Abstract
Costunolide (CTD) is a sesquiterpene lactone isolated from costus root and exhibits various biological activities including anti-inflammation. Since atherosclerosis is a chronic inflammatory disease, we herein investigated the anti-atherosclerotic effects of CTD and the underlying mechanism. Atherosclerosis was induced in ApoE−/− mice by feeding them with a high-fat diet (HFD) for 8 weeks, followed by administration of CTD (10, 20 mg ·kg−1·d−1, i.g.) for 8 weeks. We showed that CTD administration dose-dependently alleviated atherosclerosis in HFD-fed ApoE−/− mice. Furthermore, we found that CTD dose-dependently reduced inflammatory responses in aortas of the mice, as CTD prevented infiltration of inflammatory cells in aortas and attenuated oxLDL uptake in macrophages, leading to reduced expression of pro-inflammatory and pro-fibrotic molecules in aortas. Similar results were observed in oxLDL-stimulated mouse primary peritoneal macrophages (MPMs) in vitro. We showed that pretreatment with CTD (2.5, 5. 10 μM) restrained oxLDL-induced inflammatory responses in MPMs by blocking pro-inflammatory NF-κB/p65 signaling pathway. We further demonstrated that CTD inactivated NF-κB via covalent binding to cysteine 179 on IKKβ, a canonical upstream regulator of NF-κB, reducing its phosphorylation and leading to conformational change in the active loop of IKKβ. Our results discover IKKβ as the target of CTD for its anti-inflammatory activity and elucidate a molecular mechanism underlying the anti-atherosclerosis effect of CTD. CTD is a potentially therapeutic candidate for retarding inflammatory atherosclerotic diseases.
Similar content being viewed by others
Introduction
Atherosclerosis is a major cause of cardiovascular diseases, which are leading drivers of morbidity and mortality worldwide [1]. One critical event in the development of atherosclerosis is the chronic inflammatory response [2], which is characterized by the infiltration of leukocytes and the secretion of proinflammatory mediators [3]. The key process is initiated when vascular endothelium is activated through the accumulation of low-density lipoproteins (LDL). Subsequently, leukocytes such as macrophages, neutrophils and monocytes are recruited into lesions and secrete a plethora of proinflammatory factors, which dominates both atherosclerotic initiation and progression [4, 5]. Accordingly, anti-inflammation treatment may be a potential strategy for managing patients with atherosclerosis [6].
Nuclear factor kappa B (NF-κB), a classical transcriptional factor regulating the expression of a lot of inflammatory genes, has been shown to play a significant role in the development of atherosclerosis [7]. Canonically, NF-κB activity is regulated by the upstream NF-kappa-B inhibitor (IκB) and inhibitor of NF-kappa B kinase (IKK). IKK is a multiple protein complex comprised of IKKα, IKKβ, and NF-κB essential modulator (NEMO/IKKγ) [8]. Among these three proteins, IKKβ is the primary IKK catalytic subunit for NF-κB activation in response to proinflammatory stimuli such as tumor necrosis factor α (TNF-α), interleukin (IL)−1, and toll-like receptor agonists including lipopolysaccharide (LPS) and oxidized LDL (oxLDL) [9, 10]. Activated IKKβ phosphorylates IκBα, resulting in the ubiquitination and degradation of IκBα [11]. Subsequently, the NF-κB p65 subunit, which is normally sequestered in the cytoplasm by IκBα, is released and rapidly translocates into the nucleus. Phosphorylated NF-κB p65 binds to DNA and induces the transcription of its target inflammatory genes [12]. IKKβ has been reported to be involved in many diseases, including chronic inflammatory diseases such as atherosclerosis [13,14,15]. The deletion of IKKβ in macrophages has been shown to attenuate atherosclerosis in LDL receptor-deficient mice [16]. Thus, IKKβ could be a therapeutic target for the treatment of atherosclerosis.
Costunolide (CTD, in Fig. 1a), a natural compound first isolated from costus root and then found in various other plant species [17], is a bioactive sesquiterpene lactone with a wide range of biological activities [18]. CTD has been known to possess potential therapeutic benefits in various inflammatory diseases. Chen et al. found that CTD attenuated acute lung injury via inhibition of MAPK signaling pathway [19]. Besides, CTD was reported to be a potential candidate for alleviating LPS- and D-galactosamine-induced acute liver injury [20]. Claudia et al. found that CTD neutralized the pro-inflammatory effects of IFN-γ and IL-22 on keratinocytes [21]. Interestingly, CTD has been reported to prevent acute ulcerative colitis [22] and pulmonary fibrosis [23] through inhibiting NF-κB-dependent inflammation. However, the effect of CTD on atherosclerosis and the underlying mechanism as well as its molecular target has not been identified.
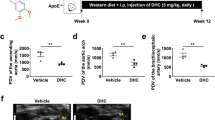
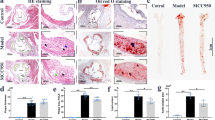
a The chemical structure of CTD (CTD). Representative en face Oil Red O staining (b) and quantification of Oil Red O-positive plaque lesion area in the aortas from ApoE−/− mice (c). Plaque area was defined as percentage of total surface area of the aorta (n = 5). d Representative images of Oil Red O staining of atherosclerotic lesion in the aortic roots (scale bar = 500 μm) and quantification lesions area (e), n = 7. Representative images of Masson’s Trichrome staining (f) and Sirius Red staining (h) for collagen deposition (scale bar = 100 μm). Quantification of fibrotic area as highlighted by Masson’s Trichrome staining (g) and Sirius Red staining (i), n = 7. Statistical data were shown as mean ± SEM; *P < 0.05; ns not significant.
In this study, we investigated the pharmacological effects of CTD against atherosclerosis and its underlying mechanism. We showed that CTD notably alleviated atherosclerosis via reducing inflammatory response in mouse aortas. Interestingly, we have also discovered that CTD elicits its anti-inflammatory activity by directly binding to IKKβ and inhibiting IKKβ/NF-κB activation in macrophages.
Materials and methods
Reagents
Oxidized low-density lipoproteins (oxLDL) and DiI-labeled oxLDL (DiI-oxLDL) were purchased from Peking Union-Biology (Beijing, China). Recombinant human IKKβ (rhIKKβ) was purchased from Cusabio Biotech (Wuhan, China). Antibodies against GAPDH (5174), p-TAK1 (9339), TAK1 (5206), p-IKKα/β (2697), IKKβ (8943), p-p65 (3033), NF-κB p65 (8242) and IκBα (9242) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against CD68 (ab955) and Lamin B1 (ab133741) were purchased from Abcam (Cambridge, UK). Antibodies against Ly6G (sc-53515) and Ly6C (sc-271811) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against Flag (20543-1-AP) and HA (51064-2-AP) were purchased from Proteintech (Wuhan, China). Fluorescent-labeled primary antibodies against APC-CD45 (157606), PerCP-CD11b (101230), PE-Ly6G (127608) and FITC-Ly6C (128006) were purchased from BioLegend (San Diego, CA). Costunolide (CTD, AB0612) was purchased from Alfa Biotechnology (Chengdu, China). Biotinylated-costunolide (Bio-CTD) and reduced costunolide (R-CTD) were synthesized independently according to protocols reported previously [24, 25]. Biotin was purchased from Energy Chemical (Shanghai, China).
