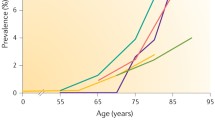
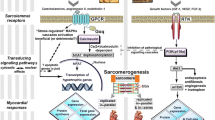
Abstract
Purpose of Review
This review summarizes the pathophysiology of calcific aortic valve stenosis (CAVS) and surveys relevant clinical data and basic research that explain how CAVS arises.
Recent Findings
Lipoprotein(a) [Lp(a)], lipoprotein-associated phospholipase A2 (Lp-PLA2), oxidized phospholipids (OxPL), autotaxin, and genetic driving forces such as mutations in LPA gene and NOTCH gene seem to play a major role in the development of CAVS. These factors might well become targets of medical therapy in the coming years.
Summary
CVAS seems to be a multifactorial disease that has much in common with coronary artery disease, mainly regarding lipidic accumulation and calcium deposition. No clinical trials conducted to date have managed to answer the key question of whether Lp(a) lowering and anti-calcific therapies confer a benefit in terms of reducing incidence or progression of CAVS, although additional outcome trials are ongoing.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cho KI, Sakuma I, Sohn IS, Jo SH, Koh KK. Inflammatory and metabolic mechanisms underlying the calcific aortic valve disease. Atherosclerosis. 2018;277:60–5.
Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343–56.
Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, et al. Scottish aortic stenosis and lipid lowering trial, impact on regression (SALTIRE) investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352(23):2389–97.
•• Capoulade R, Chan KL, Mathieu P, Bosse Y, Dumesnil JG, Tam JW, et al. Autoantibodies and immune complexes to oxidation-specific epitopes and progression of aortic stenosis: results from the ASTRONOMER trial. Atherosclerosis. 2017;260:1–7 This study failed to show that the reduction of LDL cholesterol can reduce the incidence of CAVS, and indirect markers of oxidation-specific epitopes are predictive of its progression.
Ferretti G, Bacchetti T, Johnston TP, Banach M, Pirro M, Sahebkar A. Lipoprotein(a): a missing culprit in the management of athero-thrombosis? J Cell Physiol. 2018;233:2966–81.
Perrot N, Thériault S, Dina C, Chen HU, Boekholdt SM, Rigade S, et al. Genetic variation in LPA, calcific aortic valve stenosis in patients undergoing cardiac surgery, and familial risk of aortic valve microcalcification. JAMA Cardiol. 2019. https://doi.org/10.1001/jamacardio.2019.1581.
Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63:470–7.
Arsenault BJ, Boekholdt SM, Dubé MP, Rhéaume E, Wareham NJ, Khaw KT, et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7(3):304–10.
Yu B, Hafiane A, Thanassoulis G, Ott L, Filwood N, Cerruti M, et al. Lipoprotein(a) induces human aortic valve interstitial cell calcification. J Am Coll Cardiol Basic Trans Science. 2017;2:358–71.
Yu B, Khan K, Hamid Q, Mardini A, Siddique A, Aguilar-Gonzalez LP, et al. Pathological significance of lipoprotein(a) in aortic valve stenosis. Atherosclerosis. 2018;272:168–74.
• Després AA, Perrot N, Poulin A, Tastet L, Shen M, Chen HY, et al. Lipoprotein(a), oxidized phospholipids, and aortic valve microcalcification assessed by 18F-sodium fluoride positron emission tomography and computed tomography. CJC Open. 2019;1(3):131–40 This study suggests that elevated Lp(a) and OxPL levels are associated with prevalent CAVS in a “real-world” setting, and showed that aortic valve microcalcification is present before the development of macroscopic and clinically manifested CAVS.
Mahmut A, Mahmut A, Husseini DE, Fournier D, Bouchareb R, Després JP, et al. Elevated expression of lipoprotein-associated phospholipase A2 in calcific aortic valve disease. Implications for valve mineralization. J Am CollCardiol. 2014;63:460–9.
Capoulade R, Mahmut A, Tastet L, Arsenault M, Bédard E, Dumesni JG, et al. Impact of plasma Lp-PLA2 activity on the progression of aortic stenosis the PROGRESSA study. J Am Coll Cardiol Img. 2015;8:26–33.
