Abstract
Polypharmacy (PP) use is very common in older people and may lead to drug-drug interactions (DDIs) and anticholinergic burden (ACB) that may affect cognitive function. We aimed to determine the occurrence of PP, potential DDIs and ACB and their role in cognitive outcomes in an older population. Cross-sectional data from 636 community-dwelling adults (73.2 ± 6.0 SD, 58.6% women) participating in the NutBrain study (2019–2023) were analyzed. Participants were asked about their medication use, and data on potential DDIs and ACB were extracted. The associations of PP (≥ 5 drugs/day), potential DDIs, and ACB with mild cognitive impairment (MCI) and specific cognitive domains were assessed using logistic regression adjusted for confounders. Sex-stratified analysis was performed. Overall, 27.2% of the participants were exposed to PP, 42.3% to potential DDIs and 19% to cumulative ACB. Women were less exposed to PP and more exposed to ACB than men. In multivariate analysis, the odds of having MCI (24%) were three times higher in those with severe ACB (≥ 3) (OR 3.34, 95%CI 1.35–8.25). ACB was positively associated with poor executive function (OR 4.45, 95%CI 1.72–11.49) and specifically with the Frontal Assessment Battery and neuropsychological tests of phonological and semantic fluency. In sex-stratified analysis, ACB was statistically significantly associated with MCI and executive function in women and with memory in men. PP, potential DDIs and anticholinergics use are very common in community-dwelling older people. ACB exposure is associated with MCI, particularly with poor executive function. Clinicians are encouraged to be vigilant when prescribing anticholinergics.
Trial registration: Trial registration number NCT04461951, date of registration July 7, 2020 (retrospectively registered, ClinicalTrials.gov).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
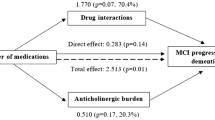
The use of multiple medications, commonly referred to as polypharmacy (PP), is very common among older adults, especially those with chronic conditions (Masnoon et al. 2017). A prevalence survey in 17 European countries showed that between 26.3% and 39.9% of the population aged ≥ 65 years reported taking at least five different medicines on a typical day, including prescribed drugs, over-the-counter medicines, and dietary supplements (Midão et al. 2018). PP, commonly defined as five or more medications per day (Masnoon et al. 2017), may increase the risk of inappropriate prescribing, poor adherence to treatment, and potential adverse drug events, including potential drug-drug interactions (DDIs), which occur when “the effects of one drug are changed by the presence of another drug” (MedicinesComplete—Log in, s.d. Recuperato 10 ottobre 2023, da https://www.medicinescomplete.com/log-in/#/browse/stockley). Prolonged use of PP may lead to a range of multiple adverse effects on physical and functional capacity (Sganga et al. 2014; Fabbietti et al. 2018a, b) and readmission to the hospital (Fabbietti et al. 2018a; b) in older patients. PP has also been found to be associated with poor global cognitive function (Jyrkkä et al. 2011; Cheng et al. 2018) and cognitive ability (Rawle et al. 2018), greater cognitive decline, and dementia or delirium (Oyarzun-Gonzalez et al. 2015). It has also been reported that people with cognitive impairment or dementia take on more drugs than people without cognitive impairment (Lau et al. 2010) due to comorbidity, misinterpretation of adverse drug reactions or newly prescribed medications. This is a risk factor that has been found to be associated with the presence and increased risk of having DDIs (Sönnerstam et al. 2018). In addition, PP may increase the risk of progression from mild cognitive impairment (MCI) to dementia in community-dwelling older adults and the effect appears to be mediated mainly through potential DDIs (Trevisan et al. 2021). However, a clear understanding of the role of PP and DDIs in MCI in free-living older people is far from conclusive.
Many of the medications commonly prescribed to older adults have an anticholinergic (AC) effect, which is the result of a drug interacting with the central nervous system by inhibiting the action of the neurotransmitter acetylcholine that is crucial for learning and memory(López-Álvarez et al. 2019). It is estimated that around 10% of people aged 65 years and older regularly use AC drugs (Chuang et al. 2017), mainly for the treatment of Parkinson's disease, urinary incontinence, Alzheimer’s disease, sleep disorders, depression, and gastrointestinal disorders (O'Donnell et al. 2017). Numerous studies have reported an association between the use of AC drugs and an increased risk of cardiovascular disease and mortality (Myint et al. 2015; Corsonello et al. 2019; D’Alia et al. 2020), as well as potential adverse effects such as cognitive dysfunction, dementia and delirium/confusion (Taylor-Rowan et al. 2021) (Campbell et al. 2009; Pieper et al. 2020). The Anticholinergic Burden (ACB) scale has been developed to identify drugs with AC properties and to guide clinical decisions to reduce and prevent the risk of cognitive adverse events, such as cognitive decline, dementia, and delirium. The ACB scale provides a list of drugs that have AC activity and, because they can cross the blood–brain barrier, may have adverse effects on the central nervous system (Pasina et al. 2019). Several observational studies have investigated the relationship between ACB and cognitive impairment in both clinical (Pasina et al. 2013) and community-dwelling older people, mainly using specific neuropsychological tests without measures of global function or clinical support for neurological assessment (Dos Santos et al. 2022; Han et al. 2008; Koyama et al. 2014; Shah et al. 2013; Uusvaara et al. 2013). A recent systematic review and meta-analysis performed on observational studies, showed that the short- and long-term use of anticholinergics was associated with an increased incidence of dementia and cognitive decline, but no significant association was observed with the incidence of MCI (Pieper et al. 2020).