Cell culture
Mouse primary peritoneal macrophages (MPMs) were isolated as described previously [26, 27]. Briefly, mice received a single intraperitoneal injection of 6% thioglycolate solution. Two days later, mice were euthanized and peritoneal cavity was flushed with RPMI-1640 medium (Gibco, Eggenstein, Germany). Samples were centrifuged, and cell suspension was plated in RPMI-1640 medium containing 10% fetal bovine serum (FBS, Gibco, Eggenstein, Germany) and 1% penicillin/streptomycin (Invitrogen, Waltham, MA). Nonadherent cells were removed 2 h after seeding the cell suspension. Mouse macrophage cell line RAW264.7 (SCSP-650) and 293 T cells (GNHu17) were purchased from Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China) and cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Eggenstein, Germany) with 10% FBS and 1% penicillin/streptomycin. All cells were cultured in a humidified incubator maintained at 37 °C with 5% CO2.
Cell viability determination
MPMs were seeded into 96-well plates at a density of 2 × 105 cells per well. Cells were allowed to attach before exposure to costunolide at increasing concentrations for 24 h. MTT reagent was added into each well and cells were incubated for 4 h. Absorbance at 490 nm was measured. Cell viability was defined as the percentage of absorbance compared to the non-treated control.
Mouse model of atherosclerosis
All animal care and experimental procedures were approved by the Wenzhou Medical University Animal Policy and Welfare Committee (Approved ID: wydw2021-0057). All the animal studies were performed in accordance to the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health). Male ApoE−/− mice on C57BL/6 background were obtained from Gempharmatech (Nanjing, China).
For studies involving costunolide, eight-week-old ApoE−/− mice were randomly divided into five groups (n = 12 for each group): (i) LFD group: mice fed with a low-fat diet (LFD) containing 10 kcal.% fat, 20 kcal.% protein and 70 kcal.% carbohydrate (H10010, HFK Bioscience, Beijing, China) and intragastrically treated with 1% CMC-Na vehicle control; (ii) LFD + CTD-20 group: mice fed with a LFD and intragastrically treated with 20 mg/kg every 2 d CTD; (iii) HDF group: mice fed with a high-fat diet (HFD) containing 40 kcal.% fat, 20 kcal.% protein, 40 kcal.% carbohydrate and 1.25% cholesterol (H10540, HFK Bioscience, Beijing, China) and intragastrically treated with 1% CMC-Na vehicle control; (iv) HFD + CTD-10 group: mice fed with a HFD and intragastrically treated with 10 mg/kg every 2 d CTD reconstituted in 1% CMC-Na solution; (v) HFD + CTD-20 group: mice fed with a HFD and intragastrically treated with 20 mg/kg every 2 d CTD. The doses of CTD were selected based on previous studies [19]. All the CTD-treated mice were firstly fed with LFD or HFD for 8 weeks, followed by CTD treatments for another 8 weeks. Body weights were recorded weekly. All the mice were sacrificed under sodium pentobarbital anesthesia at week 16. Blood samples were collected. Aortas were fixed in 4% paraformaldehyde or snap-frozen in liquid nitrogen. The levels of serum lipids including total triglycerides (TG), total cholesterol (TCH), LDL, and high-density lipoprotein were measured using respective assay kits obtained from Nanjing Jiancheng (Jiangsu, China).
Atherosclerotic lesion analysis
For en face lesion analysis of the aorta, the whole aorta and aortic sinus were dissected out, opened longitudinally from heart to the iliac arteries, and stained with Oil Red O (G1260, Solarbio, Beijing, China). The heart and proximal aorta were collected and embedded in optimum cutting temperature compound for quantification of plaque lesion. Serial 5 μm-thick cryosections of aortic sinus from each mouse were obtained. Sections were stained with Oil Red O and hematoxylin for analysis of plaque sizes.
Paraffin-embedded sections were used for Ly6G and Ly6C immunohistochemistry and Sirius red (S8060, Solarbio) and Masson’s Trichrome staining (G1340, Solarbio,). For immunohistochemistry assay, sections were deparaffinized and rehydrated, followed by heat-induced antigen retrieval using 10 mM sodium citrate buffer (pH 6.5). Sections were blocked in 3% H2O2 and then in 5% bovine serum albumin for 30 min. Primary Ly6G (1:200) and Ly6C antibodies (1:200) were added. Sections were incubated at 4 °C overnight. Horseradish peroxidase-conjugated secondary antibody and DAB were used for detection.
Frozen sections were used for NF-κB p65 and CD68 staining. Slides were fixed in cold methanol and permeabilized using 0.5% Triton-X. Then, slides were blocked using 5% bovine serum albumin for 30 min and incubated overnight with primary NF-κB p65 (1:200) and CD68 antibodies (1:200). Alexa-488 and Alexa-647 conjugated secondary antibodies (Abcam, 1:200) were used for detection. Slides were counterstained with DAPI. The images were viewed using a light microscope (Nikon, Tokyo, Japan).
Flow cytometry assay
Aorta samples were harvested and the single-cell suspension was prepared using a 70 μm cell strainer (352350, BD Biosciences, San Jose, CA). All cell suspensions were carefully washed and stained with FACS staining buffer (PBS with 2% FBS) and combinations of antibodies against CD45, CD11b, Ly6G and Ly6C for 30 min on ice. Flow cytometry analysis was performed using BD Accuri™ C6 Cytometer (BD Biosciences, San Jose, CA) under appropriate fluorescence compensation. Leukocyte subsets were gated using FlowJo software (TreeStar, San Carlos, CA). Neutrophils were identified as the CD45+CD11b+Ly6G+ subsets and monocytes as CD45+Cd11b+Ly6C+ subsets, respectively.
Cytokine measurements
The levels of TNF-α and IL-6 in the serum and cell culture media were detected using ELISA kits (Cat#. 88-7324-76 and 88-7064-76, Thermo Fisher, Carlsbad, CA).
