Abstract
Adipose tissue (AT) dysfunction, characterized by loss of its homeostatic functions, is a hallmark of non-communicable diseases. It is characterized by chronic low-grade inflammation and is observed in obesity, metabolic disorders such as insulin resistance and diabetes. While classically it has been identified by increased cytokine or chemokine expression, such as increased MCP-1, RANTES, IL-6, interferon (IFN) gamma or TNFα, mechanistically, immune cell infiltration is a prominent feature of the dysfunctional AT. These immune cells include M1 and M2 macrophages, effector and memory T cells, IL-10 producing FoxP3+ T regulatory cells, natural killer and NKT cells and granulocytes. Immune composition varies, depending on the stage and the type of pathology. Infiltrating immune cells not only produce cytokines but also metalloproteinases, reactive oxygen species, and chemokines that participate in tissue remodelling, cell signalling, and regulation of immunity. The presence of inflammatory cells in AT affects adjacent tissues and organs. In blood vessels, perivascular AT inflammation leads to vascular remodelling, superoxide production, endothelial dysfunction with loss of nitric oxide (NO) bioavailability, contributing to vascular disease, atherosclerosis, and plaque instability. Dysfunctional AT also releases adipokines such as leptin, resistin, and visfatin that promote metabolic dysfunction, alter systemic homeostasis, sympathetic outflow, glucose handling, and insulin sensitivity. Anti-inflammatory and protective adiponectin is reduced. AT may also serve as an important reservoir and possible site of activation in autoimmune-mediated and inflammatory diseases. Thus, reciprocal regulation between immune cell infiltration and AT dysfunction is a promising future therapeutic target.
Introduction
Physiologically, adipose tissue (AT) stores energy to support metabolic requirements in the times of need. From an evolutionary point of view, this is beneficial, but with increased nutrient intake and reduced energy expenditure in our modern world, AT function becomes altered leading to obesity.1 Such alteration is a result of complex interactions of metabolic and immune factors. Understanding of the importance of immunity in metabolic regulation, and the role of metabolism in immune regulation, underlies the rapidly developing biological field of immunometabolism. For example, T cell or M1 macrophage activation is typically associated with a switch from oxidative phosphorylation to anaerobic glycolysis.2 This has been reviewed in depth elsewhere,3,4 and, in the present paper, we will focus on the role of interactions of immune cells with dysfunctional AT.
AT can be typically classified as white, brown, or beige based on its metabolic activity, number of mitochondria, and uncoupling protein 1 (UCP-1) content, all of which affect adipocyte size and function. Brown AT plays a key role in thermogenesis, while white AT serves primarily for lipid storage. Brown AT is sparse in adult humans, in contrast to its periaortic location in rodents.5 In spite of this, the protective properties of brown fat have been demonstrated in cardiovascular disease.6 White AT is widely distributed as visceral (VAT) and subcutaneous AT (SAT).7 These compartments differ in their functional importance for metabolic health and in their immunometabolic properties. VAT is metabolically more active than SAT and it harbours significantly more immune cells in both health and pathology.8 This is closely linked with increased glucose uptake and fatty acid generation in VAT and greater adrenergic innervation, all of which are important in the regulation of insulin sensitivity.7 SAT in turn absorbs circulating free fatty acids and triglycerides.7 Numerous studies have shown that the retroperitoneal content of VAT is linked to cardiovascular risk.9 This is mediated by chronic low-grade inflammation, characterized by an excessive immune cell infiltration, overproduction of detrimental adipokines and cytokines (TNF-α, IL-6) that can be detected systemically as biomarkers of inflammation.10,11 Mechanistically such low-grade inflammation alters metabolic functions of AT, leading not only to insulin resistance and diabetes but also to cardiovascular pathology.12,13 More recently, attention has been focused on a very specific compartment of VAT, the perivascular AT (pVAT), due to its close proximity to blood vessels and its unique embryonic origin from vascular smooth muscle cell SM22+ precursors.8 Dynamic interplay between white and beige/brown adipocytes within pVAT results in unique metabolic and pro-inflammatory properties that make pVAT an important regulator of vascular function and plaque stability.8 Human perivascular coronary adipocytes exhibit reduced differentiation, more irregular shape, and smaller size than in the SAT or typical peri-renal VAT. This translates into smaller lipid droplet accumulation and increased synthetic capacity.14 pVAT provides a microenvironment for recruitment and activation of immune cells which in concert with adipokines affect vascular tone and other aspects of vascular homeostasis.15–17
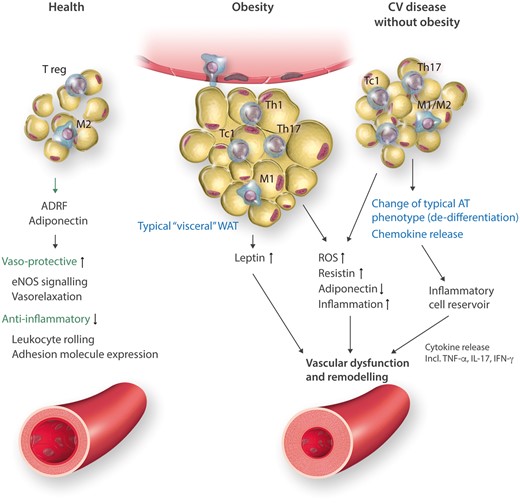
In summary, all compartments of AT: SAT, VAT as well as pVAT serve physiological functions in vascular and metabolic homeostasis. When these protective functions are disturbed, dysfunctional AT promotes the development of metabolic and vascular disease (Figure 1).
Figure 1
Triple functions of adipose tissue (VAT/pVAT) in health, obesity and in cardiovascular (CV) disease without obesity. AT compartments differ in characteristics of infiltrating immune cells, characteristics of adipocytes and adipokine profile. In health, protective adipokines and cytokines are important in maintaining vascular homeostasis. In obesity, enlarged adipocytes produce leptin and do not release adiponectin and enhance M1 macrophage accumulation in crown-like structures as well as T effector cells. In CVD without obesity macrophages are atypical, adipocytes are synthetic and create microenvironment for development of TLOs and immune cell activation.
Physiological roles of immune cells in AT
In health, AT contains numerous cell types, including not only adipocytes but also endothelial cells, fibroblasts, pre-adipocytes, stem cells, and regulatory/naive immune cells.18 Immune cells including M2 macrophages and T regulatory cells (Treg) release anti-inflammatory cytokines such as interleukin (IL)-10 and transforming growth factor beta (TGF-β), which increase insulin sensitivity and inhibit AT inflammation and dysfunction (Figure 1).19 In lean conditions, M2 cells are characterized by a lack of CD11c and the presence of CD206 and arginase 1.20 M2 and Treg polarization are reciprocally enhanced in physiological conditions by adiponectin released from IAT.21 IL-10 modulates insulin signalling through insulin receptor/IRS1-IRS2/PI3-kinase/Akt/FOXO1, in the context of hepatic gluconeogenesis and lipid synthesis. These actions are partially direct and in part indirect, through modulation of TNF, IL-6, IL-1β, and M1 macrophage polarization.22 M2 macrophages control adipocyte lipolysis.23 Upon cold exposure, M2 macrophages secrete catecholamines, to stimulate adipocyte lipolysis. This is important because, in concert with eosinophils, M2 macrophages can orchestrate generation of beige AT.24 As discussed above, in lean, insulin-sensitive AT T cells present are primarily T regulatory cells that secrete IL-10 and transforming growth factor-β (TGFβ) and Th2 cells producing anti-inflammatory cytokines such as IL-4, IL-5, IL-13, and IL-10. These play an important role in homeostasis of AT.25 Tregs in normal AT have a unique mRNA expression profile, characterizing their regulatory function, such as CD25, glucocorticoid-induced tumor necrosis factor receptor (GITR), cytotoxic T lymphocyte antigen-4 (CTLA-4), killer cell lectin-like receptor G1 and OX40 in addition to classical FoxP3.25 T regs also exhibit chemokine sensitivity as evidenced by high CC chemokine receptor expression.25 Other immune cells in lean AT include potentially protective eosinophils and to a lesser extent neutrophils. To date, the role of these cells has been less well defined. Likewise, the role of immune cells present in healthy pVAT in the regulation of vascular function has not yet been clearly defined, apart from potential effects on the release of protective adipokines from adipocytes. Immune cell content in lean subcutaneous AT has also been described but is very low. Dynamic changes of immune cells in the AT underpin their involvement in pathologies associated with AT dysfunction.
Defining dysfunctional AT
Functional changes within the AT associated with altered paracrine and endocrine properties contribute to the development of cardiovascular disease and cancer.26,27,ATdysfunction is thus characterized by decreased release of homeostatic protective factors such as adiponectin, nitric oxide, or protective prostaglandins and increased activation of stress-related pathways leading to pathological adipokine release (resistin, visfatin, leptin) and development of low-grade inflammation (Figure 1),28 which is not only a feature of dysfunctional AT but also promotes metabolic and vascular dysfunction. While this phenomenon is particularly evident in pVAT, it has also been well defined in other VAT depots26,29 in obesity.8 Adipocyte–immune cell interactions are therefore bi-directional and depend on nutritional mechanisms, neuro-hormonal pathways, and locally secreted humoural factors.8,26,29 In pathological conditions, adipocytes produce inflammatory cytokines and extracellular matrix proteins, supporting infiltration and activation of immune cells, therefore, creating an optimal microenvironment for inflammation.5 At the same time, activated immune cells secrete cytokines that influence adipocyte function, and differentiation and adipokine secretion. Links between adipokines and immune cell infiltration in the AT have been discussed elsewhere and are summarized in Table 1. The characteristics of AT inflammatory responses differ between classical inflammatory disease such as Crohn’s disease and cancer or cardiovascular disease. Common feature is, however, that dysfunctional, inflamed AT provides a microenvironment permissive for the development of pathology. These effects can be localized, for example linking pVAT to adjacent vessel dysfunction in hypertension or atherosclerosis38,39 or systemic, such as the effects of VAT dysfunction on the development of diabetes, cancer, autoimmune diseases, or signalling within the CNS.
Table 1
Summary of the effects of adipokines on immune responses. Expertly reviewed and discussed elsewhere.30,31–37
| Adipokine . |
Immune cell recruitment . |
Immune cell activation . |
Summary . |
| Leptin |
|
|
Pro-inflammatory |
| Adiponectin |
|
↓ IL-17 production from γ/δ T cells ↑ IL-8 in synovial fibroblasts ↓ Antitumour DC immunity Mf activation resembling M1 (but with M2 elements; ↑mannose receptor) ↑ CD4 T cell activation |
|
| Resistin |
|
Expressed in Mf and T cells Induced by IL-1/IL-6/TNF ↑ IL-6, IL-27, IL-23 and IL-5 in Mf (↑) Th17 and Th1 |
Pro-inflammatory |
| Visfatin (PBEF-1) |
↑ICAM-1; VCAM-1 on EC and VSMC |
↑ B-cell maturation ↑ Leukocyte activation ↑ TNF/IL-6/IL-1b ↑ NFkB |
Pro-inflammatory |
| Chemerin (RARRES2 or TIG2) |
Direct chemotaxis through CMKLR1; chemR23 especially on DCs; NK; Mf |
↓TNF/IL-6/ ↑ NFkB ↑ Adiponectin ↑ TGFβ |
Pro-inflammatory and anti-inflammatory |
| RBP4 |
? |
|
Pro-inflammatory? |
| Adipokine . |
Immune cell recruitment . |
Immune cell activation . |
Summary . |
| Leptin |
|
|
Pro-inflammatory |
| Adiponectin |
|
↓ IL-17 production from γ/δ T cells ↑ IL-8 in synovial fibroblasts ↓ Antitumour DC immunity Mf activation resembling M1 (but with M2 elements; ↑mannose receptor) ↑ CD4 T cell activation |
|
| Resistin |
|
Expressed in Mf and T cells Induced by IL-1/IL-6/TNF ↑ IL-6, IL-27, IL-23 and IL-5 in Mf (↑) Th17 and Th1 |
Pro-inflammatory |
| Visfatin (PBEF-1) |
↑ICAM-1; VCAM-1 on EC and VSMC |
↑ B-cell maturation ↑ Leukocyte activation ↑ TNF/IL-6/IL-1b ↑ NFkB |
Pro-inflammatory |
| Chemerin (RARRES2 or TIG2) |
Direct chemotaxis through CMKLR1; chemR23 especially on DCs; NK; Mf |
↓TNF/IL-6/ ↑ NFkB ↑ Adiponectin ↑ TGFβ |
Pro-inflammatory and anti-inflammatory |
| RBP4 |
? |
|
Pro-inflammatory? |
Table 1
Summary of the effects of adipokines on immune responses. Expertly reviewed and discussed elsewhere.30,31–37
| Adipokine . |
Immune cell recruitment . |
Immune cell activation . |
Summary . |
| Leptin |
|
|
Pro-inflammatory |
| Adiponectin |
|
↓ IL-17 production from γ/δ T cells ↑ IL-8 in synovial fibroblasts ↓ Antitumour DC immunity Mf activation resembling M1 (but with M2 elements; ↑mannose receptor) ↑ CD4 T cell activation |
|
| Resistin |
|
Expressed in Mf and T cells Induced by IL-1/IL-6/TNF ↑ IL-6, IL-27, IL-23 and IL-5 in Mf (↑) Th17 and Th1 |
Pro-inflammatory |
| Visfatin (PBEF-1) |
↑ICAM-1; VCAM-1 on EC and VSMC |
↑ B-cell maturation ↑ Leukocyte activation ↑ TNF/IL-6/IL-1b ↑ NFkB |
Pro-inflammatory |
| Chemerin (RARRES2 or TIG2) |
Direct chemotaxis through CMKLR1; chemR23 especially on DCs; NK; Mf |
↓TNF/IL-6/ ↑ NFkB ↑ Adiponectin ↑ TGFβ |
Pro-inflammatory and anti-inflammatory |
| RBP4 |
? |
|
Pro-inflammatory? |
| Adipokine . |
Immune cell recruitment . |
Immune cell activation . |
Summary . |
| Leptin |
|
|
Pro-inflammatory |
| Adiponectin |
|
↓ IL-17 production from γ/δ T cells ↑ IL-8 in synovial fibroblasts ↓ Antitumour DC immunity Mf activation resembling M1 (but with M2 elements; ↑mannose receptor) ↑ CD4 T cell activation |
|
| Resistin |
|
Expressed in Mf and T cells Induced by IL-1/IL-6/TNF ↑ IL-6, IL-27, IL-23 and IL-5 in Mf (↑) Th17 and Th1 |
Pro-inflammatory |
| Visfatin (PBEF-1) |
↑ICAM-1; VCAM-1 on EC and VSMC |
↑ B-cell maturation ↑ Leukocyte activation ↑ TNF/IL-6/IL-1b ↑ NFkB |
Pro-inflammatory |
| Chemerin (RARRES2 or TIG2) |
Direct chemotaxis through CMKLR1; chemR23 especially on DCs; NK; Mf |
↓TNF/IL-6/ ↑ NFkB ↑ Adiponectin ↑ TGFβ |
Pro-inflammatory and anti-inflammatory |
| RBP4 |
? |
|
Pro-inflammatory? |
Immune cells in AT dysfunction
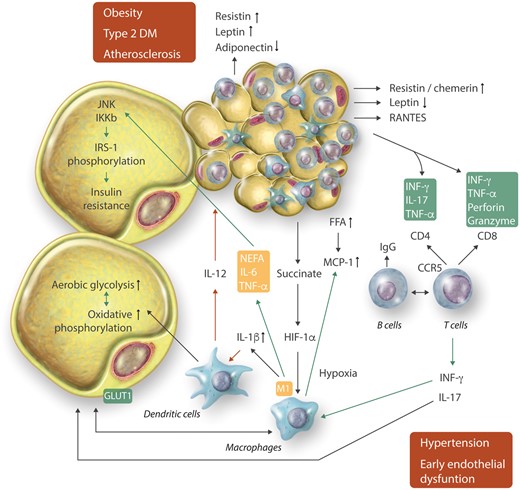
Immune cells that infiltrate dysfunctional AT are the key drivers of AT inflammation (Figure 2 and Table 2). The cellular players of such responses differ depending on the anatomical location as well as on the type and the stage of pathology.77,78
Figure 2
Interactions between adipocytes and immune cells at different stages of metabolic and cardiovascular disease. Interactions involve important immunometabolic regulation.
