Abstract
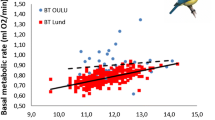
In this study we examined the allometry of basal metabolic rate (BMR) of 31 parrot species. Unlike previous reports, we show that parrots per se do not display BMRs that are any different to other captive-raised birds of their body size. An ordinary least squares regression fitted the data best and body mass explained 95% of the variation in BMR. There was no phylogenetic signal in the BMR data. We also provide new data for the Greater Vasa Parrot (Coracopsis vasa) of Madagascar. We tested the hypotheses that C. vasa may, because of its insular existence, display conservative energetic traits (low BMR, use of adaptive heterothermy) similar to those observed in several Malagasy mammals. However, this was not the case. C. vasa had a higher BMR than other parrots, especially during summer, when BMR was up-regulated by 50.5% and was 95.7% higher than predicted from an ordinary least squares (OLS) allometry of parrots (BMR = 0.042M 0.649b , BMR in Watts, M b in grammes). Compared with BMR data for 94 captive-raised bird species, the winter and summer BMRs were, respectively, 45.5 and 117.8% higher than predicted by a phylogenetic generalised least squares (PGLS) allometry (BMR = 0.030M 0.687b , BMR in Watts, M b in grammes). The summer up-regulation of BMR is the highest recorded for a bird of any size to date. We suggest that the costs of a high summer BMR may be met by the unusual cooperative breeding system of C. vasa in which groups of males feed the female and share paternity. The potential breeding benefits of a high summer BMR are unknown.






Similar content being viewed by others
References
Arnold KE, Owens IPF (1999) Cooperative breeding in birds: the role of ecology. Behav Ecol 10:465–471
Astheimer LB, Buttemer WA (2002) Changes in latitude, changes in attitude: a perspective on ecophysiological studies of Australian birds. EMU 102:19–27
Aujard F, Vasseur F (2001) Effect of ambient temperature on the body temperature rhythm of male gray mouse lemurs (Microcebus murinus). Int J Primatol 22:43–56
Bennett PM, Harvey PH (1987) Active and resting metabolism in birds: allometry, phylogeny and ecology. J Zool 213:327–363
Blomberg SP, Garland T (2002) Tempo and mode in evolution: phylogentic inertia, adaptation, and comparative methods. J Evol Biol 15:899–910
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioural traits are more labile. Evolution 57:717–745
Bush NG, Brown M, Downs CT (2008) Seasonal effects on thermoregulatory responses of the Rock Kestrel, Falco rupicolis. J Therm Biol 33:404–412
Chamane SC, Downs CT (2009) Seasonal effects on metabolism and thermoregulation abilities of the red-winged starling (Onychognathus morio). J Therm Biol 34:337–341
Chambers GK, Boon WM, Buckley TR, Hitchmough RA (2001) Using molecular methods to understand the Gondwanan affinities of the New Zealand biota: three case studies. Aust J Bot 49:377–387
Cockburn A (1998) Evolution of helping behavior in cooperatively breeding birds. Annu Rev Ecol Syst 29:141–177
Cockburn A (2006) Prevalence of different modes of parental care in birds. Proc R Soc B 273:1375–1383
Cockburn A, Floyd RB, Sheppard AW, Debarro PJ (1996) Why do so many Australian birds cooperate: social evolution in the Corvida? Frontiers of population ecology. CSIRO, Melbourne, pp 451–472
Daniels HL (1984) Oxygen consumption in Lemur fulvus: deviation from the ideal model. J Mamm 65:584–592
Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G (2004) Hibernation in a tropical primate. Nature 429:825–826
Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G (2005) Hibernation in the tropics: lessons from a primate. J Comp Physiol B 175:147–155
Dawson WR, Bennett AF (1973) Roles of metabolic level and temperature regulation in the adjustment of western plumed pigeons (Lophophaps ferruginea) to desert conditions. Comp Biochem Physiol A 44:249–266
Dewar RE, Richard AF (2007) Evolution in the hypervariable environment of Madagascar. Proc Natl Acad Sci USA 104:13723–13727
Dowsett RJ, Dowsett-Lamaire F (2000) The status of the Greater and Lesser Vasa parrots, Coracopsis vasa and C. nigra and the Grey-headed Lovebird Agapornis canus in Madagascar. BirdLife International, IUCN, London, pp 1–49
Edwards SV, Naeem S (1993) The phylogentic component of cooperative breeding in perching birds. Am Nat 141:754–789