Transcriptome sequencing
Cells were collected using RNAiso Plus (9109, Takara, Shiga, Japan) and subjected to a genome-wide transcriptomics analysis by LC-Bio (Hangzhou, China). The differentially expressed genes were selected with fold change >2 or fold change <0.5 and P value < 0.05. DAVID (https://david.ncifcrf.gov/) was utilized to perform the KEGG and GO enrichment analyses among the total DEGs, as described by LC-Bio (https://www.lc-bio.cn/). Transcription factors prediction analysis was performed using TRRUST (https://www.grnpedia.org/trrust/) and ChEA3 (https://maayanlab.cloud/chea3/).
oxLDL uptake assay
For uptake detection, MPMs were incubated with 50 μg/mL DiI-oxLDL for 3 h at 37 °C. Cells were washed with PBS and imaged using Leica TCS SP8 confocal laser scanning microscope (Buffalo Grove, IL). Cells under identical conditions were also dislodged and analyzed by flow cytometry. The results of flow cytometry were expressed as mean fluorescence intensity after subtracting auto-fluorescence of cells (absence of DiI-oxLDL). Oil Red O staining in MPMs was performed according to the instructions of an Oil Red O stain kit (G1262, Solarbio). Images were captured using Nikon microscope equipped with a digital camera (Tokyo, Japan).
Generation of stable NF-κB EGFP RAW264.7 cells
RAW264.7 cells were transfected with pGL3-NF-κB-EGFP lentiviral particles to obtain stable expression of NF-κB reporter. Briefly, lentivirus containing the response element of NF-κB was first generated by co-transfecting 293 T cells with p-LV-NF-κB-RE-EGFP (Invitrogen, Carlsbad, CA) and packaging plasmids (psPAX2 and pMD2.G) using PEI. Supernatant was collected 48 h later and filtered using a 0.45 μm filter. Then, RAW264.7 cells were incubated with the supernatant and 8 μg/mL polybrene (Sigma-Aldrich, St. Louis, MO) for 12 h. Cells were selected with 2 μg/mL puromycin (Invitrogen, San Diego, CA). Following transfection, cells were challenged with 50 μg/mL oxLDL for 6 h. Mean fluorescence intensity was evaluated using flow cytometry.
Real time quantitative PCR
Total RNA was extracted from cells or aorta tissues using RNAiso Plus. Reverse transcription was carried out using PrimeScript™ RT reagent Kit with gDNA Eraser (RR047A, Takara, Shiga, Japan). Quantitative PCR was conducted using TB Green® Premix Ex Taq™ II (RR820A, Takara, Shiga, Japan) in QuantStudioTM 3 Real-Time PCR System (Thermo Fisher, Carlsbad, CA). Primers were obtained from Thermo Fisher. Primer sequences used in this study are listed in Supplementary Table S1.
Western blot and co-immunoprecipitation
Total protein from cells were extracted using lysis buffer (AR0103, Boster Biological Technology, Pleasanton, CA). Proteins were separated using 10% SDS-PAGE and then transferred to polyvinylidene fluoride membranes. Before adding specific primary antibodies, membranes were blocked in Tris-buffered saline for 1.5 h at room temperature. Protein bands were then detected by incubating with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagent (Bio-Rad, Hercules, CA). Band densities were quantified using Image J software (Version 1.38e, NIH, Bethesda, MD) and normalized to loading controls.
For co-immunoprecipitation assays, cell extracts prepared following treatments were incubated with indicated antibodies at 4 °C overnight. Then the proteins were immunoprecipitated with Protein A + G Agarose (P2012, Beyotime, Shanghai, China) at 4 °C for 2 h. Immunoprecipitation samples were further subjected to immunoblotting for the detection of co-precipitated proteins. Total lysates were also performed for Western blot analysis as an input control. Protein interactions were quantified using Image J software (Version 1.38e, NIH, Bethesda, MD).
IKKβ knockdown and overexpression
Gene silencing in cells was achieved by transfecting specific siRNA (Gene Pharma, Shanghai, China). Custom siRNAs were synthesized for mouse IKKβ (5′-AUCUAGUAGAGCGGAUGAUTT-3′ and 5′-AUCAUCCGCUCUACUAGAUTT-3′). Control cells were transfected with negative control siRNA. Transfection of RAW264.7 cells was carried out using Lipofectamine™ 2000 (Thermo Fisher, Carlsbad, CA). IKKβ overexpression in 293 T cells was achieved by transfecting cells with IKKβ plasmids (pCMV3-humanIKKβ-Flag, HG11595-CF; Sino Biological, Beijing, China). And the mutant IKKβ plasmids (pCMV3-human IKKβ-Flag-C179A, pCMV3-human IKKβ-Flag-S177E/S181E, pCMV3-human IKKβ (KD) [1-457AA]-Flag, pCMV3-human IKKβ-HA) were designed and obtained from GENEWIZ (Suzhou, China).
CTD-IKKβ binding assay
The binding ability of CTD to IKKβ was detected through pull-down assay using BeaverBeads™ Streptavidin Beads (22308, BEAVER, Suzhou, China). 5 µL of 10 mM biotinylated-CTD was added to 50 μL streptavidin beads and incubated at room temperature for 1 h. Biotin alone was used as the negative control. Lysates prepared from MPMs, 293 T cells or recombinant human IKKβ were then added to the streptavidin beads with bio-CTD. The mixture was incubated at 4 °C for 24 h with gentle rocking. Beads were then washed with 0.05% Tween 20 in PBS for five times and boiled in the loading buffer. The samples were loaded on a 10% polyacrylamide gel for Western Blot analysis. Total lysates were used as an input control.
Surface plasmon resonance (SPR) analysis
The binding affinity of CTD to recombinant human IKKβ (rhIKKβ) was determined using the Biacore T200 Protein Interaction Assay system (GE Healthcare, Pittsburgh, PA) with a CM7 sensor chip (29-1470-20). rhIKKβ protein was dissolved in 10 mM acetate acid buffer (pH 5.0), and then amine coupling kit (GE BR-1000-50, GE Healthcare) was used to immobilize rhIKKβ on the chip. Different concentrations of CTD were prepared with running buffer (PBS containing 5% DMSO). Sensor and sample plates were placed in the instrument. The interactions were determined at a flow rate of 30 μL/min for 250 s during the association phase, followed by 250 s for the dissociation phase at 25 °C. The data were analyzed using Biacore T200 manager software (GE Healthcare). Binding kinetic parameters were calculated by global fitting of the kinetic data from various concentrations of CTD using a 1:1 Langmuir binding model.
Drug affinity responsive target stability (DARTS) assay
DARTS was carried out according to the published protocol [28]. 293 T cells were harvested 24 h after transfection and lysed with lysis buffer. Lysates were incubated with CTD at 4 °C overnight with gentle rocking. Then, the pronase (10165921001, Sigma, St. Louis, MO) was added to the lysates and incubated for 5 min at room temperature. The reaction was stopped by the addition of loading buffer. Then, the samples were analyzed via immunoblotting.
Computational docking and molecular simulation
The crystal structure of IKKβ (PDB code 4KIK/4E3C) was derived from Protein Data Bank repository. Input files of ligand and receptor for docking were prepared using Graphical User Interface program AutoDock Tools 1.5.6 (The Scripps Research Institute, La Jolla, CA) [29]. Molecular docking was performed via AutoDock Vina 1.0.2.