Table 2
Key cell types infiltrating adipose tissue in health and disease – selected metabolic and cardiovascular (CV) effects. See Table 1 legend for abbreviations
| Cell type . |
Preferential localisation . |
Metabolic effects . |
Role in CV pathology . |
|
|
|
Insulin resistance (M1) Higher AT ROS production41 Increased lactate production41 Regulate differentiation of adipocytes via GM-CSF signalling42 ATMs can inhibit adipogenesis43 |
Polarising M1 phenotype in atherosclerosis and hypertension Role in hypoxia Promote vascular Th17 response44 M2 Mf in vascular fibrosis45 |
| T cells |
CD8+ |
VAT>SAT40 |
|
initiate inflammatory cascades46 role in macrophages differentiation, activation and migration46 impair vascular function39 |
| Th1 |
VAT>SAT49,50 |
• Promote insulin resistance48 |
• impair vascular function39Promote atherosclerosis51,52 |
| Th17 |
|
Associated with cholesterol level54 Promote insulin resistance53 Promote diabetes and autoimmune responses enhance obesity55; Suppress adipocyte differentiation53 |
|
| Th2 |
VAT>SAT49,50 |
|
• Improve vascular function; Increase or decrease atherosclerosis58–60 |
| γ/δ T cells |
VAT>SAT53 |
Promote insulin resistance61 |
• Induce vascular dysfunction and hypertension62role in atherosclerosis unclear63 |
| Tregs |
VAT>SAT40,64 |
|
Decrease vascular inflammation65 Prevent atherosclerosis52,66,67 |
| B cells |
pVAT>VAT7VAT>SAT40 |
|
Higher production of IgG68 Activate vascular CD8+ and Th1 cells68 promote atherosclerosis52 |
| NK cells |
VAT>SAT69Epi.AT>Ing.AT70 |
• Insulin resistance69 |
|
| NKT cells |
Epi.AT>Ing.AT70 |
Insulin resistance72 Hepatic steatosis47,72 |
• Contribute to vascular production of IFN-γ, IL-4, and TNF-α72 |
| Eosinophils |
VAT>SAT73 |
Insulin sensitivity73 Reduce body weight24 Increase beiging24 |
IL-4 and IL-13 release perivascularly (Th2) Polarization of M2 macrophages73—possibly profibrotic In pVAT—anti contractile; improve vascular function74 |
| Neutrophils |
VAT>SAT75 |
Insulin resistance76 Decreased adiposity76 |
|
| Cell type . |
Preferential localisation . |
Metabolic effects . |
Role in CV pathology . |
|
|
|
Insulin resistance (M1) Higher AT ROS production41 Increased lactate production41 Regulate differentiation of adipocytes via GM-CSF signalling42 ATMs can inhibit adipogenesis43 |
Polarising M1 phenotype in atherosclerosis and hypertension Role in hypoxia Promote vascular Th17 response44 M2 Mf in vascular fibrosis45 |
| T cells |
CD8+ |
VAT>SAT40 |
|
initiate inflammatory cascades46 role in macrophages differentiation, activation and migration46 impair vascular function39 |
| Th1 |
VAT>SAT49,50 |
• Promote insulin resistance48 |
• impair vascular function39Promote atherosclerosis51,52 |
| Th17 |
|
Associated with cholesterol level54 Promote insulin resistance53 Promote diabetes and autoimmune responses enhance obesity55; Suppress adipocyte differentiation53 |
|
| Th2 |
VAT>SAT49,50 |
|
• Improve vascular function; Increase or decrease atherosclerosis58–60 |
| γ/δ T cells |
VAT>SAT53 |
Promote insulin resistance61 |
• Induce vascular dysfunction and hypertension62role in atherosclerosis unclear63 |
| Tregs |
VAT>SAT40,64 |
|
Decrease vascular inflammation65 Prevent atherosclerosis52,66,67 |
| B cells |
pVAT>VAT7VAT>SAT40 |
|
Higher production of IgG68 Activate vascular CD8+ and Th1 cells68 promote atherosclerosis52 |
| NK cells |
VAT>SAT69Epi.AT>Ing.AT70 |
• Insulin resistance69 |
|
| NKT cells |
Epi.AT>Ing.AT70 |
Insulin resistance72 Hepatic steatosis47,72 |
• Contribute to vascular production of IFN-γ, IL-4, and TNF-α72 |
| Eosinophils |
VAT>SAT73 |
Insulin sensitivity73 Reduce body weight24 Increase beiging24 |
IL-4 and IL-13 release perivascularly (Th2) Polarization of M2 macrophages73—possibly profibrotic In pVAT—anti contractile; improve vascular function74 |
| Neutrophils |
VAT>SAT75 |
Insulin resistance76 Decreased adiposity76 |
|
Table 2
Key cell types infiltrating adipose tissue in health and disease – selected metabolic and cardiovascular (CV) effects. See Table 1 legend for abbreviations
| Cell type . |
Preferential localisation . |
Metabolic effects . |
Role in CV pathology . |
|
|
|
Insulin resistance (M1) Higher AT ROS production41 Increased lactate production41 Regulate differentiation of adipocytes via GM-CSF signalling42 ATMs can inhibit adipogenesis43 |
Polarising M1 phenotype in atherosclerosis and hypertension Role in hypoxia Promote vascular Th17 response44 M2 Mf in vascular fibrosis45 |
| T cells |
CD8+ |
VAT>SAT40 |
|
initiate inflammatory cascades46 role in macrophages differentiation, activation and migration46 impair vascular function39 |
| Th1 |
VAT>SAT49,50 |
• Promote insulin resistance48 |
• impair vascular function39Promote atherosclerosis51,52 |
| Th17 |
|
Associated with cholesterol level54 Promote insulin resistance53 Promote diabetes and autoimmune responses enhance obesity55; Suppress adipocyte differentiation53 |
|
| Th2 |
VAT>SAT49,50 |
|
• Improve vascular function; Increase or decrease atherosclerosis58–60 |
| γ/δ T cells |
VAT>SAT53 |
Promote insulin resistance61 |
• Induce vascular dysfunction and hypertension62role in atherosclerosis unclear63 |
| Tregs |
VAT>SAT40,64 |
|
Decrease vascular inflammation65 Prevent atherosclerosis52,66,67 |
| B cells |
pVAT>VAT7VAT>SAT40 |
|
Higher production of IgG68 Activate vascular CD8+ and Th1 cells68 promote atherosclerosis52 |
| NK cells |
VAT>SAT69Epi.AT>Ing.AT70 |
• Insulin resistance69 |
|
| NKT cells |
Epi.AT>Ing.AT70 |
Insulin resistance72 Hepatic steatosis47,72 |
• Contribute to vascular production of IFN-γ, IL-4, and TNF-α72 |
| Eosinophils |
VAT>SAT73 |
Insulin sensitivity73 Reduce body weight24 Increase beiging24 |
IL-4 and IL-13 release perivascularly (Th2) Polarization of M2 macrophages73—possibly profibrotic In pVAT—anti contractile; improve vascular function74 |
| Neutrophils |
VAT>SAT75 |
Insulin resistance76 Decreased adiposity76 |
|
| Cell type . |
Preferential localisation . |
Metabolic effects . |
Role in CV pathology . |
|
|
|
Insulin resistance (M1) Higher AT ROS production41 Increased lactate production41 Regulate differentiation of adipocytes via GM-CSF signalling42 ATMs can inhibit adipogenesis43 |
Polarising M1 phenotype in atherosclerosis and hypertension Role in hypoxia Promote vascular Th17 response44 M2 Mf in vascular fibrosis45 |
| T cells |
CD8+ |
VAT>SAT40 |
|
initiate inflammatory cascades46 role in macrophages differentiation, activation and migration46 impair vascular function39 |
| Th1 |
VAT>SAT49,50 |
• Promote insulin resistance48 |
• impair vascular function39Promote atherosclerosis51,52 |
| Th17 |
|
Associated with cholesterol level54 Promote insulin resistance53 Promote diabetes and autoimmune responses enhance obesity55; Suppress adipocyte differentiation53 |
|
| Th2 |
VAT>SAT49,50 |
|
• Improve vascular function; Increase or decrease atherosclerosis58–60 |
| γ/δ T cells |
VAT>SAT53 |
Promote insulin resistance61 |
• Induce vascular dysfunction and hypertension62role in atherosclerosis unclear63 |
| Tregs |
VAT>SAT40,64 |
|
Decrease vascular inflammation65 Prevent atherosclerosis52,66,67 |
| B cells |
pVAT>VAT7VAT>SAT40 |
|
Higher production of IgG68 Activate vascular CD8+ and Th1 cells68 promote atherosclerosis52 |
| NK cells |
VAT>SAT69Epi.AT>Ing.AT70 |
• Insulin resistance69 |
|
| NKT cells |
Epi.AT>Ing.AT70 |
Insulin resistance72 Hepatic steatosis47,72 |
• Contribute to vascular production of IFN-γ, IL-4, and TNF-α72 |
| Eosinophils |
VAT>SAT73 |
Insulin sensitivity73 Reduce body weight24 Increase beiging24 |
IL-4 and IL-13 release perivascularly (Th2) Polarization of M2 macrophages73—possibly profibrotic In pVAT—anti contractile; improve vascular function74 |
| Neutrophils |
VAT>SAT75 |
Insulin resistance76 Decreased adiposity76 |
|
Macrophages were the first immune cells identified in AT.79 They are also the most abundant cell type in typical visceral and subcutaneous AT, representing more than 50% of all leukocytes. Their content in SAT is several folds lower than in typical VAT in both health and disease, suggesting their metabolic role. Resident AT macrophages (ATMs) play immune and scavenger functions. They present antigens to lymphocytes, phagocytose foreign organisms, release antimicrobial peptides, and attract other immune cells to areas of inflammation.10,80 In lean animals and humans, ATMs characterized by the surface markers F4/80 or CD68 constitute less than 5% of all AT cells. A dramatic increase (up to 40% of all AT cells) is observed in metabolic stress.10,81 Such an increase is also associated with qualitative changes of ATMs. In lean AT, M2-like producing IL-10 macrophages are dispersed, while in dysfunctional AT, M1 macrophages predominate and form crown-like aggregates, surrounding necrotic adipocytes/lipid droplets.13,20,82 In pathological conditions, these classically activated, M1 polarized, CD11c+ macrophages increases,83 produce pro-inflammatory TNF-α and IL-6 and IL1β.13,84 Such simple dichotomous division of ATMs into protective M2 and damaging M1 cells appears to be an oversimplification, especially when it concerns human pathology. Several studies point to the role of M2 cells in dysfunctional AT and insulin resistance82 or vascular remodelling and fibrosis45 indicating the need for further phenotypic characterization of ATM that may include Ly6C, CD34, CCR2, and CX3CR1.85 Macrophages also promote further propagation of AT inflammation through numerous humoural and cellular mechanisms including release of metalloproteinases such as ADAMTS13 and others.77,86–89 Discussion continues what proportion of these cells is chemotactically recruited and what proportion is proliferating from resident ATMs.90,91
Other types of innate immune cells in VAT and pVAT include neutrophils, representing about 2% of visceral stromal, non-adipocyte, cell fraction. In contrast to resident macrophages and dendritic cells (DCs), their presence may be transient,75 but they may still contribute to insulin resistance76 (Table 2). Especially, in lean conditions, AT harbours eosinophils and mast cells, cells that are typically involved in allergic reactions. Eosinophils secrete IL-4 and IL-13 and contribute to the anti-inflammatory, insulin-sensitive AT phenotype that supports the expansion of M2 ATMs.73 Their content in pathology is decreased. Mast cells in turn increase in dysfunctional AT and have been linked to atherosclerosis and metabolic dysfunction92 by promoting monocyte recruitment.93–95
While the role of macrophages in AT dysfunction is predominantly linked to their innate functions, these cells also serve as antigen-presenting cells leading to the activation of the adaptive immune system in AT. This is particularly evident in pVAT, where tertiary lymphoid structures have been identified.96,97 Dendritic cells, which are the most efficient antigen presenting cells, have also been identified both in typical VAT98 and in pVAT.8,38,39 Thus, dysfunctional AT, creates a microenvironment permissive for T and B lymphocyte activation,98 and lymphocytes constitute the second most abundant immune cell population in VAT.99 In some diseases, their content in the AT exceeds the number of macrophages38,39 allowing for the propagation of inflammation.100,101 T cells that expand in pathology and promote development of insulin resistance, atherosclerosis, and hypertension include predominantly IFN-γ-producing Th1 (CD4+) and Tc1 (CD8+) cells, producing IFNγ and TNF, and IL-17 producing Th17 cells (Figures 1 and 3). These cells initiate an inflammatory cascade that may precede ATM infiltration.46 Another subset of T cells, key to AT dysfunction, include invariant natural killer T (iNKT) cells (Table 2). These lymphocytes express a semi-invariant TCR and proteins typical of NK cells but recognize lipid and glycolipids presented in the context of CD1d MHC-like molecule.102 They can produce both Th2- and Th1-type cytokines.103 In healthy human omentum, up to 10% of T cells are iNKT cells and their number is reduced in patients with obesity and cancer.104 Their exact role is not fully recognized but link to immune activation by lipids makes them a critical candidates for important immuno-metabolic cells.105 Recently, gamma-delta (γ/δ TcR) T cells have been demonstrated to represent substantial proportion of T cells in the AT and their number increases in metabolic and vascular pathologies.61–63, 106 Importantly, these cells are an important source of strongly pro-inflammatory Il-17 and may further regulate immune responses. T cell presence and activation in dysfunctional AT is also closely linked to inflammasome activation.107 Nlrp3 in regulates IL-18 and IFN-γ in the AT and promotes effector T cell accumulation in AT.107 Finally, there is a small number of B cells in the VAT of lean animals, where they provide immunity against infections, including bacteria from peritoneal space.108 B-cell content increases in dysfunctional AT, where they promote activation of other immune cells and may affect metabolic status (Table 2).
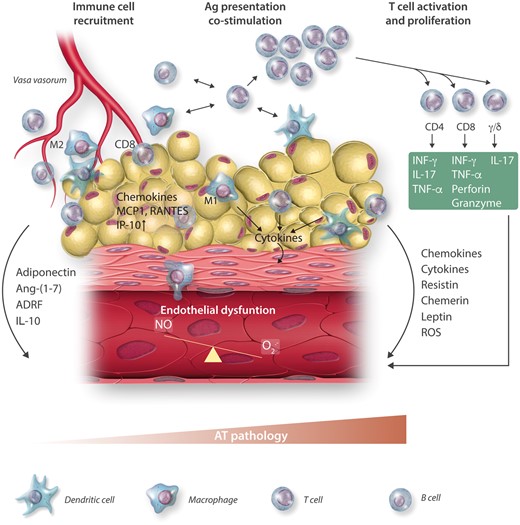
Figure 3
Perivascular AT inflammation as a mechanism of endothelial dysfunction.
The mechanisms of immune cell recruitment and the metabolic and functional consequences of their presence in AT vary in different pathological conditions which are briefly summarized below.