Ekstrom JMM, Burke T, Randrianaina L, Birkhead TR (2007) Unusual sex roles in a highly promiscuous parrot: the Greater Vasa Parrot Coracopsis vasa. Ibis 149:313–320
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Fry CH (1972) Social organization of bee-eaters (Meropidae) and cooperative breeding in hot-climate birds. Ibis 114:1
Garland T, Ives AR (2000) Using the past to predict the present: confidence intervals for regression equations in phylogenetic comparative methods. Am Nat 155:346–365
Garland T, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol 41:18–32
Garland T, Dickerman AW, Janis CM, Jones JA (1993) Phylogenetic analysis of covariance by computer simulation. Syst Biol 42:265–292
Greene TC (1999) Aspects of the ecology of Antipodes Island parakeet (Cyanoramphus unicolor) and Reischek’s parakeet (C. novaezelandiae novaezelandiae) on Antipodes Island, October–November 1995. Notornis 46:301–310
Greene TC (2003) Breeding biology of red-crowned parakeets (Cyanoramphus novaezelandiae novaezelandiae) on Little Barrier Island, Hauraki Gulf, New Zealand. Notornis 50:83–99
Hackett SJ, Kimball RT, Reddy S, Bowie RCK, Braun EL, Braun MJ, Chojnowski JL, Cox WA, Han KL, Harshman J, Huddleston CJ, Marks BD, Miglia KJ, Moore WS, Sheldon FH, Steadman DW, Witt CC, Yuri T (2008) A phylogenomic study of birds reveals their evolutionary history. Science 320:1763–1768
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University, Oxford
Jetz W, Freckleton RP, McKechnie AE (2008) Environment, migratory tendency, phylogeny and basal metabolic rate in birds. PloS One 3(9):e3261
Kvist A, Lindstrom A (2001) Basal metabolic rate in migratory waders: intra-individual, intraspecific, interspecific and seasonal variation. Funct Ecol 15:465–473
Lavin SR, Karasov WH, Ives AR, Middleton KM, Garland T (2008) Morphometrics of the avian small intestine compared with that of nonflying mammals: a phylogenetic approach. Physiol Biochem Zool 81:526–550
Lindsay CV, Downs CT, Brown M (2009) Physiological variation in amethyst sunbirds (Chalcomitra amethystina) over an altitudinal gradient in winter. J Exp Biol 212:483–493
Lovegrove BG (2000) The zoogeography of mammalian basal metabolic rate. Am Nat 156:201–219
Lovegrove BG (2003) The influence of climate on the basal metabolic rate of small mammals: a slow-fast metabolic continuum. J Comp Physiol B 173:87–112
Lovegrove BG (2009) Modification and miniaturization of Thermochron iButtons for surgical implantation into small animals. J Comp Physiol B 179:451–458
Lovegrove BG, Génin F (2008) Torpor and hibernation in a basal placental mammal, the lesser hedgehog tenrec Echinops telfairi. J Comp Physiol B 178:691–698
Lovegrove BG, Heldmaier G, Ruf T (1991) Perspectives of endothermy revisited: the endothermic temperature range. J Therm Biol 16:185–197
Lovegrove BG, Lawes MJ, Roxburgh L (1999) Confirmation of pleisiomorphic daily torpor in mammals: the round-eared elephant shrew Macroscelides proboscideus (Macroscelidea). J Comp Physiol B 169:453–460
Maddison WP, Maddison DR (2009) Mesquite: a modular system for evolutionary analysis. Version 1:12
Maddocks TA, Geiser F (2000) Seasonal variations in thermal energetics of Australian silvereyes (Zosterops lateralis). J Zool 252:327–333
McCormick SA (1981) Oxygen consumption and torpor in the fat tailed dwarf lemur (Cheirogaleus medius): rethinking prosimian metabolism. Comp Biochem Physiol A 68:605–610
McKechnie AE (2008) Phenotypic flexibility in basal metabolic rate and the changing view of avian physiological diversity: a review. J Comp Physiol B 178:235–247
McKechnie AE, Wolf BO (2004) The allometry of avian basal metabolic rate: good predictions need good data. Physiol Biochem Zool 77:502–521
McKechnie AE, Freckleton RP, Jetz W (2006) Phenotypic plasticity in the scaling of avian basal metabolic rate. Proc R Soc B 473:931–937
McKechnie AE, Chetty K, Lovegrove BG (2007) Phenotypic flexibility in the basal metabolic rate of laughing doves: responses to short-term thermal acclimation. J Exp Biol 210:97–106
McNab BK, Salisbury CA (1995) Energetics of New Zealand’s temperate parrots. N Z J Zool 22:339–349
Ortmann S, Schmid J, Ganzhorn JU, Heldmaier G, Geiser F, Hulbert AJ, Nicol SC (1996) Body temperature and torpor in a Malagasy small primate, the mouse lemur. Adaptations to the cold. In: Tenth International Hibernation Symposium, University of New England Press, Armidale, pp 55–61
Prinzinger R, Pressmar A, Schleucher E (1991) Body temperature in birds. Comp Biochem Physiol A 99:499–506