Statistical analysis
All experiments were randomized and blinded. Data represented at least three independent experiments and were expressed as mean ± SEM. Statistical analyses were performed with GraphPad Prism 8.0 software (GraphPad, San Diego, CA, USA). Comparisons between two groups were analyzed using Student’s t test. One-way ANOVA followed by Dunnett’s post hoc test was used when comparing more than two groups of data. P value < 0.05 was considered significant. Post-tests were run only if F achieved P < 0.05 and there was no significant variance in homogeneity.
Results
CTD alleviates atherosclerotic progression in HFD-fed ApoE−/− mice
Firstly, CTD was applied to HFD-fed ApoE−/− mice to explore the potential effects on atherosclerosis. The doses of CTD (10 and 20 mg/kg) were selected according to a previous report, where oral administration with CTD at 10 and 20 mg/kg efficaciously inhibited pulmonary fibrosis [23]. As shown in Supplementary Fig. 1a, feeding with a HFD increased the body weight of mice compared to the LFD group, while CTD exhibited no effect on body weight change in HFD-fed ApoE−/− mice. In addition, the serum levels of total triglycerides, TCH, and LDL were markedly increased in both ApoE−/− mice and CTD-treated mice fed with HFD, and there was no significant difference between HFD group and HFD + CTD groups (Supplementary Fig. 1b–d), indicating that CTD treatment did not affect the HFD-induced hyperlipidemia profile in mice. However, CTD treatment significantly reduced the plaque area in the aorta in a dose-dependent manner (Fig. 1b, c). Similarly, Oil Red O staining of aortic roots showed that CTD treatment alleviated atherosclerotic lesions compared to vehicle-treated HFD-fed mice (Fig. 1d, e). Masson’s Trichome and Sirius Red staining also revealed reduced collagen deposition in atherosclerotic plaques in the CTD-treated group (Fig. 1f–i). Collectively, these results indicate that CTD attenuated the development of atherosclerosis without affecting lipid metabolism in the HFD-fed ApoE−/− mice.
CTD prevents the infiltration of inflammatory cells and reduces inflammatory response in aortas
In view of that leukocyte recruitment and inflammation play an important role in atherosclerotic development [4, 5, 30], we next probed into the anti-inflammatory effect of CTD in atherosclerotic mice. Immunofluorescence staining showed that CTD markedly reduced the infiltration of CD68-positive macrophages into the aortic roots of HFD-fed ApoE−/− mice (Fig. 2a, b). Also, recruitment of neutrophils (Fig. 2c, d) and monocytes (Fig. 2e, f) into atherosclerotic plaques was dose-dependently inhibited by CTD treatment, which was consistent with the immunohistochemical results for Ly6G (Fig. 2g) and Ly6C (Fig. 2h) staining. CTD administration also reduced the HFD-induced increase in the mRNA levels of adhesion molecules (Icam1, Vcam1) and chemokines (Cxcl1, Ccl2) (Fig. 2i, j), which might be the reason for the reduced invasion of inflammatory cells into the plaque lesions. In addition, CTD treatment dose-dependently suppressed the up-regulation of proinflammatory cytokines (TNF-α, IL-6) at both the mRNA and protein levels (Fig. 2k–n). Taken together, these data suggest that CTD exhibits the anti-atherosclerosis effect through reducing inflammatory cell infiltration and expression of inflammatory response in atherosclerotic lesions.
a Representative immunofluorescence staining images of CD68 (green) in aortic roots. Tissues were counterstained with DAPI (blue). b Quantification of CD68-positive area in aortic roots (n = 7). Flow cytometry analysis and quantification of Ly6G+ cells (c–d) and Ly6C+ cells (e–f) in aortas (n = 5). Representative immunohistochemistry images of Ly6G+ (g) and Ly6C+ (h) staining in aortic roots. mRNA levels of adhesion molecules (i) and proinflammatory chemokines (j) in aortas (n = 5). Rn18s was used as the loading control. Protein (k–l) and mRNA (m–n) levels of inflammatory cytokines TNF-α and IL-6 in serum and aortas (n = 7). Rn18s was used as the loading control. Data were shown as mean ± SEM; *P < 0.05; ns not significant.
CTD attenuates oxLDL-induced inflammation in macrophages
Since macrophages play fundamental roles in inflammatory atherosclerosis [31,32,33], we next used oxLDL-challenged mouse primary macrophages as an in vitro model to explore the pharmacological mechanism of CTD in atherosclerosis. Firstly, based on the results of toxicity of CTD in MPMs (Supplementary Fig. S2a), we chose 2.5, 5 and 10 μM as the treatment concentrations of CTD in subsequent in vitro studies. Next, we utilized transcriptome sequencing to identify candidate pathways that are regulated by CTD in oxLDL-treated MPMs. Differentially expressed genes (DEGs) were compared between MPMs stimulated with oxLDL with or without pretreatment of CTD. There were 1987 differentially expressed genes, including 1392 upregulated genes and 595 downregulated genes when comparing oxLDL group and oxLDL+CTD group (Fig. 3a). KEGG enrichment analyses of the RNA-seq gene expression profiles revealed that CTD downregulated ‘inflammatory response’ and key inflammatory pathways including ‘chemokine signaling pathway’ and ‘cell adhesion molecules’ (Fig. 3b, c), which likewise confirmed the findings obtained in our in vivo study. As expected, CTD reduced the oxLDL-induced expression of pro-inflammatory cytokines at both mRNA and protein levels in cultured macrophages (Fig. 3d, e). CTD also dose-dependently lowered the transcriptional levels of pro-inflammatory chemokines and adhesion molecules in oxLDL-challenged macrophages (Fig. 3f, g). Finally, CTD substantially inhibited the uptake of oxLDL in MPMs (Fig. 3h, j, Supplementary Fig. S2b), leading to less foam cell formation.
a Mouse primary peritoneal macrophages (MPMs) were pretreated with CTD or vehicle (DMSO, 1‰) for 1 h, followed by stimulation of oxLDL (50 μg/mL) for 6 h. Total RNA was sequenced to identify differentially expressed genes (DEGs). Volcano plot analysis of DEGs in oxLDL-treated MPMs with or without pretreatment of CTD. FC, fold change. GO (b) and KEGG (c) enrichments of DEGs. d MPMs were pretreated with CTD (2.5, 5 and 10 μM) or vehicle (DMSO, 1‰) for 1 h, followed by exposure of oxLDL (50 μg/mL) for 24 h. Protein levels of TNF-α and IL-6 were analyzed using ELISA (n = 4). e–g MPMs were pretreated the same as described in (d), followed by exposure of oxLDL (50 μg/mL) for 6 h. mRNA levels of inflammatory cytokines (e), adhesion molecules (f) and proinflammatory chemokines (g) were determined via RT-qPCR (n = 3). β-actin was used as the loading control. h–j MPMs were pretreated the same as described in (d), followed by DiI-oxLDL (50 μg/mL) stimulation for 3 h. h Fluorescence staining of DiI-oxLDL (red) in MPMs. Cells were counterstained with DAPI (blue). Flow cytometry analysis (i) and quantification of mean fluorescence intensity of DiI-oxLDL (j) were shown (n = 4). Data were shown as mean ± SEM; *P < 0.05.