Kamstrup PR, Hung MY, Witztum JL, Tsimikas S, Nordestgaard BG. Oxidized phospholipids and risk of calcific aortic valve disease the Copenhagen General Population Study. Arterioscler Thromb Vasc Biol. 2017;37:1570–8.
Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34:715–23.
Bouchareb R, Mahmut A, Nsaibia MJ, Boulanger MC, Dahou A, Lépine JL, et al. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 2015;132:677–90.
Sticchi E, Giusti B, Cordisco A, Gori AM, Sereni A, Sofi F, et al. Role of lipoprotein (a) and LPA KIV2 repeat polymorphism in bicuspid aortic valve stenosis and calcifcation: a proof of concept study. Intern Emerg Med. 2019;14:45.
• Trenkwalder T, Nelson CP, Musameh MD, Mordi IR, Kessler T, Pellegrini C, et al. Effects of the coronary artery disease associated LPA and 9p21 loci on risk of aortic valve stenosis. Int J Cardiol. 2019;276:212–7. https://doi.org/10.1016/j.ijcard.2018.11.094 This study confirmed the association of the LPA locus with risk of AS, with a higher effect in those without concomitant coronary disease.
•• Chen HU, Dufresne L, Burr H, Ambikkumar A, Yasui N, et al. Association of LPA variants with aortic stenosis a large-scale study using diagnostic and procedural codes from electronic health records. JAMA Cardiol. 2018;3(1):18–23. https://doi.org/10.1001/jamacardio.2017.4266 Large genetic study about AS including over 40,000 individuals, confirming the association between 2 LPA variants and AS.
Abraityte A, Gullestad L, Askevold ET, Nymo S, Dahl CP, Aakhus S, et al. The Notch ligand Delta-like 1 is elevated and associated with mortality in patients with symptomatic aortic stenosis. Int J Cardiol. 2015;180:18–20.
Hadji F, Boulanger MC, Guay SP, Gaudreault N, Amellah S, Mkannez G, et al. Altered DNA methylation of long noncoding RNA H19 in calcific aortic valve disease promotes mineralization by silencing NOTCH1. Circulation. 2016;134:1848–62.
Irtyuga O, Malashicheva A, Zhiduleva E, Freylikhman O, Rotar O, Bäck M, et al. NOTCH1 mutations in aortic stenosis: association with osteoprotegerin/RANK/RANKL. Biomed Res Int. 2017;2017:6917907.
Guerraty M, Mohler ER. Models of aortic valve calcification. J Investig Med. 2007;55(6):278–83.
Fazio S, Linton MF. Mouse models of hyperlipidemia and atherosclerosis. Front Biosci. 2001;6:D515–25.
Sider KL, Blaser MC, Simmons CA. Animal models of calcific aortic valve disease. Int J Inflam. 2011;2011:364310.
Hinton RB Jr, Alfieri CM, Witt SA, Glascock BJ, Khoury PR, Benson DW, et al. Mouse heart valve structure and function: echocardiographic and morphometric analyses from the fetus through the aged adult. Am J Physiol Heart Circ Physiol. 2008;294(6):H2480–8.
Drolet MC, Roussel E, Deshaies Y, Couet J, Arsenault M. A high fat/high carbohydrate diet induces aortic valve disease in C57BL/6J mice. J Am Coll Cardiol. 2006;47(4):850–5.
Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114(19):2065–9.
Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116(24):2841–50.
Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115(3):377–86.
Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119(13):1785–94.
Hjortnaes J, Butcher J, Figueiredo JL, Riccio M, Kohler RH, Kozloff KM, et al. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. Eur Heart J. 2010;31(16):1975–84.
Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011;108(11):1392–412.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evidence-Based Medicine, Clinical Trials and Their Interpretations
Rights and permissions
About this article
Cite this article
de Oliveira Sá, M.P.B., Cavalcanti, L.R.P., Perazzo, Á.M. et al. Calcific Aortic Valve Stenosis and Atherosclerotic Calcification. Curr Atheroscler Rep 22, 2 (2020). https://doi.org/10.1007/s11883-020-0821-7
Published:
DOI: https://doi.org/10.1007/s11883-020-0821-7