Immune cells in the AT and metabolic diseases
Obesity
Increased adipocyte size triggers a stress response and release of chemoattractant proteins, such as MCP-1, M-CSF-1, or RANTES,109 leading to monocyte recruitment and macrophage accumulation.10,11,110 As discussed above, Adipokines also induce chemokine expression and have key chemotactic properties themselves (Table 1).109 There is a correlation between the accumulation of AT macrophages and adipocyte size.10 Local lipid fluxes are also regulators of ATM recruitment.111 High levels of free fatty acids (FFA) elevate chemokine secretion from adipocytes inducing macrophage chemotaxis to VAT. FFAs activate TLR4 signalling in adipose cells. In TLR4 knockout mice, AT inflammation is prevented, and these animals are protected against obesity-induced insulin resistance.112 Finally, hypoxia and oxidative stress in the VAT is characteristic for obesity and can promote chronic inflammation through metabolic and classical chemokine-dependent mechanisms.113,114 Apart from chemotaxis, increased macrophage proliferation115,116 and differentiation from preadipocytes can enhance the content of macrophages.117 Obesity and insulin resistance are characterized by the predominance of M1 macrophages in the VAT.13,84 Mechanisms of M1 macrophage polarization in obesity are not entirely clear. Non-esterified fatty acids (NEFA) are produced in AT and increased systemically in obese subjects. NEFA induce the expression of IL-6, while reducing IL-10 (Figure 2).118 In contrast, PPARγ skews macrophages toward an alternative M2 phenotype by regulating fatty acid storage and, in doing so, reduces obesity and improves insulin resistance (Figure 2).119
While the metabolic state plays a role in macrophage recruitment and polarization, ATMs in turn have important effects on AT metabolism (Figure 2).3 Depletion of macrophages in AT increases the expression of adipose triglyceride lipase (ATGL) and genes regulated by FFAs. Blockade of monocyte recruitment to VAT genetically or pharmacologically, through CCR2 antagonism protects from diet-induced obesity, improves insulin sensitivity, and lowers AT genes expression related to inflammation and AT dysfunction.81,84,120 Similarly, selective depletion of M1 macrophages decreases pro-inflammatory genes expression and reduction in crown-like structures in obese AT, and consequently improves insulin sensitivity.121 Weight loss decreases macrophage content leading to improved insulin sensitivity.111 Both fasting and bariatric surgery111,122 decrease MCP-1, CSF-3, and genes related to hypoxia (HIF1-α) in AT and consequently reduce the number of ATM cells.122
While macrophages are quantitatively the most abundant immune cells in obesity, T cells also play a critical regulatory role.99 They increase significantly in the AT in obesity and tend to localize around enlarged adipocytes.123 T cells can interact with ATMs regulating inflammatory responses and metabolic dysfunction.124 Of importance are the cytotoxic CD8+ T cells that secrete TNF-α, IL-2, IFN-γ, and chemokine RANTES and CD4+ Th1 cells that secrete TNF-α, IL-12, and INF-γ. These cytokines directly affect adipocyte function and promote M1 macrophage polarization.125 T cell recruitment in obesity is partially mediated by the RANTES–CCR5 axis.99,123 T cell infiltration of AT may precede macrophage-dependent inflammation as it is present after 4–5 weeks of high-fat feeding while macrophage influx was observed after 10 weeks.126 AT T cells infiltration is strongly associated with early reduction of insulin sensitivity and impaired glucose tolerance.126 In line with this, CD8−/− mice are protected from M1 macrophage AT infiltration and subsequent AT dysfunction in obesity.46 Indeed, T cell cytokines are essential for macrophage polarization in the setting of classical inflammation.127 A specific subset of pro-inflammatory T cells (CD153 + PD-1 + CD44hiCD4+) are remarkably increased in the VAT of HFD-fed mice. These osteopontin-producing CD4+ T cells show functional and genetic features of senescent T cells.128,129 T cells in obese AT are regulated by NLRP3 inflammasome, which senses obesity-associated danger signals and contributes to obesity-induced inflammation and insulin resistance.107,130 These mechanisms also link macrophage activation to T cell role in obesity.
Other immune cells are also increased in AT in obesity. B cell AT infiltration is associated with increased IgG production in the AT. Concentrations of pro-inflammatory IgG2c in serum and VAT are elevated in obese mice. Most importantly, B cells from obese mice transferred into B cell-deficient lean mice induce insulin resistance.68 Apart from antibody-mediated mechanisms, B cells from obese mice secrete pro-inflammatory cytokines (IL-6 and INF-γ) and can directly regulate T cells and macrophages.131
Eosinophils also play an important role in the immune regulation of obesity. Mice lacking eosinophils exhibit weight gain, insulin resistance, and increased proinflammatory M1 macrophages in the AT.73 At the same time, mice with eosinophilia (overexpressing IL-5) demonstrate decreased adiposity and improved insulin sensitivity when fed a high-fat diet.73 IL-5 can be produced by AT itself but importantly by innate lymphoid type 2 cells (ILC2s). Deletion of ILC2s causes significant reductions in VAT eosinophils and alternatively activated macrophages M2. Interleukin 33, which promotes activation and recruitment of the ILC2s, leads to ILC2-dependent increases in VAT eosinophils and M2 macrophages.132 Finally, the role of iNKT cells in obesity is not clear. While they are activated by lipid, iNKT cell number is decreased in obesity104 and their depletion increases fat deposition, enhances the presence of M1 macrophages in VAT, and increases insulin resistance and glucose intolerance. Adoptive transfer of iNKT cells into obese mice causes weight loss, improvement of glucose tolerance, and insulin sensitivity.133
A link between vascular oxidative stress and obesity in the context of insulin resistance was recently reported in mice with vascular smooth muscle-targeted deletion of p22phox subunit of NADPH oxidase.134 High-fat feeding did not induce weight gain or leptin resistance in these mice which was associated with strongly reduced T-cell infiltration of pVAT. This is important as indicates causal immunometabolic linking vascular dysfunction to obesity suggesting that vascular inflammation may be primary in the development of obesity and insulin resistance.134,135 Such wide-spread participation of various immune cells in metabolic regulation demonstrates the complexity of the immune system and AT inflammation in obesity.
Diabetes and insulin resistance
Immune cell infiltration into AT provides an important link among obesity, insulin resistance, and diabetes. The number of macrophages infiltrating AT in obese patients with insulin resistance is higher than in patients with insulin-sensitive obesity, independent of the fat mass.11 Insulin levels affect AT inflammation during high-fat diet.11 Progressive macrophage infiltration in VAT preceded increase of insulin in serum, suggesting that AT inflammation is a cause rather than the consequence of insulin resistance.11 Increasing evidence supports the role of adaptive immunity in insulin resistance and diabetes, through inducing pro-inflammatory cytokines in metabolic organs, such as the AT, liver, muscle, and pancreas.136 CCR5 knockout mice are protected from insulin resistance induced by high-fat diet and this effect is mediated by reduced effector T cell accumulation with subsequent reduction of ATMs and M2 polarization of persisting macrophages.137 Clinical studies confirmed that Th1 cells are up-regulated in the AT and peripheral blood from patients with prediabetes or T2DM.138 Moreover, high fat diet and insulin resistance are associated with accumulation of Th1, Th17, and effector CD8+ lymphocytes in the AT, while anti-inflammatory Th2 and Treg cells are decreased.125 Combined anti-CD3 and glucosylceramide treatment induces IL-10 and TGF-β, reducing VAT inflammation in obese mice, and improving fasting glucose levels.101
Immune cell activation, involving the co-stimulatory molecule CD40 and its ligand CD40L, is particularly important in linking AT inflammation to diabetes.139 CD40–CD40L interactions promote pancreatic, AT, and vascular inflammation (Figure 3),140,141 increasing the expression of pro-inflammatory cytokines and chemokines (e.g. TNF-α, IL-6, MCP-1), leukotriene B4 at the same time enhancing lipid droplet accumulation and adipogenesis.142–144 These effects are mediated by reduced expression of insulin receptor substrate (IRS-1) and glucose transporter type-4 (GLUT-4).140,143 CD40L expressed on T cells may induce AT inflammation and impair insulin sensitivity (Figure 2).140
AT immune cells in vascular disease—hypertension and atherosclerosis
Hypertension
Hypertension represents an important example of immuno-metabolic vascular disease.145–147 It is associated with obesity and BMI is one of the strongest predictors of increased blood pressure. Many hypertensive subjects are not obese, but present features of metabolic dysregulation. In hypertension with or without obesity, pVAT inflammation is a prominent feature, and is involved in the pathogenesis of vascular dysfunction.39 This leads to the loss of protective properties of pVAT and promotes loss of endothelium-dependent vasodilatation and enhanced vasoconstriction.8 These functional changes are linked with morphological alterations, as pVAT becomes synthetic, pro-inflammatory, often de-differentiated, and highly metabolically active (Figure 3). This profile is characterized by changes in adipokines (increased resistin and visfatin and decreased adiponectin and leptin) and increased production of chemokines such as RANTES or IP-10 (CXCL10) that are key for recruitment of activated monocytes/macrophages and CD8+ T cells. Apart from AT-specific factors activating immune system in the pVAT, central nervous system is also involved,148 which is important in the context of high perivascular sympathetic innervation and its role in hypertension.149
In health, the immune cell infiltrate in the pVAT constitutes only about 2% of the stromal vascular fraction (SVF) cells.38,39 In vascular pathologies, such as Ang II-induced hypertension, leukocytes in pVAT increase to 7–10% of SVF cells, and, in atherosclerosis, their content reaches up to 10–20%. Hypertension is linked with a significant increase of T cell and antigen presenting cell pVAT infiltration, which mediates endothelial dysfunction150 and provides a link between hypertension and subsequent atherosclerosis. Dysfunctional endothelium promotes inflammation through a number of NFkB dependent, Notch/Jagged1-regulated integrin, and adhesion molecule expression.151,152 Both CD4+ and CD8+ T cell subpopulations are increased in the pVAT in hypertension and express higher levels of proinflammatory cytokines (TNF-α, INF-γ) and CCR5.39,153,154 T cell activation and vascular and renal recruitment is essential for the development of AngII-induced hypertension.153 This is partially mediated by RANTES, similar to obesity and insulin resistance, through which Th1, Tc1, and gamma-delta (γ/δ) T cells, lymphocytes are recruited to the vascular wall.39 Th17 cells, essential for blood pressure increase, are in turn recruited in a RANTES-independent CCR6, -dependent manner.62 Th17 cells not only participate in blood pressure increase155 but also contribute to vascular stiffening observed in hypertension.156 In contrast, adoptive transfer of suppressive, Tregs prevent AngII-induced hypertension and vascular inflammation and improves vascular function.157,158 B cells in pVAT are almost equal in percentage of SVF cells to T cells and their number is increased during hypertension.39 They may act as antigen-presenting cells, modulating T cell responses, and produce IgG2b and IgG3. Depletion of B cells protects from hypertension.159 Finally, macrophage infiltration is also significantly increased in hypertensive pVAT.39 Elevated blood pressure is correlated with pVAT expression of macrophage chemokine receptors CCR2 and its ligands CCL2, CCL7, CCL8, and CCL12. Moreover, the CCR2 antagonist INCB3344,7–9 reduces CCR2 expression and reverses macrophage accumulation in pVAT of mice with hypertension.160 Macrophages in pVAT in healthy conditions appear to be predominantly unpolarised or skewed towards M2.38,39 However, when blood pressure is elevated, the level of both M1 and M2 subpopulations is increased.39 Macrophage infiltration to the pVAT during hypertension is regulated by T cell-dependent mechanisms39 as lymphocyte adaptor protein (LNK) deficiency, leading to hyperactivated T cells increased number of macrophages in the aorta and pVAT.161
Classical antigen-presenting cells such as DCs are regulators of adaptive immune response may play an important role in initiation of inflammation by interactions with T cells. They occur in small numbers in pVAT in the healthy state and their number increases during hypertension.39 Elevated oxidative stress leads to endogenous peptide modification by isoketal (isolevuglandin) adduct formation. This occurs in AT, vessels, and kidneys and promotes antigen presentation by dendritic cells precipitating the role of the T cells in hypertension and further development of pVAT inflammation.162 Blocking the co-stimulation molecules between T cells and dendritic cells prevents pVAT inflammation and decreases blood pressure.163 Moreover, DCs secrete cytokines such as IL-1β, IL-6, IL-23 which promote polarization of T lymphocytes to Th17 cells, which plays particular role in hypertension development.155 Thus, hypertension and associated vascular dysfunction result from complex interactions between several cell types involved in inflammatory responses in hypertension. All types of cells discussed above coexist together in pVAT and they can interact with each other initiating inflammation and causing development of vascular dysfunction and disease.8
The effector mechanisms linking infiltrating immune cells to AT dysfunction in hypertension are related to the release of effector cytokines such as IL-17A, IFNγ, TNF-α, and IL-6.20,164 These cytokines also impair endothelium-dependent relaxation as demonstrated in ex vivo studies,39 as well as in vivo using INF-γ knockout mice.71,165 IL-6 is also necessary for Th17 cell differentiation.166 IL-17, a key pro-hypertensive cytokine, is a potent activator of the endothelial cells promoting the expression of adhesion molecules.167 IL-17A activates RhoA/Rho-kinase and increases inhibitory eNOS Thr495 phosphorylation in endothelial cells leading to decreased NO production.168 Inflammatory cytokines modulate smooth muscle cell constriction, proliferation, and migration.169 They also affect adipokines release from AT. For example, TNFα, IL-6, and IL-17A can all inhibit expression and release of adiponectin.170–172 One of the key adipokines, leptin, has a structure similar to IL-6, IL-12, IL-15 and can affect leukocyte activation and chemotaxis, release of oxygen radicals, VSMC proliferation, and expression of adhesion molecules on endothelial and vascular smooth muscle cells.173 IL-17A and TNF increase leptin and resistin production in AT which upregulate the expression of VCAM1 and ICAM and/or induction of CCL2 as well as endothelin-1 from endothelial cells174 and can induce vascular dysfunction and oxidative stress.8,135 All these mechanisms, besides promoting pVAT dysfunction, provide a link between hypertension and atherosclerosis, in part independently of blood pressure.
Atherosclerosis
PVAT is dysfunctional at all stages of atherogenesis. Increased levels of chemerin, visfatin, leptin, and vaspin are correlated with atherosclerosis development.175 At early stages of atherosclerosis macrophages, T cells and dendritic cells are recruited into perivascular adventita and AT surrounding vasculature.38 This precedes development of endothelial dysfunction176 and oxidative stress110,177 and can be modified by interventions targeting numerous metabolic functions such as Ang(1-7).38,178 Such perivascular inflammation of AT continues to be observed at later stages of the disease, with further increase of macrophage and B cell content.179,180 In a pivotal early study, Galkina et al. observed high leukocytes number in aorta with pVAT in old ApoE−/− mice in advanced atherosclerosis.179,180 Perivascular inflammation, in particular T cell dependent, correlates with lesion size and is clearly age dependent,180,181 and T cell depletion prevents atherosclerosis.182 Leukocyte infiltration to pVAT in atherosclerosis is mediated by similar mechanisms to those observed in hypertension. IL-8, RANTES, and MCP-1 are all increased in the pVAT from arteries with atherosclerotic plaques.183 We have recently described a key role of increase in M1 macrophage polarization in early atherosclerosis in the pVAT and measures to reduce pVAT M1 macrophage differentiation prevent plaque formation.38 Pro-inflammatory IL-17A-producing T cells are present in the adventitia and blockade of IL-17A leads to reduction of macrophage accumulation and atherosclerosis.184 At early stages, leukocytes are scattered throughout the PVAT,179,180 however, with age they seem to organize to form perivascular arterial tertiary lymphoid organs (ATLO),96,97 which can serve also suppressive functions or become dysfunctional. Molecular mechanisms of pVAT inflammation in atherosclerosis indicate several key targets linking immune responses to metabolic dysfunction. Signal transducer and activator transcription 4 (STAT4) is expressed in adipocytes and immune cells and can participate in PVAT inflammation. STAT4 deficiency reduces development of atherosclerosis and PVAT inflammation in ApoE−/− mouse and in insulin resistant obese Zucker rats.185 Interestingly, the number of CD8+ T cells is increased in pVAT of Apoe-/-mice indicating that in metabolic disease, hypertension, and atherosclerosis CD8 cells play a particularly important regulatory role. Recently, an important regulatory function has been attributed to myeloid-derived suppressor cells that can affect AT inflammation.186 Finally, the role of B cells has recently been clarified in atherosclerosis. B cells may serve as an important source of antibodies which promote plaque inflammation and development but can also contribute to antigen presentation and are important source of humoural factors such as TNF.187 The complexity of immunity of atherosclerosis is reviewed elshewhere.182,188
AT immune cells in immune and inflammatory disorders
Autoimmune and inflammatory diseases are typically associated with metabolic dysregulation.189 This is particularly evident in psoriasis, ankylosing spondylitis and rheumatoid arthritis and is linked with development of metabolic syndrome. Psoriasis is associated with significant perivascular, global arterial, and SAT inflammation.190 Similarly, AT in rheumatoid arthritis is highly infiltrated with macrophages which form crown-like structures. These macrophages are activated and express mixed characteristics with high levels of TNF, IL-1beta, but also IL-10.191 These macrophages secrete chemokines (CCL2 and RANTES) as well as IL-6, IL-8, MMP-3.191 These factors further promote macrophage infiltration and can mediate T cell recruitment and activation. T regulatory cells resident in AT may serve an important role in maintaining self-tolerance, and their impairment may promote development of autoimmunity.192 This mechanism may link epidemiological suggestions of links between obesity and autoimmune diseases.192 A key unanswered question is whether adipose tissue in autoimmune disease can create a microenviroment for T cell activation and participate in the pathogenesis of autoimmune disease, or if it is a mere manifestation of systemic inflammation.