CTD blocks oxLDL-induced NF-κB activation in macrophages
To further explore the anti-inflammatory mechanism of CTD, 86 DEGs upregulated in the oxLDL-treated group but downregulated by CTD (Fig. 4a) were selected for a predictive analysis of transcription factors using two independent methods/systems, TRRUST and ChEA3. Interestingly, both methods showed that NF-κB p65 (RELA) ranked first, suggesting that CTD might inhibit NF-κB p65 activation to downregulate inflammatory response in macrophages (Fig. 4b). We then confirmed that oxLDL activated NF-κB p65 in MPMs in a time-dependent manner (Supplementary Fig. S2c). However, MPMs pretreated with CTD had a dose-dependent reduction in oxLDL-induced NF-κB p65 phosphorylation (Fig. 4c, d). Further, we evaluated the transcriptional activity of NF-κB in macrophages by utilizing an NF-κB-EGFP reporter. Pretreatment of RAW264.7 cells stably expressing NF-κB EGFP with CTD prior to oxLDL challenge showed a CTD dose-dependent reduction in NF-κB activity compared to cells without CTD pretreatment (Fig. 4e and Supplementary Fig. S2d). In addition, CTD dose-dependently dwindled the oxLDL-induced p65 nuclear translocation in MPMs (Fig. 4f–i). The staining of p65 in aortic roots supported that CTD suppressed p65 nuclear translocation in aortic roots of HFD-fed ApoE−/− mice (Fig. 4j). Taken together, these results evidence that CTD significantly diminished NF-κB activation in oxLDL-challenged macrophages.
a Venn diagram of genes upregulated in oxLDL compared to control (pink), and genes upregulated in oxLDL compared to oxLDL+CTD (blue). b Transcription factor prediction was performed using TRRUST and ChEA3 databases. c–d MPMs were pretreated with CTD (2.5, 5 and 10 μM) or vehicle (DMSO, 1‰) for 1 h, followed by stimulation of oxLDL (50 μg/mL) for 1 h. Western blot analysis (c) and densitometric quantification (d) of p-p65 and p65 were shown. GAPDH was used as loading control. e RAW264.7 cells were transfected to express NF-κB-RE-EGFP reporter. Cells were pretreated as described in (c), followed by stimulation of oxLDL (50 μg/mL) for 6 h. The activity of NF-κB was evaluated by flow cytometry analysis. f–h MPMs were treated as described in (c). Protein levels of p65 in cytoplasm and nuclear fractions were measured via Western blot. GAPDH was used as loading control for cytosolic fractions. Lamin B1 was used as loading control for nuclear fractions. Densitometric quantification of p65 was determined in cytoplasm (g) and nucleus (h), respectively. i MPMs were treated as in (c). Representative immunofluorescence staining images of NF-κB p65 (red) in MPMs were shown. j Representative immunofluorescence staining images of NF-κB p65 (red) in aortic roots. Cells or tissues were counterstained with DAPI (blue). Statistical data were shown as mean ± SEM; n = 6; *, P < 0.05.
CTD directly binds to IKKβ via formation of covalent bond
To explore how CTD inhibits NF-κB activation, we measured the activation of canonical upstream proteins of NF-κB in oxLDL-challenged MPMs. Surprisingly, we discovered that the oxLDL-induced increase in the phosphorylation level of IKKβ was decreased by CTD treatment in a dose-dependent manner, while the phosphorylation levels of TAK1 and IKKα were not reversed (Fig. 5a, b). Observation in 293 T cells transfected with Flag-IKKβ revealed that CTD dose-dependently inhibited the oxLDL-induced phosphorylation of IKKβ, and subsequent IκBα degradation and p65 phosphorylation (Fig. 5c). As expected, knockout of IKKβ by siRNA in RAW264.7 cells (Fig. 5d) reduced overexpression of TNF-α and IL-6 response to oxLDL exposure (Fig. 5e, f). We also tested whether CTD exerts anti-inflammatory activity via inhibiting IKKβ. We found that CTD has no synergistic or additive effects in macrophages with IKKβ knockout (Fig. 5e, f), indicating that IKKβ mediates the anti-inflammatory activity of CTD.
a–b MPMs were pretreated with CTD (2.5, 5 and 10 μM) or vehicle (DMSO, 1‰) for 1 h, followed by exposure of oxLDL (50 μg/mL) for 1 h. Western blot analysis (a) and densitometric quantification (b) of IκBα, p-IKKβ and p-TAK1 were determined using Western blot. GAPDH, IKKβ and TAK1 were used as loading controls (n = 6). c 293 T cells transfected with Flag-IKKβ or control vector were treated with CTD for 1 h. Western blot analysis of p-IKKβ, p-p65 and IκBα were performed. p65, Flag and GAPDH were used as loading controls. d RAW264.7 cells were transfected with IKKβ siRNA. Control cells were transfected with negative control siRNA. Levels of IKKβ were measured following knockdown (n = 5). e–f RAW264.7 cells transfected with negative control siRNA or RAW264.7 cells with IKKβ knockdown were pretreated with CTD (2.5, 5 and 10 μM) or vehicle (DMSO, 1‰) for 1 h, followed by exposure of oxLDL (50 μg/mL) for 24 h. Levels of TNF-α (e) and IL-6 (f) in cellular supernatant were measured by ELISA (n = 4). g The chemical structure of biotinylated-CTD (Bio-CTD). h–i The interaction between IKKβ and CTD was detected by pull-down assay using streptavidin beads. Lysates prepared from MPMs (h), 293 T cells transfected with Flag-IKKβ (i) and recombinant IKKβ (j) were added to the streptavidin beads with Biotinylated CTD (Bio-CTD). The binding of IKKβ was determined via Western blot assay. k Surface plasmon resonance (SPR) analysis showing direct interaction between CTD and IKKβ. Statistical data were shown as mean ± SEM; *P < 0.05.
These data suggest that IKKβ might be the molecular target of CTD for its anti-inflammatory activity in MPMs. To confirm this hypothesis, we then performed pull-down assays to verify the direct interaction between CTD and IKKβ using biotinylated-CTD (Bio-CTD (Fig. 5g). As shown in Fig. 5h, i, Bio-CTD directly bound to IKKβ protein in lysates from both MPMs and 293 T cells that transfected with Flag-IKKβ. Furthermore, pull-down assays using cell-free rhIKKβ protein validated the direct interaction between CTD and IKKβ protein (Fig. 5j). Their direct interaction was further confirmed using an SPR assay, showing that CTD bound to rhIKKβ protein with a KD value of 1.19 × 10−8 M (Fig. 5k).