Reynolds PS, Lee RM (1996) Phylogenetic analysis of avian energetics: passerines and nonpasserines do not differ. Am Nat 147:735–759
Richardson DS, Jury FL, Blaakmeer K, Komdeur J, Burke T (2001) Parentage assignment and extra-group paternity in a cooperative breeder: the Seychelles warbler (Acrocephalus sechellensis). Mol Ecol 10:2263–2273
Saarela S, Hohtola E (2003) Seasonal thermal acclimatization in sedentary and active pigeons. Israel J Zool 49:185–193
Schleucher E, Withers PC (2001) Re-evaluation of the allometry of wet thermal conductance for birds. Comp Biochem Physiol A 129:821–827
Schmid J (1996) Oxygen consumption and torpor in mouse lemurs (Microcebus murinus and M. myoxinus): preliminary results of a study in western Madagascar. In: Geiser F, Hulbert AJ, Nicol SC (eds) Adaptations to the cold: Tenth International Hibernation Symposium. University of New England, Armidale, pp 47–54
Schmid J (2001) Daily torpor in free-ranging gray mouse lemurs (Microcebus murinus) in Madagascar. Int J Primatol 22:1021–1031
Schmidt-Nielsen K (1983) Animal physiology: adaptation and environment. Cambridge University, Cambridge
Scholl P (1974) Temperaturregulation beim madagassischen Igeltanrek Echinops telfairi (Martin, 1838). J Comp Physiol 89:175–195
Sibley CG, Ahlquist JE (1990) Phylogeny and classification of birds. Yale University, New Haven
Sinclair I, Langrand O (2003) Birds of the Indian Ocean islands. Struik, Cape Town
Smit B, McKechnie AE (2010) Avian seasonal metabolic variation in a subtropical desert: basal metabolic rates are lower in winter than in summer. Funct Ecol 24:330–339
Smit B, Brown M, Downs CT (2008) Thermoregulatory responses in seasonally acclimatized captive Southern white-faced scops-owls. J Therm Biol 33:76–86
Theuerkauf J, Rouys S, Mériot JM, Gula R, Kuehn R (2009) Cooperative breeding, mate guarding, and nest sharing in two parrot species of New Caledonia. J Ornithol 150:791–797
Tieleman BI, Williams JB (2000) The adjustment of avian metabolic rates and water fluxes to desert environments. Physiol Biochem Zool 73:461–479
Tieleman BI, Williams JB, Buschur ME (2002) Physiological adjustments to arid and mesic environments in larks (Alaudidae). Physiol Biochem Zool 75:305–313
Weathers WW (1979) Climate adaptation in avian standard metabolic rate. Oecologia 42:81–89
Weathers WW, Caccamise DF (1978) Seasonal acclimatization to temperature in Monk Parakeets. Oecologia 35:173–183
White CR, Blackburn TM, Martin GR, Butler PJ (2007) Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc R Soc B 274:287–293
Wiersma P, Munoz-Garcia A, Walker A, Williams JB (2007) Tropical birds have a slow pace of life. Proc Natl Acad Sci USA 104:9340–9345
Wilkinson R, Birkhead TR (1995) Copulation behaviour in the Vasa Parrots Coracopsis vasa and C. nigra. Ibis 137:117–119
Williams JB, Tieleman BI (2001) Physiological ecology and behavior of desert birds. Curr Ornithol 16l:299–353
Williams JB, Tieleman BI (2002) Ecological and evolutionary physiology of desert birds: a progress report. Int Comp Biol 42:68–75
Williams JB, Withers PC, Bradshaw SD, Nagy KA (1991) Metabolism and water flux of captive and free-living Australian parrots. Aust J Zool 39:131–142
Withers PC (1977) Measurement of VO2, VCO2, and evaporative water loss with a flow-through mask. J Appl Physiol 42:120–123
Withers PC, Cooper CE, Larcombe AN (2006) Environmental correlates of physiological variables in marsupials. Physiol Biochem Zool 79:437–453
Wright TF, Schirtzinger EE, Matsumoto T, Eberhard JR, Graves GR, Sanchez JJ, Capelli S, Mueller H, Scharpegge J, Chambers GK, Fleischer RC (2008) A multilocus molecular phylogeny of the parrots (Psittaciformes): Support for a Gondwanan origin during the Cretaceous. Mol Biol Evol 25:2141–2156
Zar JH (1984) Biostatistical analysis. Prentice-Hall International, New Jersey
Acknowledgments
This study was financed by an NRF and UKZN incentive grants to BGL and MRP. Thamsanqa Mjwara is thanked for assistance in bird maintenance and installing the video recording equipment. We thank Brian Boswell most sincerely for the loan of his birds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lovegrove, B.G., Perrin, M.R. & Brown, M. The allometry of parrot BMR: seasonal data for the Greater Vasa Parrot, Coracopsis vasa, from Madagascar. J Comp Physiol B 181, 1075–1087 (2011). https://doi.org/10.1007/s00360-011-0590-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-011-0590-2