Ectopic fat depots and chronic inflammation
Ectopic AT is the visceral fat surrounding intraabdominal organs and located in the liver, heart, pancreas, and muscles. Its presence is linked to low-grade inflammation and cardio-metabolic complications commonly experienced in type 2 diabetes.9 In particular, non-alcoholic fatty liver disease constitutes an important risk determinant for cardiometabolic risk. Myocardial triglyceride, epicardial, and pericardial fat depots accumulate with increasing amount of liver fat and VAT.193 Thus, the association of LV diastolic function with hepatic ectopic fat may be an indicator of systemic inflammation. Ectopic fat accumulation in the liver is linked to the infiltration of the γ/δ+ T cells, granulocytes, and CD11b+ cells in mice. It appears that IL-6 regulates recruitment of these cells and IL-17 production in the liver that promotes ectopic fat.194 This is in part regulated by decreased microRNAs (miR) such as miR26a, providing a link to cardiac injury.195 Similar regulatory properties have been attributed to other miRs expressed in the AT and cardiovascular system.49,196–199 The inflammatory nature of epicardial AT has been known for years,200 and is supported by numerous molecular mechanisms.196 Only recently, however, have we started appreciating the heterogeneity of epicardial AT which is particularly linked to its pro-inflammatory properties.30,201 It may also underlie a link between subclinical atherosclerosis and epicardial fat thickness and hepatic steatosis.202 Thus, ectopic fat accumulation in and around the heart, kidneys, muscles, and liver is a marker of increased cardiovascular risk likely linked to chronic inflammation. At the same time, through the release of adipokines and chemokines, it attracts pro-inflammatory cells like IL-17 producing γ/δ+ T cells, which contribute to the pathology.
Translational evidence
While most of data regarding immune cell infiltration of AT originate from animal models, the role of immune cells has been clearly demonstrated in humans. Similar to animal models, macrophages constitute about 4% of the total AT stromal visceral fraction and it increases up to 15% in obesity.203 There are, however, some key differences in the characteristics of immune cells infiltrating human AT. In contrast to animal studies, an ‘M2-type’ macrophage with remodelling capacity (e.g. through TGF-β and IL-10 release), but also able to secrete proinflammatory cytokines, has been identified in obese AT in humans.204 These mixed-type macrophages have CD11c+CD206+ characteristics but are pro-inflammatory and linked with insulin resistance in human obesity.82 T cell infiltration in human AT is much less characterized.99 AT T cells correlate with BMI, their recruitment is dependent on RANTES chemokine and functionally affects adipocyte and pre-adipocyte differentiation and function.99 Detailed characteristics, activation mechanisms, and effector functions of effector T cells present in human AT are still poorly defined. Adipokines have been shown to regulate human immune cell activation, for example inhibit IL-17 production from T cells and CD8+ effector cell accumulation (summarized in Table 2).
Interestingly, several studies have recently shown that vascular dysfunction, may regulate AT dysfunction, with immune cell infiltration as a key intermediate step. For example, p22phox overexpression in VSMCs leads to increased diet induced obesity that is mediated by AT T cell infiltration.134 The same has been shown in humans where oxidative stress derivated such as 5-HNE regulate adiponectin release from AT.50,205,206 Significant weight loss, in obese individuals, demonstrates clear links to reduced immune cell infiltration in the AT with concomitant improvement of insulin sensitivity and vascular function.122 Several clinical studies using immune targeted therapies in patients with type 2 diabetes confirmed experimental suggestions of the causal role of inflammation in insulin resistance and hyperglycaemia. Indeed, in patients with type 2 diabetes treated with IL-1 receptor blocker (Anakinra),207 IL-1β antagonist (gevokizumab,208 canakinumab,209 LY2189102210), TNF antagonist (CDP571,211 Ro 45-2081,212 etanercept213) or IKKβ-NF-κB inhibitor214 all have been shown to improve metabolic profile providing an important translational evidence.
Conclusions
Over the years, it has become apparent that vascular and metabolic dysfunction occur in a wide range of vascular pathologies and are closely regulated by coincident immune dysregulation. Immune cells infiltrating AT both sense and can induce metabolic disturbances, contributing to a vicious circle of AT dysfunction. Immune infiltration of AT is critical in T2D, obesity or insulin resistance it is also a primary feature of hypertension or atherosclerosis, making immuno-metabolic interventions a valuable therapeutic approach in a wide range of cardiovascular pathologies. While in animal models of metabolic disease, we have now identified the key immune cell subpopulations and their immunometabolic profiles, relatively little is known about human AT infiltration. One challenge is to identify specific immune cell populations within human AT that could be targeted and differences in their characteristics depending on anatomical location. Finally, we need to understand dynamic changes of the role of immune cells at different time points of metabolic and vascular pathology.
While specific therapeutic interventions limiting AT inflammation may be designed based on this,215,216 we already know that commonly used agents, including methotrexate, anti-TNF therapies and leflunomide limit macrophage infiltration in AT.217 Similarly, several vasoactive therapies such as ACE-inhibitors or angiotensin II receptor blockers have potential to limit inflammation in pVAT. While these approaches lead to systemic immunosuppression, more specific small molecule immune targeted therapies might prove helpful to improve the metabolic profile of AT and prevent AT dysfunction.
Acknowledgements
The paper is supported by Wellcome Trust Senior Biomedical Fellowship (to T.J.G.), National Science Centre of Poland (No. 2011/03/B/NZ4/02454) and BHF Centre of Research Excellence (RE/13/5/30177) and ‘Mobilnosc Plus’ (1300/1/MOB/IV/2015/0) to D.S.
Conflict of interest: none declared.
References
1
Stevens
GA
,
Singh
GM
,
Lu
Y
,
Danaei
G
,
Lin
JK
,
Finucane
MM
,
Bahalim
AN
,
McIntire
RK
,
Gutierrez
HR
,
Cowan
M
,
Paciorek
CJ
,
Farzadfar
F
,
Riley
L
,
Ezzati
M
,
Factors
GBMR.
National, regional, and global trends in adult overweight and obesity prevalences
.
Popul Health Metr
2012
;
10
:
22
.
2
Newton
R
,
Priyadharshini
B
,
Turka
LA.
Immunometabolism of regulatory T cells
.
Nat Immunol
2016
;
17
:
618
–
625
.
3
Norata
GD
,
Caligiuri
G
,
Chavakis
T
,
Matarese
G
,
Netea
MG
,
Nicoletti
A
,
O'neill
LA
,
Marelli-Berg
FM.
The cellular and molecular basis of translational immunometabolism
.
Immunity
2015
;
43
:
421
–
434
.
4
Guzik
TJ
,
Cosentino
F.
Epigenetics and immunometabolism in diabetes and aging
.
Antioxid Redox Signal
2017
; in revision.
5
Ronti
T
,
Lupattelli
G
,
Mannarino
E.
The endocrine function of adipose tissue: an update
.
Clin Endocrinol (Oxf)
2006
;
64
:
355
–
365
.
6
Virtanen
KA
,
Lidell
ME
,
Orava
J
,
Heglind
M
,
Westergren
R
,
Niemi
T
,
Taittonen
M
,
Laine
J
,
Savisto
NJ
,
Enerback
S
,
Nuutila
P.
Functional brown adipose tissue in healthy adults
.
N Engl J Med
2009
;
360
:
1518
–
1525
.
7
Ibrahim
MM.
Subcutaneous and visceral adipose tissue: structural and functional differences
.
Obes Rev
2010
;
11
:
11
–
18
.
8
Nosalski
R
,
Guzik
TJ.
Perivascular adipose tissue inflammation in vascular disease
.
Br J Pharmacol
2017
; doi: 10.1111/bph.13705.
9
Lim
S
,
Meigs
JB.
Ectopic fat and cardiometabolic and vascular risk
.
Int J Cardiol
2013
;
169
:
166
–
176
.
10
Weisberg
SP
,
McCann
D
,
Desai
M
,
Rosenbaum
M
,
Leibel
RL
,
Ferrante
AW.
Obesity is associated with macrophage accumulation in adipose tissue
.
J Clin Invest
2003
;
112
:
1796
–
1808
.
11
Xu
HY
,
Barnes
GT
,
Yang
Q
,
Tan
Q
,
Yang
DS
,
Chou
CJ
,
Sole
J
,
Nichols
A
,
Ross
JS
,
Tartaglia
LA
,
Chen
H.
Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance
.
J Clin Invest
2003
;
112
:
1821
–
1830
.
12
Hotamisligil
GS
,
Shargill
NS
,
Spiegelman
BM.
Adipose expression of tumor-necrosis-factor-alpha–direct role in obesity-linked insulin resistance
.
Science
1993
;
259
:
87
–
91
.
13
Lumeng
CN
,
DeYoung
SM
,
Bodzin
JL
,
Saltiel
AR.
Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity
.
Diabetes
2007
;
56
:
16
–
23
.
14
Chatterjee
TK
,
Aronow
BJ
,
Tong
WS
,
Manka
D
,
Tang
Y
,
Bogdanov
VY
,
Unruh
D
,
Blomkalns
AL
,
Piegore
MG
Jr.,
Weintraub
DS
,
Rudich
SM
,
Kuhel
DG
,
Hui
DY
,
Weintraub
NL.
Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis
.
Physiol Genomics
2013
;
45
:
697
–
709
.
15
Brown
NK
,
Zhou
Z
,
Zhang
JF
,
Zeng
R
,
Wu
JR
,
Eitzman
DT
,
Chen
YE
,
Chang
L.
Perivascular adipose tissue in vascular function and disease—a review of current research and animal models
.
Arterioscl Throm Vas
2014
;
34
:
1621
–
1630
.
16
Watanabe
K
,
Watanabe
R
,
Konii
H
,
Shirai
R
,
Sato
K
,
Matsuyama
TA
,
Ishibashi-Ueda
H
,
Koba
S
,
Kobayashi
Y
,
Hirano
T
,
Watanabe
T.
Counteractive effects of omentin-1 against atherogenesisdagger
.
Cardiovasc Res
2016
;
110
:
118
–
128
.
17
Hiramatsu-Ito
M
,
Shibata
R
,
Ohashi
K
,
Uemura
Y
,
Kanemura
N
,
Kambara
T
,
Enomoto
T
,
Yuasa
D
,
Matsuo
K
,
Ito
M
,
Hayakawa
S
,
Ogawa
H
,
Otaka
N
,
Kihara
S
,
Murohara
T
,
Ouchi
N.
Omentin attenuates atherosclerotic lesion formation in apolipoprotein E-deficient mice
.
Cardiovasc Res
2016
;
110
:
107
–
117
.
18
Kanneganti
TD
,
Dixit
VD.
Immunological complications of obesity
.
Nat Immunol
2012
;
13
:
707
–
712
.
19
Hong
EG
,
Ko
HJ
,
Cho
YR
,
Kim
HJ
,
Ma
Z
,
Yu
TY
,
Friedline
RH
,
Kurt-Jones
E
,
Finberg
R
,
Fischer
MA
,
Granger
EL
,
Norbury
CC
,
Hauschka
SD
,
Philbrick
WM
,
Lee
CG
,
Elias
JA
,
Kim
JK.
Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle
.
Diabetes
2009
;
58
:
2525
–
2535
.
20
Lumeng
CN
,
Bodzin
JL
,
Saltiel
AR.
Obesity induces a phenotypic switch in adipose tissue macrophage polarization
.
J Clin Invest
2007
;
117
:
175
–
184
.
21
Wolf
AM
,
Wolf
D
,
Rumpold
H
,
Enrich
B
,
Tilg
H.
Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes
.
Biochem Biophys Res Commun
2004
;
323
:
630
–
635
.
22
Cintra
DE
,
Pauli
JR
,
Araujo
EP
,
Moraes
JC
,
de Souza
CT
,
Milanski
M
,
Morari
J
,
Gambero
A
,
Saad
MJ
,
Velloso
LA.
Interleukin-10 is a protective factor against diet-induced insulin resistance in liver
.
J Hepatol
2008
;
48
:
628
–
637
.
23
Nguyen
KD
,
Qiu
Y
,
Cui
X
,
Goh
YP
,
Mwangi
J
,
David
T
,
Mukundan
L
,
Brombacher
F
,
Locksley
RM
,
Chawla
A.
Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis
.
Nature
2011
;
480
:
104
–
108
.
24
Qiu
Y
,
Nguyen
KD
,
Odegaard
JI
,
Cui
X
,
Tian
X
,
Locksley
RM
,
Palmiter
RD
,
Chawla
A.
Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat
.
Cell
2014
;
157
:
1292
–
1308
.
25
Feuerer
M
,
Herrero
L
,
Cipolletta
D
,
Naaz
A
,
Wong
J
,
Nayer
A
,
Lee
J
,
Goldfine
AB
,
Benoist
C
,
Shoelson
S
,
Mathis
D.
Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters
.
Nat Med
2009
;
15
:
930
–
939
.
26
Guzik
TJ
,
Marvar
PJ
,
Czesnikiewicz-Guzik
M
,
Korbut
R.
Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction
.
J Physiol Pharmacol
2007
;
58
:
591
–
610
.
27
Ignacak
A
,
Kasztelnik
M
,
Sliwa
T
,
Korbut
RA
,
Rajda
K
,
Guzik
TJ.
Prolactin–not only lactotrophin. A “new” view of the “old” hormone
.
J Physiol Pharmacol
2012
;
63
:
435
–
443
.
28
Sun
K
,
Tordjman
J
,
Clement
K
,
Scherer
PE.
Fibrosis and Adipose Tissue Dysfunction
.
Cell Metab
2013
;
18
:
470
–
477
.
29
Guzik
TJ
,
Mangalat
D
,
Korbut
R.
Adipocytokines – novel link between inflammation and vascular function?
.
J Physiol Pharmacol
2006
;
57
:
505
–
528
.
30
Hatem
SN
,
Redheuil
A
,
Gandjbakhch
E.
Cardiac adipose tissue and atrial fibrillation: the perils of adiposity
.
Cardiovasc Res
2016
;
109
:
502
–
509
.
31
Naylor
C
,
Petri
WA.
Jr.
Leptin regulation of immune responses
.
Trends Mol Med
2016
;
22
:
88
–
98
.
32
Ouchi
N
,
Parker
JL
,
Lugus
JJ
,
Walsh
K.
Adipokines in inflammation and metabolic disease
.
Nat Rev Immunol
2011
;
11
:
85
–
97
.
33
Shibata
S
,
Tada
Y
,
Hau
CS
,
Mitsui
A
,
Kamata
M
,
Asano
Y
,
Sugaya
M
,
Kadono
T
,
Masamoto
Y
,
Kurokawa
M
,
Yamauchi
T
,
Kubota
N
,
Kadowaki
T
,
Sato
S.
Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from gammadelta-T cells
.
Nat Commun
2015
;
6
:
7687.