CTD is covalently coupled with cysteine 179 on IKKβ
Next, we explored the nature of the interaction between CTD and IKKβ. We have noted that the chemical structure of CTD possesses an electrophilic functional group, αβ-unsaturated carbonyl (Fig. 6a, shown in red), which, as a Michael acceptor, may react with the nucleophilic sulfhydryl functional group of an active-site cysteine [34,35,36]. Considering that CTD may form covalent bond with cysteines in IKKβ protein, we investigated the reversibility of the inhibition of CTD against IKKβ. Our data showed that CTD maintained its anti-inflammatory effect independent of wash after administration (Fig. 6b, c), suggesting that the inhibitory effect of CTD in oxLDL-challenged MPMs is irreversible, at least in the first 24 h. Similarly, CTD irreversibly suppressed the oxLDL-induced phosphorylation of both IKKβ and p65 in macrophages (Fig. 6d, Supplementary Fig. S3). All these data suggest that CTD may exhibit its anti-inflammatory effects via covalently binding to IKKβ. Then, we synthesized a reduced CTD (R-CTD) without the αβ-unsaturated carbonyl (Fig. 6e) to evaluate if it retains anti-inflammatory effect. As expected, the reduced-CTD abolished its inhibitory effects on the overexpression of proinflammatory cytokines caused by oxLDL (Fig. 6f, g). Collectively, these results certify that the αβ-unsaturated carbonyl, as a Michael acceptor, is essential for the covalent binding between CTD and IKKβ, which is important for the anti-inflammatory effect of CTD.
a The chemical structure of red denotes cysteine-reactive enone (red) in CTD. b–c MPMs were pretreated with CTD (2.5, 5 and 10 μM) or vehicle (DMSO, 1‰) for 1 h. With or without wash of PBS, cells were then stimulated by oxLDL (50 μg/mL) for 24 h. Levels of TNF-α (b) and IL-6 (c) in cellular supernatant were measured. d MPMs were pretreated as described in (b), followed by exposure of oxLDL (50 μg/mL) for 1 h. Phosphorylated IKKβ and p65 were detected using Western blot. IKKβ, p65 and GAPDH were used as loading controls. e The chemical structures of CTD and CTD without carbon–carbon double-bond (R-CTD). f–g MPMs were pretreated with R-CTD (2.5, 5 and 10 μM) or vehicle (DMSO, 1‰) for 1 h, followed by exposure of oxLDL (50 μg/mL) for 24 h. Levels of TNF-α (f) and IL-6 (g) in cellular supernatant of MPMs were measured by ELISA. Statistical data were shown as mean ± SEM; n = 4; *P < 0.05.
It is unclear which cysteine in IKKβ protein is involved in CTD’s inhibitory activity. IKKβ protein consists of three domains [37, 38] (Fig. 7a, upper panel). Since CTD inhibits IKKβ phosphorylation, we speculated that the kinase domain of IKKβ might be the CTD-binding domain. We designed a Flag-labeled plasmid encoding the kinase domain of IKKβ. As expected, the kinase domain of IKKβ could be pulled down by Bio-CTD (Fig. 7a, lower panel), suggesting that CTD binds to the kinase domain. The dimerization of IKKβ is afforded by the LZ domain of IKKβ, prior to the kinase phosphorylation and independent of the kinase activity [39, 40]. Co-immunoprecipitation data showed that CTD did not affect the dimerization of IKKβ (Fig. 7b), further confirming the specificity of CTD binding to the kinase domain.
a The major structural domains of IKKβ (upper panel), including kinase domain (KD), leucine zipper (LZ) and helix-loop-helix (HLH). The binding between Bio-CTD and Flag-IKKβ (KD) was evaluated by pull-down analysis in Flag-IKKβ (KD) expressing 293 T cells (lower panel). b 293 T cells were transfected with Flag-IKKβ and HA-IKKβ plasmids, followed by treatment of CTD (10 μM) or vehicle for 1 h. IKKβ dimerization was determined by co-immunoprecipitation assay. c A model of IKKβ regulation and the sequence of the active loop in kinase domain. d Molecular docking of CTD with IKKβ was carried out using Tripos molecular modeling packages Sybyl-x.v1.1.1083. e 293 T cells transfected with Flag-(C179A)IKKβ or control vector were treated with CTD (2.5, 5 and 10 μM) or vehicle (DMSO, 1‰) for 1 h. The phosphorylated IKKβ and p65, and levels of IκBα were determined by Western blot analysis. p65, Flag and GAPDH were used as loading controls. f–g 293 T cells transfected with Flag-IKKβ (f) or Flag-(C179A)IKKβ (g) plasmids were treated with CTD (10 μM) or vehicle (DMSO, 1‰) for 1 h. A DARTS assay was performed. h 293 T cells were transfected with either Flag-IKKβ or Flag-(C179A)IKKβ plasmids. The cells were treated with CTD (2.5, 5 or 10 μM) and bio-CTD (10 μM). The competitive interaction between IKKβ and bio-CTD was detected by pull-down assay using streptavidin beads. i–j 293 T cells transfected with Flag-(S177E/S181E)IKKβ or control vector were treated with CTD (2.5, 5 or 10 μM) for 1 h. i The phosphorylated p65 and levels of IκBα were determined by Western blot analysis. p65, Flag and GAPDH were used as loading controls. j The densitometric quantification of IκBα and p-p65 was shown. Statistical data were shown as mean ± SEM; n = 3; *P < 0.05.
We then analyzed the structure of activation loop in kinase domain of IKKβ (Fig. 7c). In the activation loop, two phosphorylating sites, serine 177 (Ser177) and serine 181 (Ser181), are indispensable for IKKβ phosphorylation [37, 38]. There is only one cysteine (Cys179) in the activation loop, and interestingly, it is precisely located in the middle of Ser177 and Ser181. Consistent with this finding, our molecular docking simulation results revealed that CTD could covalently bind to Cys179 on IKKβ, which hindered the phosphorylating conformation of both Ser177 and Ser181 (Fig. 7d). We then constructed a mutant plasmid in which Cys179 was replaced by alanine. Co-immunoprecipitation assay showed that the mutation of Cys179Ala abrogated the inhibitory effect of CTD on IKKβ phosphorylation and subsequent NF-κB activation (Fig. 7e). Furthermore, the DARTS assay validated that CTD protected IKKβ, but not IKKβ C179A, against protease-mediated proteolysis (Fig. 7f, g). Pull-down assay also showed that Bio-CTD failed to interact with C179A-mutant IKKβ protein (Fig. 7h). Finally, constitutively activated IKKβ was constructed by substituting Ser177 and Ser181 with glutamic acids to mimic the phosphorylated structure at these sites [37, 41]. Binding to Cys179 by CTD remained restraining constitutively activated IKKβ as evidenced by the dose-dependent inhibition of NF-κB activation (Fig. 7i, j), possibly because the active conformation changes of S177E/S181E were also hindered by CTD binding to Cys179 (Supplementary Fig. S4). In summary, these data indicate Cys179 of IKKβ is the covalent binding site of CTD and is responsible for the IKKβ/NF-κB inhibition of CTD.
