dot-MnO2 Nanosheet-Based Optical Sensing Platform: A Sensitive Fluorescence “Turn Off-On” Nanosensor for Glutathione Detection and Intracellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 21990–21996. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kong, X.J.; Yu, R.Q.; Chen, T.T.; Chu, X. MnO2 Nanosheet-based Fluorescence Sensing Platform for Sensitive Detection of Endonuclease. Anal. Sci. 2017, 33, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Zhuang, J.; Jiang, X.; Liu, X.; Tang, D. A three-dimensional DNA walker amplified FRET sensor for detection of telomerase activity based on the MnO2 nanosheet-upconversion nanoparticle sensing platform. Chem. Commun. 2019, 55, 9857–9860. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, L.; Ahmad, W.; Rong, Y.; Li, H.; Hu, Y.; Chen, Q. A highly sensitive detection of carbendazim pesticide in food based on the upconversion-MnO(2) luminescent resonance energy transfer biosensor. Food Chem. 2021, 349, 129157. [Google Scholar] [CrossRef]
- Li, J.; Du, B.; Li, Y.; Wang, Y.; Wu, D.; Wei, Q. A turn-on fluorescent sensor for highly sensitive mercury(ii) detection based on a carbon dot-labeled oligodeoxyribonucleotide and MnO2 nanosheets. New J. Chem. 2018, 42, 1228–1234. [Google Scholar] [CrossRef]
- Paissoni, C.; Spiliotopoulos, D.; Musco, G.; Spitaleri, A. GMXPBSA 2.0: A GROMACS tool to perform MM/PBSA and computational alanine scanning. Comput. Phys. Commun. 2014, 185, 105–107. [Google Scholar] [CrossRef]
- Elkarhat, Z.; Charoute, H.; Elkhattabi, L.; Barakat, A.; Rouba, H. Potential inhibitors of SARS-CoV-2 RNA dependent RNA polymerase protein: Molecular docking, molecular dynamics simulations and MM-PBSA analyses. J. Biomol. Struct. Dyn. 2020, 40, 361–374. [Google Scholar] [CrossRef]
- Yan, X.; Yang, L.; Tang, J.; Wen, X.; Chen, X.; Zheng, X.; Chen, L.; Li, J.; Le, T. High-Sensitive FAM Labeled Aptasensor Based on Fe3O4/Au/g-C3N4 for the Detection of Sulfamethazine in Food Matrix. Biosensors 2022, 12, 759. [Google Scholar] [CrossRef]
- Chen, X.; Yang, L.; Tang, J.; Wen, X.; Zheng, X.; Chen, L.; Li, J.; Xie, Y.; Le, T. An AuNPs-Based Fluorescent Sensor with Truncated Aptamer for Detection of Sulfaquinoxaline in Water. Biosensors 2022, 12, 513. [Google Scholar] [CrossRef]
- Yang, L.; Chen, X.; Wen, X.; Tang, J.; Zheng, X.; Li, J.; Chen, L.; Jiang, S.; Le, T. A label-free dual-modal aptasensor for colorimetric and fluorescent detection of sulfadiazine. J. Mater. Chem. B 2022, 10, 6187–6193. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kou, Q.; Chen, X.; Wang, Y.; Yang, L.; Wen, X.; Zheng, X.; Yan, X.; Le, T. A novel fluorescent aptasensor based on mesoporous silica nanoparticles for the selective detection of sulfadiazine in edible tissue. Arab. J. Chem. 2022, 15, 104067. [Google Scholar] [CrossRef]







| Method | LOD (ng/mL) | References |
|---|---|---|
| HPLC | 0.9 | Alipanahpour et al. (2021) [8] |
| Spectrophotometry | 19 | Errayess et al. (2017) [9] |
| Chitosan-based ELISA | 8.64 | Zeng et al. (2021) [10] |
| Electrochemical | 2.25 | Kokulnathan et al. (2021) [2] |
| Aptasensor based on gold nanoparticles | 2 | Yang et al. (2022) [32] |
| Aptasensor based on MnO2 | 3.25 | Present work |
| Sample | Spiked (µg/kg) | This Work | HPLC | ||||
|---|---|---|---|---|---|---|---|
| Found (µg/kg) | Recovery (%) | CV (%) | Found (µg/kg) | Recovery (%) | CV (%) | ||
| Soil | 10 | 8.96 | 89.58 | 12.71 | 10.55 | 105.45 | 3.39 |
| 20 | 20.47 | 102.36 | 8.03 | 19.27 | 96.36 | 9.51 | |
| 30 | 31.75 | 105.84 | 7.45 | 28.73 | 95.76 | 9.38 | |
| Lake water | 10 | 8.72 | 87.19 | 11.21 | 9.45 | 94.55 | 8.33 |
| 20 | 19.88 | 99.39 | 7.81 | 19.82 | 99.09 | 8.33 | |
| 30 | 31.27 | 104.23 | 9.98 | 30.55 | 101.82 | 4.67 | |
| River water | 10 | 10.35 | 98.71 | 11.99 | 9.64 | 96.36 | 8.98 |
| 20 | 21.33 | 106.65 | 9.28 | 18.18 | 90.91 | 9.60 | |
| 30 | 30.62 | 102.07 | 3.13 | 28.55 | 95.15 | 7.73 | |
| Egg | 10 | 10.22 | 102.18 | 7.74 | 9.27 | 92.73 | 3.79 |
| 20 | 20.90 | 104.50 | 7.66 | 18.00 | 90.00 | 5.33 | |
| 30 | 32.78 | 109.26 | 13.14 | 27.82 | 92.73 | 7.08 | |
| Beef | 10 | 10.74 | 107.39 | 9.10 | 10.00 | 100.00 | 9.05 |
| 20 | 20.85 | 104.23 | 7.16 | 19.64 | 98.18 | 6.39 | |
| 30 | 32.46 | 108.19 | 5.49 | 26.91 | 89.70 | 2.68 | |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
|
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Yang, L.; Sun, Q.; Zhang, L.; Le, T. Development and Validation of Aptasensor Based on MnO2 for the Detection of Sulfadiazine Residues. Biosensors 2023, 13, 613. https://doi.org/10.3390/bios13060613
Zheng X, Yang L, Sun Q, Zhang L, Le T. Development and Validation of Aptasensor Based on MnO2 for the Detection of Sulfadiazine Residues. Biosensors. 2023; 13(6):613. https://doi.org/10.3390/bios13060613
Chicago/Turabian StyleZheng, Xiaoling, Lulan Yang, Qi Sun, Lei Zhang, and Tao Le. 2023. "Development and Validation of Aptasensor Based on MnO2 for the Detection of Sulfadiazine Residues" Biosensors 13, no. 6: 613. https://doi.org/10.3390/bios13060613