34
Cheng
X
,
Folco
EJ
,
Shimizu
K
,
Libby
P.
Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells
.
J Biol Chem
2012
;
287
:
36896
–
36904
.
35
Walcher
D
,
Hess
K
,
Berger
R
,
Aleksic
M
,
Heinz
P
,
Bach
H
,
Durst
R
,
Hausauer
A
,
Hombach
V
,
Marx
N.
Resistin: a newly identified chemokine for human CD4-positive lymphocytes
.
Cardiovasc Res
2010
;
85
:
167
–
174
.
36
Kukla
M
,
Mazur
W
,
Buldak
RJ
,
Zwirska-Korczala
K.
Potential role of leptin, adiponectin and three novel adipokines–visfatin, chemerin and vaspin–in chronic hepatitis
.
Mol Med
2011
;
17
:
1397
–
1410
.
37
Moraes-Vieira
PM
,
Yore
MM
,
Dwyer
PM
,
Syed
I
,
Aryal
P
,
Kahn
BB.
RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance
.
Cell Metab
2014
;
19
:
512
–
526
.
38
Skiba
DS
,
Nosalski
R
,
Mikolajczyk
TP
,
Siedlinski
M
,
Rios
FJ
,
Montezano
AC
,
Jawien
J
,
Olszanecki
R
,
Korbut
R
,
Czesnikiewicz-Guzik
M
,
Touyz
RM
,
Guzik
TJ.
Antiatherosclerotic effect of Ang- (1–7) non-peptide mimetic (AVE 0991) is mediated by inhibition of perivascular and plaque inflammation in early atherosclerosis
.
Br J Pharmacol
2016
. doi: 10.1111/bph.13685.
39
Mikolajczyk
TP
,
Nosalski
R
,
Szczepaniak
P
,
Budzyn
K
,
Osmenda
G
,
Skiba
D
,
Sagan
A
,
Wu
J
,
Vinh
A
,
Marvar
PJ
,
Guzik
B
,
Podolec
J
,
Drummond
G
,
Lob
HE
,
Harrison
DG
,
Guzik
TJ.
Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension
.
Faseb J
2016
;
30
:
1987
–
1999
.
40
Bapat
SP
,
Suh
JM
,
Fang
S
,
Liu
SH
,
Zhang
Y
,
Cheng
A
,
Zhou
C
,
Liang
YQ
,
LeBlanc
M
,
Liddle
C
,
Atkins
AR
,
Yu
RT
,
Downes
M
,
Evans
RM
,
Zheng
Y.
Depletion of fat-resident T-reg cells prevents age-associated insulin resistance
.
Nature
2015
;
528
:
137.
+.
41
Freemerman
AJ
,
Johnson
AR
,
Sacks
GN
,
Milner
JJ
,
Kirk
EL
,
Troester
MA
,
Macintyre
AN
,
Goraksha-Hicks
P
,
Rathmell
JC
,
Makowski
L.
Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype
.
J Biol Chem
2014
;
289
:
7884
–
7896
.
42
Pamir
N
,
Liu
NC
,
Irwin
A
,
Becker
L
,
Peng
YF
,
Ronsein
GE
,
Bornfeldt
KE
,
Duffield
JS
,
Heinecke
JW.
Granulocyte/macrophage colony-stimulating factor-dependent dendritic cells restrain lean adipose tissue expansion
.
J Biol Chem
2015
;
290
:
14656
–
14667
.
43
Bilkovski
R
,
Schulte
DM
,
Oberhauser
F
,
Mauer
J
,
Hampel
B
,
Gutschow
C
,
Krone
W
,
Laudes
M.
Adipose tissue macrophages inhibit adipogenesis of mesenchymal precursor cells via wnt-5a in humans
.
Int J Obes Relat Metab Disord
2011
;
35
:
1450
–
1454
.
44
Chen
YH
,
Tian
J
,
Tian
XY
,
Tang
XY
,
Rui
K
,
Tong
J
,
Lu
LW
,
Xu
HX
,
Wang
SJ.
Adipose tissue dendritic cells enhances inflammation by prompting the generation of Th17 cells
.
Plos One
2014
;
9
.
45
Moore
JP
,
Vinh
A
,
Tuck
KL
,
Sakkal
S
,
Krishnan
SM
,
Chan
CT
,
Lieu
M
,
Samuel
CS
,
Diep
H
,
Kemp-Harper
BK
,
Tare
M
,
Ricardo
SD
,
Guzik
TJ
,
Sobey
CG
,
Drummond
GR.
M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure
.
Am J Physiol Heart Circ Physiol
2015
;
309
:
H906
–
H917
.
46
Nishimura
S
,
Manabe
I
,
Nagasaki
M
,
Eto
K
,
Yamashita
H
,
Ohsugi
M
,
Otsu
M
,
Hara
K
,
Ueki
K
,
Sugiura
S
,
Yoshimura
K
,
Kadowaki
T
,
Nagai
R.
CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity
.
Nat Med
2009
;
15
:
914
–
920
.
47
Wolf
MJ
,
Adili
A
,
Piotrowitz
K
,
Abdullah
Z
,
Boege
Y
,
Stemmer
K
,
Ringelhan
M
,
Simonavicius
N
,
Egger
M
,
Wohlleber
D
,
Lorentzen
A
,
Einer
C
,
Schulz
S
,
Clavel
T
,
Protzer
U
,
Thiele
C
,
Zischka
H
,
Moch
H
,
Tschop
M
,
Tumanov
AV
,
Haller
D
,
Unger
K
,
Karin
M
,
Kopf
M
,
Knolle
P
,
Weber
A
,
Heikenwalder
M.
Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes
.
Cancer Cell
2014
;
26
:
549
–
564
.
48
Revelo
XS
,
Tsai
S
,
Lei
H
,
Luck
H
,
Ghazarian
M
,
Tsui
H
,
Shi
SY
,
Schroer
S
,
Luk
CT
,
Lin
GH
,
Mak
TW
,
Woo
M
,
Winer
S
,
Winer
DA.
Perforin is a novel immune regulator of obesity-related insulin resistance
.
Diabetes
2015
;
64
:
90
–
103
.
49
Ding
W
,
Li
J
,
Singh
J
,
Alif
R
,
Vazquez-Padron
RI
,
Gomes
SA
,
Hare
JM
,
Shehadeh
LA.
miR-30e targets IGF2-regulated osteogenesis in bone marrow-derived mesenchymal stem cells, aortic smooth muscle cells, and ApoE−/− mice
.
Cardiovasc Res
2015
;
106
:
131
–
142
.
50
Antonopoulos
AS
,
Margaritis
M
,
Coutinho
P
,
Digby
J
,
Patel
R
,
Psarros
C
,
Ntusi
N
,
Karamitsos
TD
,
Lee
R
,
De Silva
R
,
Petrou
M
,
Sayeed
R
,
Demosthenous
M
,
Bakogiannis
C
,
Wordsworth
PB
,
Tousoulis
D
,
Neubauer
S
,
Channon
KM
,
Antoniades
C.
Reciprocal effects of systemic inflammation and brain natriuretic peptide on adiponectin biosynthesis in adipose tissue of patients with ischemic heart disease
.
Arterioscler Thromb Vasc Biol
2014
;
34
:
2151
–
2159
.
51
Mallat
Z
,
Gojova
A
,
Brun
V
,
Esposito
B
,
Fournier
N
,
Cottrez
F
,
Tedgui
A
,
Groux
H.
Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice
.
Circulation
2003
;
108
:
1232
–
1237
.
52
Ait-Oufella
H
,
Taleb
S
,
Mallat
Z
,
Tedgui
A.
Recent advances on the role of cytokines in atherosclerosis
.
Arterioscler Thromb Vasc Biol
2011
;
31
:
969
–
979
.
53
Zuniga
LA
,
Shen
WJ
,
Joyce-Shaikh
B
,
Pyatnova
EA
,
Richards
AG
,
Thom
C
,
Andrade
SM
,
Cua
DJ
,
Kraemer
FB
,
Butcher
EC.
IL-17 regulates adipogenesis, glucose homeostasis, and obesity
.
J Immunol
2010
;
185
:
6947
–
6959
.
54
van Bruggen
N
,
Ouyang
WJ.
Th17 cells at the crossroads of autoimmunity, inflammation, and atherosclerosis
.
Immunity
2014
;
40
:
10
–
12
.
55
Emamaullee
JA
,
Davis
J
,
Merani
S
,
Toso
C
,
Elliott
JF
,
Thiesen
A
,
Shapiro
AM.
Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice
.
Diabetes
2009
;
58
:
1302
–
1311
.
56
Taleb
S
,
Romain
M
,
Ramkhelawon
B
,
Uyttenhove
C
,
Pasterkamp
G
,
Herbin
O
,
Esposito
B
,
Perez
N
,
Yasukawa
H
,
Van Snick
J
,
Yoshimura
A
,
Tedgui
A
,
Mallat
Z.
Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis
.
J Exp Med
2009
;
206
:
2067
–
2077
.
57
Taleb
S
,
Tedgui
A
,
Mallat
Z.
IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles
.
Arterioscler Thromb Vasc Biol
2015
;
35
:
258
–
264
.
58
Wang
L
,
Gao
S
,
Xu
W
,
Zhao
S
,
Zhou
J
,
Wang
N
,
Yuan
Z.
Allergic asthma accelerates atherosclerosis dependent on Th2 and Th17 in apolipoprotein E deficient mice
.
J Mol Cell Cardiol
2014
;
72
:
20
–
27
.
59
Xiong
Q
,
Jin
L
,
Li
J
,
Fan
H
,
Cao
R
,
Wu
J
,
Li
T
,
Liu
J.
A Th2 immune shift to heat shock protein 65 fails to arrest atherosclerosis: proatherogenic role of Th2-deviated autoantibodies
.
Autoimmunity
2009
;
42
:
475
–
483
.
60
Daugherty
A
,
Rateri
DL.
T lymphocytes in atherosclerosis: the yin-yang of Th1 and Th2 influence on lesion formation
.
Circ Res
2002
;
90
:
1039
–
1040
.
61
Mehta
P
,
Nuotio-Antar
AM
,
Smith
CW.
gammadelta T cells promote inflammation and insulin resistance during high fat diet-induced obesity in mice
.
J Leukoc Biol
2015
;
97
:
121
–
134
.
62
Caillon
A
,
Mian
MO
,
Fraulob-Aquino
JC
,
Huo
KG
,
Barhoumi
T
,
Ouerd
S
,
Sinnaeve
PR
,
Paradis
P
,
Schiffrin
EL.
Gamma delta T cells mediate angiotensin ii-induced hypertension and vascular injury
.
Circulation
2017
;
135
:
2155
–
2162
.
63
Cheng
HY
,
Wu
R
,
Hedrick
CC.
Gammadelta (gammadelta) T lymphocytes do not impact the development of early atherosclerosis
.
Atherosclerosis
2014
;
234
:
265
–
269
.
64
Feuerer
M
,
Herrero
L
,
Cipolletta
D
,
Naaz
A
,
Wong
J
,
Nayer
A
,
Lee
J
,
Goldfine
A
,
Benoist
C
,
Shoelson
S
,
Mathis
D.
Fat T(reg) cells: a liaison between the immune and metabolic systems
.
Nat Med
2009
;
15
:
930
–
939
.
65
Qi
L.
Tipping the balance in metabolic regulation: regulating regulatory T cells by costimulation
.
Diabetes
2014
;
63
:
1179
–
1181
.
66
Caligiuri
G
,
Rudling
M
,
Ollivier
V
,
Jacob
MP
,
Michel
JB
,
Hansson
GK
,
Nicoletti
A.
Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice
.
Mol Med
2003
;
9
:
10
–
17
.
67
Ait-Oufella
H
,
Salomon
BL
,
Potteaux
S
,
Robertson
AK
,
Gourdy
P
,
Zoll
J
,
Merval
R
,
Esposito
B
,
Cohen
JL
,
Fisson
S
,
Flavell
RA
,
Hansson
GK
,
Klatzmann
D
,
Tedgui
A
,
Mallat
Z.
Natural regulatory T cells control the development of atherosclerosis in mice
.
Nat Med
2006
;
12
:
178
–
180
.
68
Winer
DA
,
Winer
S
,
Shen
L
,
Wadia
PP
,
Yantha
J
,
Paltser
G
,
Tsui
H
,
Wu
P
,
Davidson
MG
,
Alonso
MN
,
Leong
HX
,
Glassford
A
,
Caimol
M
,
Kenkel
JA
,
Tedder
TF
,
McLaughlin
T
,
Miklos
DB
,
Dosch
HM
,
Engleman
EG.
B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies
.
Nat Med
2011
;
17
:
610
–
U134
.
69
Wensveen
FM
,
Jelencic
V
,
Valentic
S
,
Sestan
M
,
Wensveen
TT
,
Theurich
S
,
Glasner
A
,
Mendrila
D
,
Stimac
D
,
Wunderlich
FT
,
Bruning
JC
,
Mandelboim
O
,
Polic
B.
NK cells link obesity-induced adipose stress to inflammation and insulin resistance
.
Nat Immunol
2015
;
16
:
376
–
385
.
70
Caspar-Bauguil
S
,
Cousin
B
,
Galinier
A
,
Segafredo
C
,
Nibbelink
M
,
Andre
A
,
Casteilla
L
,
Penicaud
L.
Adipose tissues as an ancestral immune organ: site-specific change in obesity
.
Febs Lett
2005
;
579
:
3487
–
3492
.
71
Kossmann
S
,
Schwenk
M
,
Hausding
M
,
Karbach
SH
,
Schmidgen
MI
,
Brandt
M
,
Knorr
M
,
Hu
H
,
Kroller-Schon
S
,
Schonfelder
T
,
Grabbe
S
,
Oelze
M
,
Daiber
A
,
Munzel
T
,
Becker
C
,
Wenzel
P.
Angiotensin II-induced vascular dysfunction depends on interferon-gamma-driven immune cell recruitment and mutual activation of monocytes and NK-cells
.
Arterioscler Thromb Vasc Biol
2013
;
33
:
1313
–
1319
.
72
Wu
L
,
Parekh
VV
,
Gabriel
CL
,
Bracy
DP
,
Marks-Shulman
PA
,
Tamboli
RA
,
Kim
S
,
Mendez-Fernandez
YV
,
Besra
GS
,
Lomenick
JP
,
Williams
B
,
Wasserman
DH
,
Van Kaer
L.
Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice
.
P Natl Acad Sci USA
2012
;
109
:
E1143
–
E1152
.
73
Wu
D
,
Molofsky
AB
,
Liang
HE
,
Ricardo-Gonzalez
RR
,
Jouihan
HA
,
Bando
JK
,
Chawla
A
,
Locksley
RM.
Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis
.
Science
2011
;
332
:
243
–
247
.
74
Withers
SB
,
Forman
R
,
Meza-Perez
S
,
Sorobetea
D
,
Sitnik
K
,
Hopwood
T
,
Lawrence
CB
,
Agace
WW
,
Else
KJ
,
Heagerty
AM
,
Svensson-Frej
M
,
Cruickshank
SM.
Eosinophils are key regulators of perivascular adipose tissue and vascular functionality
.
Sci Rep
2017
;
7
:
44571.
75
Elgazar-Carmon
V
,
Rudich
A
,
Hadad
N
,
Levy
R.
Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding
.
J Lipid Res
2008
;
49
:
1894
–
1903
.
76
Talukdar
S
,
Oh
DY
,
Bandyopadhyay
G
,
Li
D
,
Xu
J
,
McNelis
J
,
Lu
M
,
Li
P
,
Yan
Q
,
Zhu
Y
,
Ofrecio
J
,
Lin
M
,
Brenner
MB
,
Olefsky
JM.
Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase
.
Nat Med
2012
;
18
:
1407
–
1412
.
77
Scheiermann
C
,
Frenette
PS
,
Hidalgo
A.
Regulation of leucocyte homeostasis in the circulation
.
Cardiovasc Res
2015
;
107
:
340
–
351
.
78
Rossaint
J
,
Zarbock
A.