Discussion
In this study, we evaluated the protective effects of CTD in HFD-induced atherosclerosis in vivo and in vitro. The two key findings include: (1) blockade of IKKβ by CTD significantly attenuates HFD-induced pro-inflammatory molecules and inhibited oxLDL uptake in macrophages, leading to ameliorating the development of atherosclerosis. (2) CTD covalently bind to cysteine 179 on IKKβ, leading to inhibition IKKβ phosphorylation and IKKβ/NF-κB signaling pathway. A schematic illustration of the main findings is shown in Fig. 8. Our findings present a direct target and a mechanistic explanation for CTD as a potentially therapeutic candidate for retarding atherosclerotic progression.
CTD covalently bind to cysteine 179 on IKKβ, leading to inhibition IKKβ phosphorylation and IKKβ/NF-κB signaling pathway. Blockade of NF-κB by CTD significantly attenuates HFD-induced pro-inflammatory molecules and inhibited oxLDL uptake in macrophages, finally ameliorating the development of atherosclerosis.
NF-κB pathway plays a critical role in the pathogenesis of atherosclerosis [42]. NF-κB activation has been observed in atherosclerotic plaques in both human and animal models [43]. In addition, NF-κB activation in human atherosclerosis has been confirmed to be IKKβ-dependent, which is associated with the up-regulation of proinflammatory and prothrombotic responses [44]. Thus, IKKβ, as a critical molecule linking inflammation and atherosclerosis, could be a promising therapeutic target for the clinical management of atherosclerosis. As a bioactive natural product, CTD has been used to treat many inflammatory diseases via inhibiting NF-κB pathway. For example, Fan et al. found that CTD administration improves dextran sulfate sodiium-induced acute ulcerative colitis via inhibiting the phosphorylation of NF-κB p65 and degradation of IκB [22]. He et al. reported that CTD attenuates osteoarthritis via suppressing the NF‑κB and Wnt/β‑catenin signaling pathways [45]. Liu et al. proved that CTD inhibited pulmonary fibrosis through ameliorating NF-κB dependent inflammation [23]. However, none of these studies explained the mechanism of how CTD inhibits NF-κB. Here, we showed that CTD attenuates inflammatory atherosclerosis via inhibiting the NF-κB pro-inflammatory signaling pathway in macrophages. Most importantly, we have first identified that CTD inactivates NF-κB p65 through selectively and covalently targeting IKKβ at Cys179, rather than TAK1 and IKKα. To our best acknowledge, it is the first time to clearly illustrate the molecular target as well as the binding mode of CTD for its anti-inflammatory activity.
So far, most small-molecule IKKβ inhibitors, such as SC-514 [46], ML120B [47], TPCA-1 [48], restrain IKKβ activity through competing for ATP-binding site. In addition, two IKKβ inhibitors, 15d-PGJ2 and PGA1, have been reported to covalently bind to Cys179 on IKKβ [49]. In this study, we illustrated that CTD was covalently bound to IKKβ since the inhibitory effect of CTD in oxLDL-challenged MPMs was irreversible. Interestingly, our data also show that CTD does not affect the dimerization of IKKβ, afforded by the LZ domain of IKKβ. Furthermore, we found that the reactive αβ-unsaturated carbonyl, as a Michael acceptor, was essential for the covalent bond formation between CTD and Cys179 in IKKβ kinase domain. Replacement of cysteine 179 with alanine abolished the binding between CTD and IKKβ. A molecular docking and simulation study showed that, after binding to cysteine 179, CTD altered the conformation of the activation loop of IKKβ, suppressing its phosphorylation. This work provides a new lead compound for designing and discovering new IKKβ inhibitors. However, further studies are required to uncover the exact mechanisms or binding mode using CTD-IKKβ(KD) complex crystal structural biology.
Oxidative stress has also been shown to contribute to atherosclerosis [50]. CTD has been reported to exhibit anti-oxidant activity in other disease models [18]. Although our transcriptome sequencing showed that CTD treatment mainly affects the inflammatory signaling pathway rather than oxidation-related signals (Fig. 4b), it is still possible that anti-oxidant activity partly contributes to the pharmacological effects of CTD against atherosclerosis. In the other hands, generally, bioactive natural compounds possess multiple molecular targets to afford their multi-functional activities. In addition to IKKβ, CTD may also attenuate atherosclerosis through targeting other proteins. A limitation of this study is that we did not exclude the contribution of other potential targets in vivo. It is imperative for future studies to utilize macrophage-specific IKKβ-knockout mice with CTD administration to rule out other mechanisms involved in the anti-atherosclerosis effect of CTD.
In addition to macrophages, vascular smooth muscle cells (VSMCs) are also closely associated with the whole stage of atherosclerosis [51]. Inflammation triggered by macrophages leads to VSMCs differentiation which in turn contributes to microcalcification deposits in the intimal wall [52]. Moreover, VSMCs promote foam cell production and possess inflammatory-like cell characteristics [53]. Although we found that CTD attenuated inflammation in macrophages in this study, the potential effects of CTD on the pathophysiological changes in VSMCs remain unknown, which may also contribute to the anti-atherosclerosis activity of CTD. This is a limitation of this study and deserves attentions in further studies.
In conclusion, our study demonstrated that CTD prevented inflammatory atherosclerosis by covalently binding to cysteine 179 on IKKβ. To the best of our knowledge, it is the first time to report CTD as an anti-atherosclerosis agent and a covalent inhibitor of IKKβ. While this study lacked a positive drug control group, a potential limitation of this study, we identified the excellent pharmacological effect of CTD against atherosclerosis in mice. Our data proved that CTD could be a potential therapeutic candidate for retarding atherosclerotic progression. However, the clinical application of CTD requires further researches. One potential concern regarding the clinical use of CTD may be its possible adverse effects. Some historical covalent inhibitors were considered undesirable due to potential toxicity issues [54]. Although 20 mg/kg of CTD showed no significant toxicity in our in vivo study, dose reaching 50 mg/kg may get adverse effects as reported recently [55, 56]. Prospective investigation focusing on chemical analogues, targeted release, and combination therapy would be favorable for the further development of CTD as a candidate or lead compound.
References
Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–46.
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74.
Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329.
Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on A. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38.
Kutuk O, Basaga H. Inflammation meets oxidation: NF-kappaB as a mediator of initial lesion development in atherosclerosis. Trends Mol Med. 2003;9:549–57.
Liu F, Xia Y, Parker AS, Verma IM. IKK biology. Immunol Rev. 2012;246:239–53.
Cole JE, Kassiteridi C, Monaco C. Toll-like receptors in atherosclerosis: a ‘Pandora’s box’ of advances and controversies. Trends Pharmacol Sci. 2013;34:629–36.
Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13.
