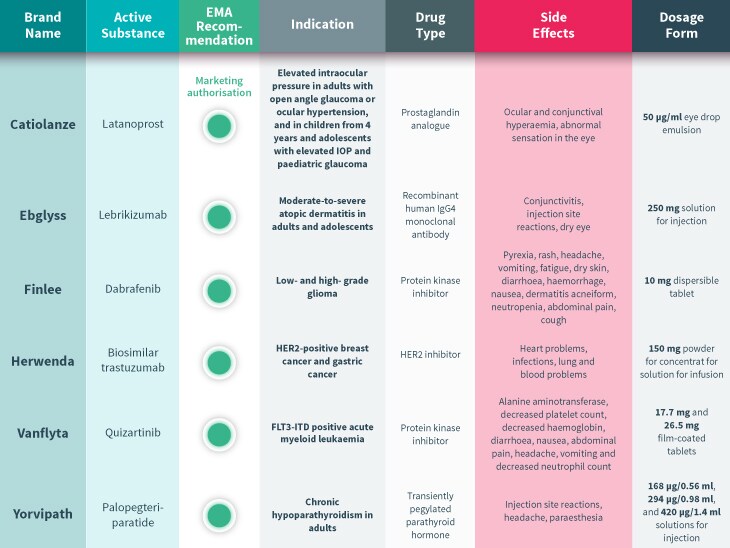
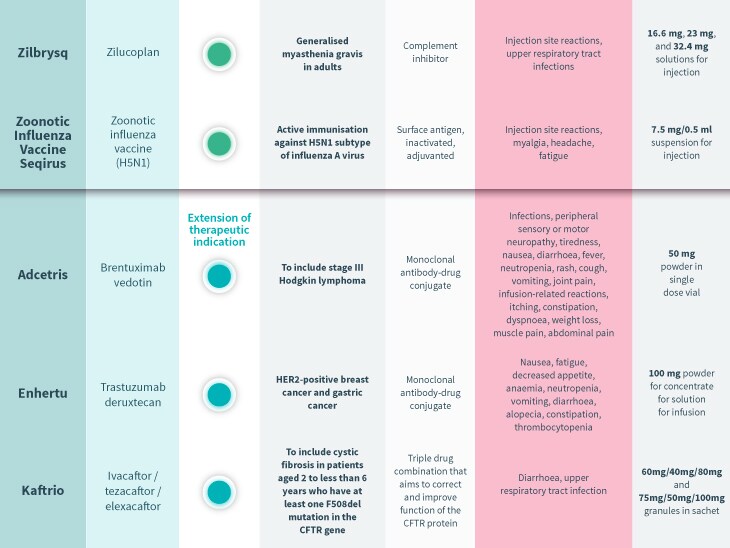
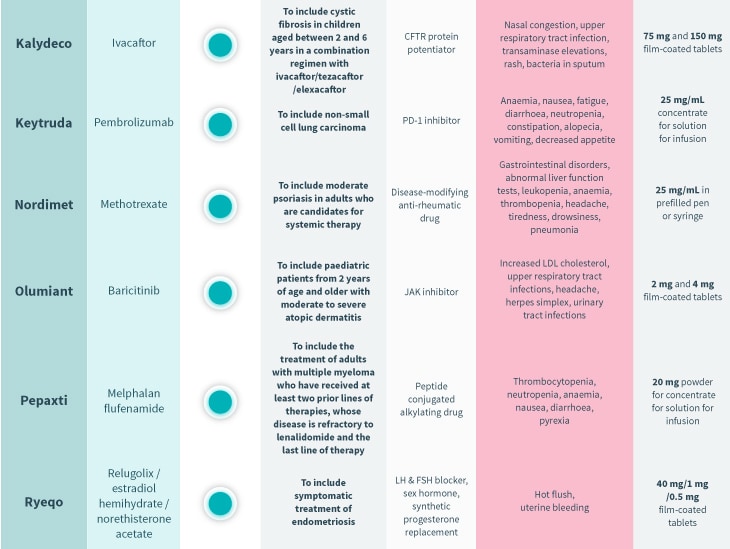
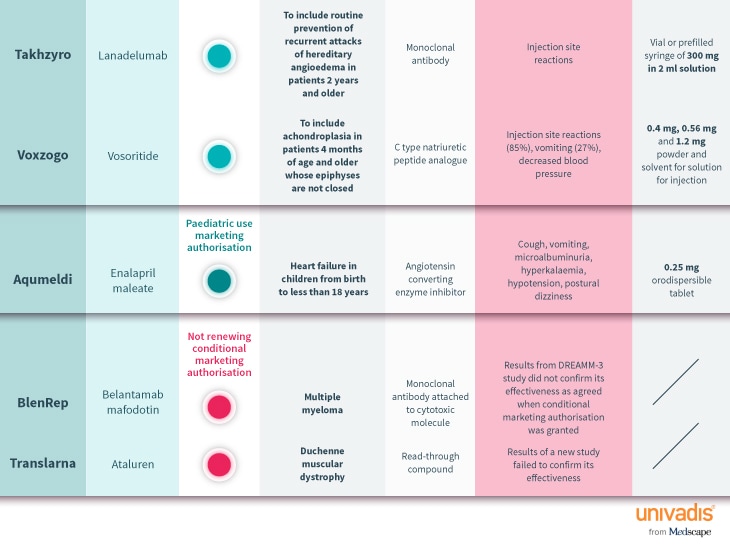
Every month, the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) assesses drugs for use on the EU-wide market. Univadis has assembled some highlights from this month’s decisions.
All CHMP opinions are forwarded to the European Commission, which issues a final, legally binding decision applicable in all EU Member States.