Platelets in leucocyte recruitment and function
.
Cardiovasc Res
2015
;
107
:
386
–
395
.
79
Bornstein
SR
,
Abu-Asab
M
,
Glasow
A
,
Path
G
,
Hauner
H
,
Tsokos
M
,
Chrousos
GP
,
Scherbaum
WA.
Immnohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells in primary culture
.
Diabetes
2000
;
49
:
532
–
538
.
80
Gordon
S.
The role of the macrophage in immune regulation
.
Res Immunol
1998
;
149
:
685
–
688
.
81
Weisberg
SP
,
Hunter
D
,
Huber
R
,
Lemieux
J
,
Slaymaker
S
,
Vaddi
K
,
Charo
I
,
Leibel
RL
,
Ferrante
AW.
Jr.
CCR2 modulates inflammatory and metabolic effects of high-fat feeding
.
J Clin Invest
2006
;
116
:
115
–
124
.
82
Wentworth
JM
,
Naselli
G
,
Brovvn
WA
,
Doyle
L
,
Phipson
B
,
Smyth
GK
,
Wabitsch
M
,
O'brien
PE
,
Harrison
LC.
Pro-inflammatory CD11c(+)CD206(+) adipose tissue macrophages are associated with insulin resistance in human obesity
.
Diabetes
2010
;
59
:
1648
–
1656
.
83
Shaul
ME
,
Bennett
G
,
Strissel
KJ
,
Greenberg
AS
,
Obin
MS.
Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet-induced obesity in mice
.
Diabetes
2010
;
59
:
1171
–
1181
.
84
Oh
DY
,
Morinaga
H
,
Talukdar
S
,
Bae
EJ
,
Olefsky
JM.
Increased macrophage migration into adipose tissue in obese mice
.
Diabetes
2012
;
61
:
346
–
354
.
85
Weber
C
,
Shantsila
E
,
Hristov
M
,
Caligiuri
G
,
Guzik
T
,
Heine
GH
,
Hoefer
IE
,
Monaco
C
,
Peter
K
,
Rainger
E
,
Siegbahn
A
,
Steffens
S
,
Wojta
J
,
Lip
GY.
Role and analysis of monocyte subsets in cardiovascular disease. Joint consensus document of the European Society of Cardiology (ESC) Working Groups “Atherosclerosis & Vascular Biology”
.
Thromb Haemost
2016
;
116
:
626
–
637
.
86
Eerenberg
ES
,
Teunissen
PF
,
van den Born
BJ
,
Meijers
JC
,
Hollander
MR
,
Jansen
M
,
Tijssen
R
,
Belien
JA
,
van de Ven
PM
,
Aly
MF
,
Kamp
O
,
Niessen
HW
,
Kamphuisen
PW
,
Levi
M
,
van Royen
N.
The role of ADAMTS13 in acute myocardial infarction: cause or consequence?
.
Cardiovasc Res
2016
;
111
:
194
–
203
.
87
Duca
L
,
Blaise
S
,
Romier
B
,
Laffargue
M
,
Gayral
S
,
El Btaouri
H
,
Kawecki
C
,
Guillot
A
,
Martiny
L
,
Debelle
L
,
Maurice
P.
Matrix ageing and vascular impacts: focus on elastin fragmentation
.
Cardiovasc Res
2016
;
110
:
298
–
308
.
88
Di Gregoli
K
,
George
SJ
,
Jackson
CL
,
Newby
AC
,
Johnson
JL.
Differential effects of tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 on atherosclerosis and monocyte/macrophage invasion
.
Cardiovasc Res
2016
;
109
:
318
–
330
.
89
De Caterina
R
,
Madonna
R.
Von Willebrand factor, ADAMTS13, and coronary microvascular obstruction: beautiful hypotheses, ugly facts
.
Cardiovasc Res
2016
;
111
:
169
–
171
.
90
Ensan
S
,
Li
A
,
Besla
R
,
Degousee
N
,
Cosme
J
,
Roufaiel
M
,
Shikatani
EA
,
El-Maklizi
M
,
Williams
JW
,
Robins
L
,
Li
C
,
Lewis
B
,
Yun
TJ
,
Lee
JS
,
Wieghofer
P
,
Khattar
R
,
Farrokhi
K
,
Byrne
J
,
Ouzounian
M
,
Zavitz
CC
,
Levy
GA
,
Bauer
CM
,
Libby
P
,
Husain
M
,
Swirski
FK
,
Cheong
C
,
Prinz
M
,
Hilgendorf
I
,
Randolph
GJ
,
Epelman
S
,
Gramolini
AO
,
Cybulsky
MI
,
Rubin
BB
,
Robbins
CS.
Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth
.
Nat Immunol
2016
;
17
:
159
–
168
.
91
Robbins
CS
,
Hilgendorf
I
,
Weber
GF
,
Theurl
I
,
Iwamoto
Y
,
Figueiredo
JL
,
Gorbatov
R
,
Sukhova
GK
,
Gerhardt
LM
,
Smyth
D
,
Zavitz
CC
,
Shikatani
EA
,
Parsons
M
,
van Rooijen
N
,
Lin
HY
,
Husain
M
,
Libby
P
,
Nahrendorf
M
,
Weissleder
R
,
Swirski
FK.
Local proliferation dominates lesional macrophage accumulation in atherosclerosis
.
Nat Med
2013
;
19
:
1166
–
1172
.
92
Wu
J
,
Grassia
G
,
Cambrook
H
,
Ialenti
A
,
MacRitchie
N
,
Carberry
J
,
Wadsworth
RM
,
Lawrence
C
,
Kennedy
S
,
Maffia
P.
Perivascular mast cells regulate vein graft neointimal formation and remodeling
.
PeerJ
2015
;
3
:
e1192.
93
Bot
I
,
de Jager
SC
,
Zernecke
A
,
Lindstedt
KA
,
van Berkel
TJ
,
Weber
C
,
Biessen
EA.
Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice
.
Circulation
2007
;
115
:
2516
–
2525
.
94
Layne
K
,
Di Giosia
P
,
Ferro
A
,
Passacquale
G.
Anti-platelet drugs attenuate the expansion of circulating CD14highCD16+ monocytes under pro-inflammatory conditions
.
Cardiovasc Res
2016
;
111
:
26
–
33
.
95
Gerhardt
T
,
Ley
K.
Monocyte trafficking across the vessel wall
.
Cardiovasc Res
2015
;
107
:
321
–
330
.
96
Hu
D
,
Mohanta
SK
,
Yin
C
,
Peng
L
,
Ma
Z
,
Srikakulapu
P
,
Grassia
G
,
MacRitchie
N
,
Dever
G
,
Gordon
P
,
Burton
FL
,
Ialenti
A
,
Sabir
SR
,
McInnes
IB
,
Brewer
JM
,
Garside
P
,
Weber
C
,
Lehmann
T
,
Teupser
D
,
Habenicht
L
,
Beer
M
,
Grabner
R
,
Maffia
P
,
Weih
F
,
Habenicht
AJ.
Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin beta receptors
.
Immunity
2015
;
42
:
1100
–
1115
.
97
Akhavanpoor
M
,
Wangler
S
,
Gleissner
CA
,
Korosoglou
G
,
Katus
HA
,
Erbel
C.
Adventitial inflammation and its interaction with intimal atherosclerotic lesions
.
Front Physiol
2014
;
5
:
296.
98
Bertola
A
,
Ciucci
T
,
Rousseau
D
,
Bourlier
V
,
Duffaut
C
,
Bonnafous
S
,
Blin-Wakkach
C
,
Anty
R
,
Iannelli
A
,
Gugenheim
J
,
Tran
A
,
Bouloumie
A
,
Gual
P
,
Wakkach
A.
Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients
.
Diabetes
2012
;
61
:
2238
–
2247
.
99
Wu
H
,
Ghosh
S
,
Perrard
XD
,
Feng
L
,
Garcia
GE
,
Perrard
JL
,
Sweeney
JF
,
Peterson
LE
,
Chan
L
,
Smith
CW
,
Ballantyne
CM.
T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity
.
Circulation
2007
;
115
:
1029
–
1038
.
100
Han
JM
,
Wu
D
,
Denroche
HC
,
Yao
Y
,
Verchere
CB
,
Levings
MK.
IL-33 reverses an obesity-induced deficit in visceral adipose tissue ST2+ T regulatory cells and ameliorates adipose tissue inflammation and insulin resistance
.
JI
2015
;
194
:
4777
–
4783
.
101
Ilan
Y
,
Maron
R
,
Tukpah
AM
,
Maioli
TU
,
Murugaiyan
G
,
Yang
K
,
Wu
HY
,
Weiner
HL.
Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice
.
Proc Natl Acad Sci USA
2010
;
107
:
9765
–
9770
.
102
Li
Y
,
Kanellakis
P
,
Hosseini
H
,
Cao
A
,
Deswaerte
V
,
Tipping
P
,
Toh
BH
,
Bobik
A
,
Kyaw
T.
A CD1d-dependent lipid antagonist to NKT cells ameliorates atherosclerosis in ApoE−/− mice by reducing lesion necrosis and inflammation
.
Cardiovasc Res
2016
;
109
:
305
–
317
.
103
Bendelac
A
,
Savage
PB
,
Teyton
L.
The biology of NKT cells
.
Annu Rev Immunol
2007
;
25
:
297
–
336
.
104
Lynch
L
,
O'shea
D
,
Winter
DC
,
Geoghegan
J
,
Doherty
DG
,
O'farrelly
C.
Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity
.
Eur J Immunol
2009
;
39
:
1893
–
1901
.
105
Vieth
JA
,
Das
J
,
Ranaivoson
FM
,
Comoletti
D
,
Denzin
LK
,
Sant'angelo
DB.
TCRalpha-TCRbeta pairing controls recognition of CD1d and directs the development of adipose NKT cells
.
Nat Immunol
2017
;
18
:
36
–
44
.
106
Li
Y
,
Wu
Y
,
Zhang
C
,
Li
P
,
Cui
W
,
Hao
J
,
Ma
X
,
Yin
Z
,
Du
J.
gammadeltaT Cell-derived interleukin-17A via an interleukin-1beta-dependent mechanism mediates cardiac injury and fibrosis in hypertension
.
Hypertension
2014
;
64
:
305
–
314
.
107
Vandanmagsar
B
,
Youm
YH
,
Ravussin
A
,
Galgani
JE
,
Stadler
K
,
Mynatt
RL
,
Ravussin
E
,
Stephens
JM
,
Dixit
VD.
The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance
.
Nat Med
2011
;
17
:
179
–
188
.
108
Exley
MA
,
Hand
L
,
O'shea
D
,
Lynch
L.
Interplay between the immune system and adipose tissue in obesity
.
J Endocrinol
2014
;
223
:
R41
–
R48
.
109
Skurk
T
,
Alberti-Huber
C
,
Herder
C
,
Hauner
H.
Relationship between adipocyte size and adipokine expression and secretion
.
J Clin Endocrinol Metab
2007
;
92
:
1023
–
1033
.
110
McEver
RP.
Selectins: initiators of leucocyte adhesion and signalling at the vascular wall
.
Cardiovasc Res
2015
;
107
:
331
–
339
.
111
Kosteli
A
,
Sugaru
E
,
Haemmerle
G
,
Martin
JF
,
Lei
J
,
Zechner
R
,
Ferrante
AW.
Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue
.
J Clin Invest
2010
;
120
:
3466
–
3479
.
112
Shi
H
,
Kokoeva
MV
,
Inouye
K
,
Tzameli
I
,
Yin
H
,
Flier
JS.
TLR4 links innate immunity and fatty acid-induced insulin resistance
.
J Clin Invest
2006
;
116
:
3015
–
3025
.
113
Ye
JP
,
Gao
ZG
,
Yin
J
,
He
Q.
Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am.
J Physiol Endocrinol Metab
2007
;
293
:
E1118
–
E1128
.
114
O'Rourke
RW
,
White
AE
,
Metcalf
MD
,
Olivas
AS
,
Mitra
P
,
Larison
WG
,
Cheang
EC
,
Varlamov
O
,
Corless
CL
,
Roberts
CT
,
Marks
DL.
Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells
.
Diabetologia
2011
;
54
:
1480
–
1490
.
115
Amano
SU
,
Cohen
JL
,
Vangala
P
,
Tencerova
M
,
Nicoloro
SM
,
Yawe
JC
,
Shen
YF
,
Czech
MP
,
Aouadi
M.
Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation
.
Cell Metab
2014
;
19
:
162
–
171
.
116
Zheng
C
,
Yang
Q
,
Cao
J
,
Xie
N
,
Liu
K
,
Shou
P
,
Qian
F
,
Wang
Y
,
Shi
Y.
Local proliferation initiates macrophage accumulation in adipose tissue during obesity
.
Cell Death Dis
2016
;
7
.
117
Charriere
G
,
Cousin
B
,
Arnaud
E
,
Andre
M
,
Bacou
F
,
Penicaud
L
,
Casteilla
L.
Preadipocyte conversion to macrophage – evidence of plasticity
.
J Biol Chem
2003
;
278
:
9850
–
9855
.
118
Nguyen
MTA
,
Favelyukis
S
,
Nguyen
AK
,
Reichart
D
,
Scott
PA
,
Jenn
A
,
Liu-Bryan
R
,
Glass
CK
,
Neels
JG
,
Olefsky
JM.
A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways
.
J Biol Chem
2007
;
282
:
35279
–
35292
.
119
Odegaard
JI
,
Ricardo-Gonzalez
RR
,
Goforth
MH
,
Morel
CR
,
Subramanian
V
,
Mukundan
L
,
Eagle
AR
,
Vats
D
,
Brombacher
F
,
Ferrante
AW
,
Chawla
A.
Macrophage-specific PPAR gamma controls alternative activation and improves insulin resistance
.
Nature
2007
;
447
:
1116
–
U1112
.
120
Ito
A
,
Suganami
T
,
Yamauchi
A
,
Degawa-Yamauchi
M
,
Tanaka
M
,
Kouyama
R
,
Kobayashi
Y
,
Nitta
N
,
Yasuda
K
,
Hirata
Y
,
Kuziel
WA
,
Takeya
M
,
Kanegasaki
S
,
Kamei
Y
,
Ogawa
Y.
Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue
.
J Biol Chem
2008
;
283
:
35715
–
35723
.
121
Patsouris
D
,
Li
PP
,
Thapar
D
,
Chapman
J
,
Olefsky
JM
,
Neels
JG.
Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell
Metab
2008
;
8
:
301
–
309
.
122
Cancello
R
,
Henegar
C
,
Viguerie
N
,
Taleb
S
,
Poitou
C
,
Rouault
C
,
Coupaye
M
,
Pelloux
V
,
Hugol
D
,
Bouillot
JL
,
Bouloumie
A
,
Barbatelli
G
,
Cinti
S
,
Svensson
PA
,
Barsh
GS
,
Zucker
JD
,
Basdevant
A
,
Langin
D
,
Clement
K.
Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery induced weight loss
.
Diabetes
2005
;
54
:
2277
–
2286
.
123
Rausch
ME
,
Weisberg
S
,
Vardhana
P
,
Tortoriello
DV.
Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration
.
Int J Obes Relat Metab Disord
2008
;
32
:
451
–
463
.
124
Monney
L
,
Sabatos
CA
,
Gaglia
JL
,
Ryu
A
,
Waldner
H
,
Chernova
T
,
Manning
S
,
Greenfield
EA
,
Coyle
AJ
,
Sobel
RA
,
Freeman
GJ
,
Kuchroo
VK.
Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease
.
Nature
2002
;
415
:
536
–
541
.
125
Harford
KA
,
Reynolds
CM
,
McGillicuddy
FC
,
Roche
HM.
Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue
.
Proc Nutr Soc
2011
;
70
:
408
–
417
.
126
Kintscher
U
,
Hartge
M
,
Hess
K
,
Foryst-Ludwig
A
,
Clemenz
M
,
Wabitsch
M
,
Fischer-Posovszky
P
,
Barth
TFE
,
Dragun
D
,
Skurk
T
,
Hauner
H
,
Bluher
M
,
Unger
T
,
Wolf
AM
,
Knippschild
U
,
Hombach
V
,
Marx
N.