Napetschnig J, Wu H. Molecular basis of NF-kappaB signaling. Annu Rev Biophys. 2013;42:443–68.
Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224.
Durand JK, Baldwin AS. Targeting IKK and NF-kappaB for therapy. Adv Protein Chem Struct Biol. 2017;107:77–115.
Strnad J, Burke JR. IkappaB kinase inhibitors for treating autoimmune and inflammatory disorders: potential and challenges. Trends Pharmacol Sci. 2007;28:142–8.
Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98.
Park SH, Sui Y, Gizard F, Xu J, Rios-Pilier J, Helsley RN, et al. Myeloid-specific IkappaB kinase beta deficiency decreases atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2869–76.
Mao J, Zhan H, Meng F, Wang G, Huang D, Liao Z, et al. Costunolide protects against alcohol-induced liver injury by regulating gut microbiota, oxidative stress and attenuating inflammation in vivo and in vitro. Phytother Res. 2022;36:1268–83.
Kim DY, Choi BY. Costunolide-A bioactive sesquiterpene lactone with diverse therapeutic potential. Int J Mol Sci. 2019;20:2926.
Chen YT, Du Y, Zhao B, Gan LX, Yu KK, Sun L, et al. Costunolide alleviates HKSA-induced acute lung injury via inhibition of macrophage activation. Acta Pharmacol Sin. 2019;40:1040–8.
Mao J, Yi M, Wang R, Huang Y, Chen M. Protective effects of costunolide against D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Front Pharmacol. 2018;9:1469.
Scarponi C, Butturini E, Sestito R, Madonna S, Cavani A, Mariotto S, et al. Inhibition of inflammatory and proliferative responses of human keratinocytes exposed to the sesquiterpene lactones dehydrocostuslactone and costunolide. PLoS ONE. 2014;9:e107904.
Xie F, Zhang H, Zheng C, Shen XF. Costunolide improved dextran sulfate sodium-induced acute ulcerative colitis in mice through NF-kappaB, STAT1/3, and Akt signaling pathways. Int Immunopharmacol. 2020;84:106567.
Liu B, Rong Y, Sun D, Li W, Chen H, Cao B, et al. Costunolide inhibits pulmonary fibrosis via regulating NF-κB and TGF-beta1/Smad2/Nrf2-NOX4 signaling pathways. Biochem Biophys Res Commun. 2019;510:329–33.
He H, Jiang H, Chen Y, Ye J, Wang A, Wang C, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9:2550.
Macías FA, Galindo JCG, Massanet GM. Potential allelopathic activity of several sesquiterpene lactone models. Phytochemistry. 1992;31:1969–77.
Chen T, Huang W, Qian J, Luo W, Shan P, Cai Y, et al. Macrophage-derived myeloid differentiation protein 2 plays an essential role in ox-LDL-induced inflammation and atherosclerosis. EBioMedicine. 2020;53:102706.
Pan Y, Wang Y, Cai L, Cai Y, Hu J, Yu C, et al. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br J Pharmacol. 2012;166:1169–82.
Lomenick B, Hao R, Jonai N, Chin RM, Aghajan M, Warburton S, et al. Target identification using drug affinity responsive target stability (DARTS). Proc Natl Acad Sci USA 2009;106:21984–9.
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91.
Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–26.
Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–67.
Koelwyn GJ, Corr EM, Erbay E, Moore KJ. Regulation of macrophage immunometabolism in atherosclerosis. Nat Immunol. 2018;19:526–37.
Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262:153–66.
Coricello A, Adams JD, Lien EJ, Nguyen C, Perri F, Williams TJ, et al. A walk in nature: sesquiterpene lactones as multi-target agents involved in inflammatory pathways. Curr Med Chem. 2020;27:1501–14.
Hall IH, Lee KH, Starnes CO, Sumida Y, Wu RY, Waddell TG, et al. Anti-inflammatory activity of sesquiterpene lactones and related compounds. J Pharm Sci. 1979;68:537–42.
Quintana J, Estevez F. Recent advances on cytotoxic sesquiterpene lactones. Curr Pharm Des. 2018;24:4355–61.
Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–13.
Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25:6685–705.
Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res. 2008;42:3–18.
Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–74.
Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80.
Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22.
Hernandez R, Zhou C. Recent advances in understanding the role of IKKbeta in cardiometabolic diseases. Front Cardiovasc Med. 2021;8:752337.
Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, et al. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci USA 2004;101:5634–9.
He Y, Moqbel SAA, Xu L, Ran J, Ma C, Xu K, et al. Costunolide inhibits matrix metalloproteinases expression and osteoarthritis via the NFkappaB and Wnt/betacatenin signaling pathways. Mol Med Rep. 2019;20:312–22.
Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, et al. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem. 2003;278:32861–71.
Wen D, Nong Y, Morgan JG, Gangurde P, Bielecki A, Dasilva J, et al. A selective small molecule IkappaB Kinase beta inhibitor blocks nuclear factor kappaB-mediated inflammatory responses in human fibroblast-like synoviocytes, chondrocytes, and mast cells. J Pharmacol Exp Ther. 2006;317:989–1001.
Podolin PL, Callahan JF, Bolognese BJ, Li YH, Carlson K, Davis TG, et al. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB Kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]−5-(4-fluorophenyl)−3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell proliferation. J Pharmacol Exp Ther. 2005;312:373–81.
Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26.
Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19:42.
Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702.
Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114:590–600.
Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat ML. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 2018;114:540–50.
Lonsdale R, Ward RA. Structure-based design of targeted covalent inhibitors. Chem Soc Rev. 2018;47:3816–30.
Yan Z, Xu T, An Z, Hu Y, Chen W, Ma J, et al. Costunolide induces mitochondria-mediated apoptosis in human gastric adenocarcinoma BGC-823 cells. BMC Complement Alter Med. 2019;19:151.
Xu C, Huang X, Lei X, Jin Z, Wu M, Liu X, et al. Costunolide-induced apoptosis via promoting the reactive oxygen species and inhibiting AKT/GSK3beta pathway and activating autophagy in gastric cancer. Front Cell Dev Biol. 2021;9:722734.
Acknowledgements
This study was supported by National Natural Science Foundation of China (21961142009 to GL, 82000793 to WL, 81900331 to ZW) and Zhejiang Provincial Key Scientific Project (2021C03041 to GL).
Author information
Authors and Affiliations
Contributions
GL and WJH contributed to the literature search and study design. GL, ZQH, and YW participated in the drafting of the paper. ZQH, WL, WXL, PC,and ZW carried out the experiments. RJC and YW revised the paper. WL and WJH contributed to data collection and analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Huang, Zq., Luo, W., Li, Wx. et al. Costunolide alleviates atherosclerosis in high-fat diet-fed ApoE−/− mice through covalently binding to IKKβ and inhibiting NF-κB-mediated inflammation. Acta Pharmacol Sin 44, 58–70 (2023). https://doi.org/10.1038/s41401-022-00928-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00928-0