T-lymphocyte infiltration in visceral adipose tissue – a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance
.
Arterioscl Throm Vas
2008
;
28
:
1304
–
1310
.
127
Meshkani
R
,
Vakili
S.
Tissue resident macrophages: key players in the pathogenesis of type 2 diabetes and its complications
.
Clin Chim Acta
2016
;
462
:
77
–
89
.
128
Shirakawa
K
,
Yan
XX
,
Shinmura
K
,
Endo
J
,
Kataoka
M
,
Katsumata
Y
,
Yamamoto
T
,
Anzai
A
,
Isobe
S
,
Yoshida
N
,
Itoh
H
,
Manabe
I
,
Sekai
M
,
Hamazaki
Y
,
Fukuda
K
,
Minato
N
,
Sano
M.
Obesity accelerates T cell senescence in murine visceral adipose tissue
.
J Clin Invest
2016
;
126
:
4626
–
4639
.
129
Wei
K
,
Diaz-Trelles
R
,
Liu
Q
,
Diez-Cunado
M
,
Scimia
MC
,
Cai
W
,
Sawada
J
,
Komatsu
M
,
Boyle
JJ
,
Zhou
B
,
Ruiz-Lozano
P
,
Mercola
M.
Developmental origin of age-related coronary artery disease
.
Cardiovasc Res
2015
;
107
:
287
–
294
.
130
Jourdan
T
,
Godlewski
G
,
Cinar
R
,
Bertola
A
,
Szanda
G
,
Liu
J
,
Tam
J
,
Han
T
,
Mukhopadhyay
B
,
Skarulis
MC
,
Ju
C
,
Aouadi
M
,
Czech
MP
,
Kunos
G.
Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes
.
Nat Med
2013
;
19
:
1132
–
1140
.
131
DeFuria
J
,
Belkina
AC
,
Jagannathan-Bogdan
M
,
Snyder-Cappione
J
,
Carr
JD
,
Nersesova
YR
,
Markham
D
,
Strissel
KJ
,
Watkins
AA
,
Zhu
M
,
Allen
J
,
Bouchard
J
,
Toraldo
G
,
Jasuja
R
,
Obin
MS
,
McDonnell
ME
,
Apovian
C
,
Denis
GV
,
Nikolajczyk
BS.
B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile
.
P Natl Acad Sci USA
2013
;
110
:
5133
–
5138
.
132
Molofsky
AB
,
Nussbaum
JC
,
Liang
HE
,
Van Dyken
SJ
,
Cheng
LE
,
Mohapatra
A
,
Chawla
A
,
Locksley
RM.
Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages
.
J Exp Med
2013
;
210
:
535
–
549
.
133
Lynch
L
,
Nowak
M
,
Varghese
B
,
Clark
J
,
Hogan
AE
,
Toxavidis
V
,
Balk
SP
,
O'shea
D
,
O'farrelly
C
,
Exley
MA.
Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production
.
Immunity
2012
;
37
:
574
–
587
.
134
Youn
JY
,
Siu
KL
,
Lob
HE
,
Itani
H
,
Harrison
DG
,
Cai
H.
Role of vascular oxidative stress in obesity and metabolic syndrome
.
Diabetes
2014
;
63
:
2344
–
2355
.
135
Guzik
TJ
,
Olszanecki
R
,
Sadowski
J
,
Kapelak
B
,
Rudzinski
P
,
Jopek
A
,
Kawczynska
A
,
Ryszawa
N
,
Loster
J
,
Jawien
J
,
Czesnikiewicz-Guzik
M
,
Channon
KM
,
Korbut
R.
Superoxide dismutase activity and expression in human venous and arterial bypass graft vessels
.
J Physiol Pharmacol
2005
;
56
:
313
–
323
.
136
Xia
C
,
Rao
X
,
Zhong
J.
Role of T lymphocytes in type 2 diabetes and diabetes-associated inflammation
.
J Diabetes Res
2017
;
2017
:
6.
137
Kitade
H
,
Sawamoto
K
,
Nagashimada
M
,
Inoue
H
,
Yamamoto
Y
,
Sai
Y
,
Takamura
T
,
Yamamoto
H
,
Miyamoto
K
,
Ginsberg
HN
,
Mukaida
N
,
Kaneko
S
,
Ota
T.
CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status
.
Diabetes
2012
;
61
:
1680
–
1690
.
138
McLaughlin
T
,
Liu
LF
,
Lamendola
C
,
Shen
L
,
Morton
J
,
Rivas
H
,
Winer
D
,
Tolentino
L
,
Choi
O
,
Zhang
H
,
Chng
MHY
,
Engleman
E.
T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans
.
Arterioscl Throm Vas
2014
;
34
:
2637
–
2643
.
139
Seijkens
T
,
Kusters
P
,
Engel
D
,
Lutgens
E.
CD40-CD40L: linking pancreatic, adipose tissue and vascular inflammation in type 2 diabetes and its complications
.
Diabetes Vasc Dis Re
2013
;
10
:
115
–
122
.
140
Poggi
M
,
Engel
D
,
Christ
A
,
Beckers
L
,
Wijnands
E
,
Boon
L
,
Driessen
A
,
Cleutjens
J
,
Weber
C
,
Gerdes
N
,
Lutgens
E.
CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice
.
Arterioscl Throm Vas
2011
;
31
:
2251
–
U2248
.
141
Donath
MY.
Targeting inflammation in the treatment of type 2 diabetes: time to start
.
Nat Rev Drug Discov
2014
;
13
:
465
–
476
.
142
Poggi
M
,
Jager
J
,
Paulmyer-Lacroix
O
,
Peiretti
F
,
Gremeaux
T
,
Verdier
M
,
Grino
M
,
Stepanian
A
,
Msika
S
,
Burcelin
R
,
de Prost
D
,
Tanti
JF
,
Alessi
MC.
The inflammatory receptor CD40 is expressed on human adipocytes: contribution to crosstalk between lymphocytes and adipocytes
.
Diabetologia
2009
;
52
:
1152
–
1163
.
143
Missiou
A
,
Wolf
D
,
Platzer
I
,
Ernst
S
,
Walter
C
,
Rudolf
P
,
Zirlik
K
,
Kostlin
N
,
Willecke
FK
,
Munkel
C
,
Schonbeck
U
,
Libby
P
,
Bode
C
,
Varo
N
,
Zirlik
A.
CD40L induces inflammation and adipogenesis in adipose cells – a potential link between metabolic and cardiovascular disease
.
Thromb Haemost
2010
;
103
:
788
–
796
.
144
de Hoog
VC
,
Bovens
SM
,
de Jager
SC
,
van Middelaar
BJ
,
van Duijvenvoorde
A
,
Doevendans
PA
,
Pasterkamp
G
,
de Kleijn
DP
,
Timmers
L.
BLT1 antagonist LSN2792613 reduces infarct size in a mouse model of myocardial ischaemia-reperfusion injury
.
Cardiovasc Res
2015
;
108
:
367
–
376
.
145
Harrison
DG
,
Guzik
TJ
,
Lob
HE
,
Madhur
MS
,
Marvar
PJ
,
Thabet
SR
,
Vinh
A
,
Weyand
CM.
Inflammation, immunity, and hypertension
.
Hypertension
2011
;
57
:
132
–
140
.
146
Itani
HA
,
McMaster
WG
Jr.,
Saleh
MA
,
Nazarewicz
RR
,
Mikolajczyk
TP
,
Kaszuba
AM
,
Konior
A
,
Prejbisz
A
,
Januszewicz
A
,
Norlander
AE
,
Chen
W
,
Bonami
RH
,
Marshall
AF
,
Poffenberger
G
,
Weyand
CM
,
Madhur
MS
,
Moore
DJ
,
Harrison
DG
,
Guzik
TJ.
Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans
.
Hypertension
2016
;
68
:
123
–
132
.
147
Guzik
TJ
,
Mikolajczyk
T.
In search of the T cell involved in hypertension and target organ damage
.
Hypertension
2014
;
64
:
224
–
226
.
148
Carnevale
D
,
Pallante
F
,
Fardella
V
,
Fardella
S
,
Iacobucci
R
,
Federici
M
,
Cifelli
G
,
De Lucia
M
,
Lembo
G.
The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension
.
Immunity
2014
;
41
:
737
–
752
.
149
Marvar
PJ
,
Thabet
SR
,
Guzik
TJ
,
Lob
HE
,
McCann
LA
,
Weyand
C
,
Gordon
FJ
,
Harrison
DG.
Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension
.
Circ Res
2010
;
107
:
263
–
270
.
150
Wilk
G
,
Osmenda
G
,
Matusik
P
,
Nowakowski
D
,
Jasiewicz-Honkisz
B
,
Ignacak
A
,
Czesnikiewicz-Guzik
M
,
Guzik
TJ.
Endothelial function assessment in atherosclerosis: comparison of brachial artery flow-mediated vasodilation and peripheral arterial tonometry
.
Pol Arch Med Wewn
2013
;
123
:
443
–
452
.
151
Nus
M
,
Martinez-Poveda
B
,
MacGrogan
D
,
Chevre
R
,
Amato
G
,
Sbroggio
M
,
Rodriguez
C
,
Martinez-Gonzalez
J
,
Andres
V
,
Hidalgo
A
,
Luis de la Pompa J. Endothelial Jag1-RBPJ signalling promotes inflammatory leucocyte recruitment and atherosclerosis
.
Cardiovasc Res
2016
;
112
:
568
–
580
.
152
Minami
T
,
Satoh
K
,
Nogi
M
,
Kudo
S
,
Miyata
S
,
Tanaka
S
,
Shimokawa
H.
Statins up-regulate SmgGDS through beta1-integrin/Akt1 pathway in endothelial cells
.
Cardiovasc Res
2016
;
109
:
151
–
161
.
153
Guzik
TJ
,
Hoch
NE
,
Brown
KA
,
McCann
LA
,
Rahman
A
,
Dikalov
S
,
Goronzy
J
,
Weyand
C
,
Harrison
DG.
Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction
.
J Exp Med
2007
;
204
:
2449
–
2460
.
154
West
NEJ
,
Qian
HS
,
Guzik
TJ
,
Black
E
,
Cai
S
,
George
SE
,
Channon
KM.
Nitric oxide synthase (nNOS) gene transfer modifies venous bypass graft remodeling: effects on vascular smooth muscle cell differentiation and superoxide production
.
Circulation
2001
;
104
:
1526
–
1532
.
155
Madhur
MS
,
Lob
HE
,
McCann
LA
,
Iwakura
Y
,
Blinder
Y
,
Guzik
TJ
,
Harrison
DG.
Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction
.
Hypertension
2010
;
55
:
500
–
507
.
156
Wu
J
,
Thabet
SR
,
Kirabo
A
,
Trott
DW
,
Saleh
MA
,
Xiao
L
,
Madhur
MS
,
Chen
W
,
Harrison
DG.
Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase
.
Circ Res
2014
;
114
:
616
–
625
.
157
Matrougui
K
,
Zakaria
AE
,
Kassan
M
,
Choi
S
,
Nair
D
,
Gonzalez-Villalobos
RA
,
Chentoufi
AA
,
Kadowitz
P
,
Belmadani
S
,
Partyka
M.
Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice
.
Am J Pathol
2011
;
178
:
434
–
441
.
158
Barhoumi
T
,
Kasal
DA
,
Li
MW
,
Shbat
L
,
Laurant
P
,
Neves
MF
,
Paradis
P
,
Schiffrin
EL.
T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury
.
Hypertension
2011
;
57
:
469
–
476
.
159
Chan
CT
,
Sobey
CG
,
Lieu
M
,
Ferens
D
,
Kett
MM
,
Diep
H
,
Kim
HA
,
Krishnan
SM
,
Lewis
CV
,
Salimova
E
,
Tipping
P
,
Vinh
A
,
Samuel
CS
,
Peter
K
,
Guzik
TJ
,
Kyaw
TS
,
Toh
BH
,
Bobik
A
,
Drummond
GR.
Obligatory role for B cells in the development of angiotensin II-dependent hypertension
.
Hypertension
2015
;
66
:
1023
–
1033
.
160
Chan
CT
,
Moore
JP
,
Budzyn
K
,
Guida
E
,
Diep
H
,
Vinh
A
,
Jones
ES
,
Widdop
RE
,
Armitage
JA
,
Sakkal
S
,
Ricardo
SD
,
Sobey
CG
,
Drummond
GR.
Reversal of vascular macrophage accumulation and hypertension by a CCR2 antagonist in deoxycorticosterone/salt-treated mice
.
Hypertension
2012
;
60
:
1207
–
1212
.
161
Saleh
MA
,
McMaster
WG
,
Wu
J
,
Norlander
AE
,
Funt
SA
,
Thabet
SR
,
Kirabo
A
,
Xiao
L
,
Chen
W
,
Itani
HA
,
Michell
D
,
Huan
TX
,
Zhang
YH
,
Takaki
S
,
Titze
J
,
Levy
D
,
Harrison
DG
,
Madhur
MS.
Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation
.
J Clin Invest
2015
;
125
:
1189
–
1202
.
162
Kirabo
A
,
Fontana
V
,
de Faria
APC
,
Loperena
R
,
Galindo
CL
,
Wu
J
,
Bikineyeva
AT
,
Dikalov
S
,
Xiao
L
,
Chen
W
,
Saleh
MA
,
Trott
DW
,
Itani
HA
,
Vinh
A
,
Amarnath
V
,
Amarnath
K
,
Guzik
TJ
,
Bernstein
KE
,
Shen
XZ
,
Shyr
Y
,
Chen
SC
,
Mernaugh
RL
,
Laffer
CL
,
Elijovich
F
,
Davies
SS
,
Moreno
LH
,
Madhur
MS
,
Roberts
J
,
Harrison
DG.
DC isoketal-modified proteins activate T cells and promote hypertension
.
J Clin Invest
2014
;
124
:
4642
–
4656
.
163
Vinh
A
,
Chen
W
,
Blinder
Y
,
Weiss
D
,
Taylor
WR
,
Goronzy
JJ
,
Weyand
CM
,
Harrison
DG
,
Guzik
TJ.
Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension
.
Circulation
2010
;
122
:
2529
–
2537
.
164
Lumeng
CN
,
Deyoung
SM
,
Saltiel
AR.
Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins
.
Am J Physiol Endocrinol Metab
2007
;
292
:
E166
–
E174
.
165
Pietrowski
E
,
Bender
B
,
Huppert
J
,
White
R
,
Luhmann
HJ
,
Kuhlmann
CR.
Pro-inflammatory effects of interleukin-17A on vascular smooth muscle cells involve NAD(P)H- oxidase derived reactive oxygen species
.
J Vasc Res
2011
;
48
:
52
–
58
.
166
Bettelli
E
,
Carrier
Y
,
Gao
W
,
Korn
T
,
Strom
TB
,
Oukka
M
,
Weiner
HL
,
Kuchroo
VK.
Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells
.
Nature
2006
;
441
:
235
–
238
.
167
Roussel
L
,
Houle
F
,
Chan
C
,
Yao
Y
,
Berube
J
,
Olivenstein
R
,
Martin
JG
,
Huot
J
,
Hamid
Q
,
Ferri
L
,
Rousseau
S.
IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation
.
J Immunol
2010
;
184
:
4531
–
4537
.
168
Nguyen
H
,
Chiasson
VL
,
Chatterjee
P
,
Kopriva
SE
,
Young
KJ
,
Mitchell
BM.
Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension
.
Cardiovasc Res
2013
;
97
:
696
–
704
.
169
Nosalski
R
,
McGinnigle
E
,
Siedlinski
M
,
Guzik
TJ.
Novel immune mechanisms in hypertension and cardiovascular risk
.
Curr Cardiovasc Risk Rep
2017
;
11
:
12.
170
Maeda
N
,
Shimomura
I
,
Kishida
K
,
Nishizawa
H
,
Matsuda
M
,
Nagaretani
H
,
Furuyama
N
,
Kondo
H
,
Takahashi
M
,
Arita
Y
,
Komuro
R
,
Ouchi
N
,
Kihara
S
,
Tochino
Y
,
Okutomi
K
,
Horie
M
,
Takeda
S
,
Aoyama
T
,
Funahashi
T
,
Matsuzawa
Y.
Diet-induced insulin resistance in mice lacking adiponectin/ACRP30
.
Nat Med
2002
;
8
:
731
–
737
.
171
Fasshauer
M
,
Kralisch
S
,
Klier
M
,
Lossner
U
,
Bluher
M
,
Klein
J
,
Paschke
R.
Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes
.
Biochem Biophys Res Commun
2003
;
301
:
1045
–
1050
.
172
Noh
M.
Interleukin-17A increases leptin production in human bone marrow mesenchymal stem cells
.
Biochem Pharmacol
2012
;
83
:
661
–
670
.
173
La Cava
A
,
Matarese
G.
The weight of leptin in immunity
.
Nat Rev Immunol
2004
;
4
:
371
–
379
.
174
Bokarewa
M
,
Nagaev
I
,
Dahlberg
L
,
Smith
U
,
Tarkowski
A.
Resistin, an adipokine with potent proinflammatory properties
.
J Immunol
2005
;
174
:
5789
–
5795
.
175
Spiroglou
SG
,
Kostopoulos
CG
,
Varakis
JN
,
Papadaki
HH.
Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis
.
JAT
2010
;
17
:
115
–
130
.
176
Durpes
MC
,
Morin
C
,
Paquin-Veillet
J
,
Beland
R
,
Pare
M
,
Guimond
MO
,
Rekhter
M
,
King
GL
,
Geraldes
P.
PKC-beta activation inhibits IL-18-binding protein causing endothelial dysfunction and diabetic atherosclerosis
.
Cardiovasc Res
2015
;
106
:
303
–
313
.
177
Planavila
A
,
Redondo-Angulo
I
,
Ribas
F
,
Garrabou
G
,
Casademont
J
,
Giralt
M
,
Villarroya
F.
Fibroblast growth factor 21 protects the heart from oxidative stress
.
Cardiovasc Res
2015
;
106
:
19
–
31
.
178
Podolec
J
,
Kopec
G
,
Niewiara
L
,
Komar
M
,
Guzik
B
,
Bartus
K
,
Tomkiewicz-Pajak
L
,
Guzik
TJ
,
Plazak
W
,
Zmudka
K.
Chemokine RANTES is increased at early stages of coronary artery disease
.
J Physiol Pharmacol
2016
;
67
:
321
–
328
.
179
Galkina
E
,
Kadl
A
,
Sanders
J
,
Varughese
D
,
Sarembock
IJ
,
Ley
K.
Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent
.
J Exp Med
2006
;
203
:
1273
–
1282
.
180
Moos
MPW
,
John
N
,
Grabner
R
,
Nossmann
S
,
Gunther
B
,
Vollandt
D
,
Funk
CD
,
Kaiser
B
,
Habenicht
AJR.
The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice
.
Arterioscler Thromb Vasc Biol
2005
;
25
:
2386
–
2391
.
181
Lohmann
C
,
Schafer
N
,
von Lukowicz
T
,
Stein
MAS
,
Boren
J
,
Rutti
S
,
Wahli
W
,
Donath
MY
,
Luscher
TF
,
Matter
CM.
Atherosclerotic mice exhibit systemic inflammation in periadventitial and visceral adipose tissue, liver, and pancreatic islets
.
Atherosclerosis
2009
;
207
:
360
–
367
.
182
Ketelhuth
DF
,
Hansson
GK.
Adaptive response of T and B cells in atherosclerosis
.
Circ Res
2016
;
118
:
668
–
678
.
183
Henrichot
E
,
Juge-Aubry
CE
,
Pernin
AS
,
Pache
JC
,
Velebit
V
,
Dayer
JM
,
Meda
P
,
Chizzolini
C
,
Meier
CA.
Production of chemokines by perivascular adipose tissue – a role in the pathogenesis of atherosclerosis?
.
Arterioscl Throm Vas
2005
;
25
:
2594
–
2599
.
184
Smith
E
,
Prasad
KM
,
Butcher
M
,
Dobrian
A
,
Kolls
JK
,
Ley
K
,
Galkina
E.
Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice
.
Circulation
2010
;
121
:
1746
–
1755
.
185
Dobrian
AD
,
Hatcher
MA
,
Brotman
JJ
,
Galkina
EV
,
Taghavie-Moghadam
P
,
Pei
H
,
Haynes
BA
,
Nadler
JL.
STAT4 contributes to adipose tissue inflammation and atherosclerosis
.
J Endocrinol
2015
;
227
:
13
–
24
.
186
Foks
AC
,
Van Puijvelde
GH
,
Wolbert
J
,
Kroner
MJ
,
Frodermann
V
,
Van Der Heijden
T
,
Van Santbrink
PJ
,
Boon
L
,
Bot
I
,
Kuiper
J.
CD11b+Gr-1+ myeloid-derived suppressor cells reduce atherosclerotic lesion development in LDLr deficient mice
.
Cardiovasc Res
2016
;
111
:
252
–
261
.
187
Tay
C
,
Liu
YH
,
Hosseini
H
,
Kanellakis
P
,
Cao
A
,
Peter
K
,
Tipping
P
,
Bobik
A
,
Toh
BH
,
Kyaw
T.
B-cell-specific depletion of tumour necrosis factor alpha inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation
.
Cardiovasc Res
2016
;
111
:
385
–
397
.
188
Nus
M
,
Mallat
Z.
Immune-mediated mechanisms of atherosclerosis and implications for the clinic
.
Expert Rev Clin Immunol
2016
;
12
:
1217
–
1237
.
189
Malkic Salihbegovic
E
,
Hadzigrahic
N
,
Cickusic
AJ.
Psoriasis and metabolic syndrome
.
Med Arh
2015
;
69
:
85
–
87
.
190
Hjuler
KF
,
Gormsen
LC
,
Vendelbo
MH
,
Egeberg
A
,
Nielsen
J
,
Iversen
L.
Increased global arterial and subcutaneous adipose tissue inflammation in patients with moderate-to-severe psoriasis
.
Br J Dermatol
2017
;
176
:
732
–
740
.
191
Kontny
E
,
Prochorec-Sobieszek
M.
Articular adipose tissue resident macrophages in rheumatoid arthritis patients: potential contribution to local abnormalities
.
Rheumatology (Oxford)
2013
;
52
:
2158
–
2167
.
192
Procaccini
C
,
Carbone
F
,
Galgani
M
,
La Rocca
C
,
De Rosa
V
,
Cassano
S
,
Matarese
G.
Obesity and susceptibility to autoimmune diseases
.
Expert Rev Clin Immunol
2011
;
7
:
287
–
294
.
193
Graner
M
,
Nyman
K
,
Siren
R
,
Pentikainen
MO
,
Lundbom
J
,
Hakkarainen
A
,
Lauerma
K
,
Lundbom
N
,
Nieminen
MS
,
Taskinen
MR.
Ectopic fat depots and left ventricular function in nondiabetic men with nonalcoholic fatty liver disease
.
Circ Cardiovasc Imaging
2015
;
8
:e001979.
194
He
Q
,
Li
F
,
Li
J
,
Li
R
,
Zhan
G
,
Li
G
,
Du
W
,
Tan
H.
MicroRNA-26a-interleukin (IL)-6-IL-17 axis regulates the development of non-alcoholic fatty liver disease in a murine model
.
Clin Exp Immunol
2017
;
187
:
174
–
184
.
195
Crippa
S
,
Nemir
M
,
Ounzain
S
,
Ibberson
M
,
Berthonneche
C
,
Sarre
A
,
Boisset
G
,
Maison
D
,
Harshman
K
,
Xenarios
I
,
Diviani
D
,
Schorderet
D
,
Pedrazzini
T.
Comparative transcriptome profiling of the injured zebrafish and mouse hearts identifies miRNA-dependent repair pathways
.
Cardiovasc Res
2016
;
110
:
73
–
84
.
196
Vacca
M
,
Di Eusanio
M
,
Cariello
M
,
Graziano
G
,
D'amore
S
,
Petridis
FD
,
D'orazio
A
,
Salvatore
L
,
Tamburro
A
,
Folesani
G
,
Rutigliano
D
,
Pellegrini
F
,
Sabba
C
,
Palasciano
G
,
Di Bartolomeo
R
,
Moschetta
A.
Integrative miRNA and whole-genome analyses of epicardial adipose tissue in patients with coronary atherosclerosis
.
Cardiovasc Res
2016
;
109
:
228
–
239
.
197
Iaconetti
C
,
De Rosa
S
,
Polimeni
A
,
Sorrentino
S
,
Gareri
C
,
Carino
A
,
Sabatino
J
,
Colangelo
M
,
Curcio
A
,
Indolfi
C.
Down-regulation of miR-23b induces phenotypic switching of vascular smooth muscle cells in vitro and in vivo
.
Cardiovasc Res
2015
;
107
:
522
–
533
.
198
Hu
W
,
Wang
M
,
Yin
H
,
Yao
C
,
He
Q
,
Yin
L
,
Zhang
C
,
Li
W
,
Chang
G
,
Wang
S.
MicroRNA-1298 is regulated by DNA methylation and affects vascular smooth muscle cell function by targeting connexin 43
.
Cardiovasc Res
2015
;
107
:
534
–
545
.
199
Duygu
B
,
Da Costa Martins
PA.
miR-21: a star player in cardiac hypertrophy
.
Cardiovasc Res
2015
;
105
:
235
–
237
.
200
Mazurek
T
,
Zhang
L
,
Zalewski
A
,
Mannion
JD
,
Diehl
JT
,
Arafat
H
,
Sarov-Blat
L
,
O'brien
S
,
Keiper
EA
,
Johnson
AG
,
Martin
J
,
Goldstein
BJ
,
Shi
Y.
Human epicardial adipose tissue is a source of inflammatory mediators
.
Circulation
2003
;
108
:
2460
–
2466
.
201
Gaborit
B
,
Venteclef
N
,
Ancel
P
,
Pelloux
V
,
Gariboldi
V
,
Leprince
P
,
Amour
J
,
Hatem
SN
,
Jouve
E
,
Dutour
A
,
Clement
K.
Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location
.
Cardiovasc Res
2015
;
108
:
62
–
73
.
202
Baragetti
A
,
Pisano
G
,
Bertelli
C
,
Garlaschelli
K
,
Grigore
L
,
Fracanzani
AL
,
Fargion
S
,
Norata
GD
,
Catapano
AL.
Subclinical atherosclerosis is associated with epicardial fat thickness and hepatic steatosis in the general population
.
Nutr Metab Cardiovasc Dis
2016
;
26
:
141
–
153
.
203
Harman-Boehm
I
,
Bluher
M
,
Redel
H
,
Sion-Vardy
N
,
Ovadia
S
,
Avinoach
E
,
Shai
I
,
Kloting
N
,
Stumvoll
M
,
Bashan
N
,
Rudich
A.
Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity
.
J Clin Endocrinol Metab
2007
;
92
:
2240
–
2247
.
204
Zeyda
M
,
Farmer
D
,
Todoric
J
,
Aszmann
O
,
Speiser
M
,
Gyori
G
,
Zlabinger
GJ
,
Stulnig
TM.
Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production
.
Int J Obes Relat Metab Disord
2007
;
31
:
1420
–
1428
.
205
Antonopoulos
AS
,
Margaritis
M
,
Coutinho
P
,
Shirodaria
C
,
Psarros
C
,
Herdman
L
,
Sanna
F
,
De Silva
R
,
Petrou
M
,
Sayeed
R
,
Krasopoulos
G
,
Lee
R
,
Digby
J
,
Reilly
S
,
Bakogiannis
C
,
Tousoulis
D
,
Kessler
B
,
Casadei
B
,
Channon
KM
,
Antoniades
C.
Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue
.
Diabetes
2015
;
64
:
2207
–
2219
.
206
Antonopoulos
AS
,
Margaritis
M
,
Verheule
S
,
Recalde
A
,
Sanna
F
,
Herdman
L
,
Psarros
C
,
Nasrallah
H
,
Coutinho
P
,
Akoumianakis
I
,
Brewer
AC
,
Sayeed
R
,
Krasopoulos
G
,
Petrou
M
,
Tarun
A
,
Tousoulis
D
,
Shah
AM
,
Casadei
B
,
Channon
KM
,
Antoniades
C.
Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-gamma/adiponectin signalling
.
Circ Res
2016
;
118
:
842
–
855
.
207
Larsen
CM
,
Faulenbach
M
,
Vaag
A
,
Ehses
JA
,
Donath
MY
,
Mandrup-Poulsen
T.
Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes
.
Diabetes Care
2009
;
32
:
1663
–
1668
.
208
Cavelti-Weder
C
,
Babians-Brunner
A
,
Keller
C
,
Stahel
MA
,
Kurz-Levin
M
,
Zayed
H
,
Solinger
AM
,
Mandrup-Poulsen
T
,
Dinarello
CA
,
Donath
MY.
Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes
.
Diabetes Care
2012
;
35
:
1654
–
1662
.
209
Hensen
J
,
Howard
CP
,
Walter
V
,
Thuren
T.
Impact of interleukin-1 beta antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: Results of secondary endpoints from a randomized, placebo-controlled trial
.
Diabetes Metab
2013
;
39
:
524
–
531
.
210
Sloan-Lancaster
J
,
Abu-Raddad
E
,
Polzer
J
,
Miller
JW
,
Scherer
JC
,
De Gaetano
A
,
Berg
JK
,
Landschulz
WH.
Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1 beta antibody, in patients with type 2 diabetes
.
Diabetes Care
2013
;
36
:
2239
–
2246
.
211
Ofei
F
,
Hurel
S
,
Newkirk
J
,
Sopwith
M
,
Taylor
R.
Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM
.
Diabetes
1996
;
45
:
881
–
885
.
212
Paquot
N
,
Castillo
MJ
,
Lefebvre
PJ
,
Scheen
AJ.
No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients
.
J Clin Endocrinol Metab
2000
;
85
:
1316
–
1319
.
213
Dominguez
H
,
Storgaard
H
,
Rask-Madsen
C
,
Hermann
TS
,
Ihlemann
N
,
Nielsen
DB
,
Spohr
C
,
Kober
L
,
Vaag
A
,
Torp-Pedersen
C.
Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes
.
J Vasc Res
2005
;
42
:
517
–
525
.
214
Goldfine
AB
,
Fonseca
V
,
Jablonski
KA
,
Chen
YDI
,
Tipton
L
,
Staten
MA
,
Shoelson
SE
,
Salsa
TIU.
Salicylate (Salsalate) in patients with type 2 diabetes
.
Ann Intern Med
2013
;
159
:
1.
215
Passacquale
G
,
Di Giosia
P
,
Ferro
A.
The role of inflammatory biomarkers in developing targeted cardiovascular therapies: lessons from the cardiovascular inflammation reduction trials
.
Cardiovasc Res
2016
;
109
:
9
–
23
.
216
Lewis
DR
,
Petersen
LK
,
York
AW
,
Ahuja
S
,
Chae
H
,
Joseph
LB
,
Rahimi
S
,
Uhrich
KE
,
Haser
PB
,
Moghe
PV.
Nanotherapeutics for inhibition of atherogenesis and modulation of inflammation in atherosclerotic plaques
.
Cardiovasc Res
2016
;
109
:
283
–
293
.
217
Giles
JT
,
Ferrante
AW
,
Broderick
R
,
Zartoshti
A
,
Rose
J
,
Downer
K
,
Zhang
HZ
,
Winchester
RJ.
Adipose tissue macrophages in rheumatoid arthritis: prevalence, disease related indicators, and associations with cardiometabolic risk factors
.
Arthritis Care Res (Hoboken)
2017
; doi: 10.1002/acr.23253.
Author notes
© The Author. Published on behalf of the European Society of Cardiology
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited.
PDF