To the best of our knowledge, data on the occurrence of PP, potential DDIs and ACB in free-living Italian older people, have been less investigated. In addition, their role on cognitive outcomes, using an accurate diagnosis of MCI by combining the neuropsychological and neurological confirmation and examining specific cognitive domains, is rather limited. Finally, although men and women may experience different adverse effects when treated with AC drugs (Trenaman et al. 2021), the sex-specific data on this topic remain scarce. To fill these gaps, the present study aims to provide an overview of the frequency of PP, potential DDIs and AC medication use in a sample of community-dwelling older people, and to examine their independent associations with cognitive impairment and specific cognitive domains separately in men and women.
Methods
Study design, setting, and participants
The Nutrition, Gut microbiota, and Brain Aging (NutBrain) project is a population-based cohort study of community-dwelling older adults aged ≥ 65 years living in the Lombardy region (Italy), carried out between October 2019 and January 2023. The procedures of the NutBrain study have been described in detail elsewhere (Prinelli et al. 2020). Briefly, at the time of presentation, individuals were assessed for global cognitive function and specific cognitive domains using a standardized neuropsychological test battery. Socio-demographic and lifestyle characteristics, clinical information and anthropometric measurements were also recorded. All examinations were performed by trained and certified study technicians according to harmonized protocols. A total of 807 participants were recruited; excluding those with missing data on medication use (n = 149), those who did not complete the screening data collection (n = 4), and those with dementia (n = 18) for whom it was not possible to complete the neuropsychological assessment or whose questionnaires responses were not reliable, 636 subjects (n = 268 men and n = 368 women) were included in the present analysis.
Data collection
Cognitive function
MCI refers to the development of deficits in cognitive abilities, such as memory, attention, language, or executive functions, which are not severe enough to compromise the individual’s functional independence (Albert et al. 2011). In the present analysis MCI was defined as the presence of subjective cognitive complaints and objective cognitive impairment on one or two neuropsychological tests, greater than would be expected for the individual’s age and level of education, without impairment in activities of daily living according to Albert’s criteria (Albert et al. 2011). Functional assessment of activities of daily living was assessed using the Katz Index of Independence in Activities of Daily Living (ADL) scale (Katz 1983) and the Instrumental Activities of Daily Living scale (IADL) (Lawton and Brody 1969). The neuropsychological profile was assessed using a comprehensive battery of neuropsychological tests selected to assess the global cognitive function and different cognitive domains as follows: (i) Memory functions: Free and Cued Selective Reminding Test (FCSRT) (Frasson et al. 2011), Logical Memory Test (Novelli et al. 1986), Rey-Osterrieth Complex Figure Test (ROCF)—delayed recall (Caffarra et al. 2002); (ii) Executive functions: Frontal Assessment Battery (FAB) (Appollonio et al. 2005), Phonemic (Carlesimo et al. 1996) and Semantic Verbal Fluency (Novelli et al. 1986), Trail Making Test part A and B (TMT B) (Giovagnoli et al. 1996); (iii) Language: Picture Naming Test (Sartori and Job 1988); and (iv) Visuospatial abilities: Rey-Osterrieth Complex Figure Test (ROCF)—copy (Caffarra et al. 2002). The results and the participants’ medical records were reviewed by a group of experts, including neurologists and neuropsychologists, to reach a consensus on any controversial cases. All raw neuropsychological test scores were corrected for age and education and compared with those available for the Italian population (Capitani and Laiacona 1997). For each test, the corrected scores were first converted into equivalent scores on a 5-point ordinal scale (Capitani and Laiacona 1988), then the equivalent score were dichotomised into normal (0, scale = 4) and impaired (1, scale 1–4) (Perdixi et al. 2022). We created four cognitive domain scores by summing up all tests contributing to each domain: memory, executive function, language, and visuospatial ability.
Use of drugs
Data on the use of each drug were recorded in the database and included the Anatomical Therapeutic Chemical (ATC) classification code, chemical name and brand name. The INTERcheck(®) Computerized Prescription Support System (CPSS) (http://www.intercheckweb.it), a database developed by the Mario Negri Institute for Pharmacological Research, Scientific Institute for Research, Hospitalisation and Healthcare (IRCCS), was used to analyze the PP, the potential DDIs and the ACB (estimated using the ACB scale) of the registered drugs. Here a cut-off of ≥ 5 drugs was used to define PP (Masnoon et al. 2017), and the PP score was defined as the total number of drugs taken daily.
The INTERcheck(®) system lists information on DDIs based on chemical combinations of drugs. All drug-drug interactions are classified into four different categories according to their clinical significance, as follows: (A) clinically insignificant DDIs; (B) clinical relevance is unknown and/or variable; (C) clinically relevant DDIs that can be managed, for example, by individual dose adjustment; and (D) clinically relevant DDIs that should be avoided. According to previous literature (Ghibelli et al. 2013), each potential DDI was classified as 0 = no drug interaction or minor (A and B), and 1 = at least one from moderate to severe DDI (C and D). The DDI scale indicated the number of drug-drug interactions for each patient. In the ACB scale, drugs were classified according to their AC activity as follows: no activity (score 0), possible activity (score 1), moderate activity (score 2), and severe activity (score 3) (Table 1). The cumulative effect of the ACB score was calculated by summing the ACB score of each drug and then further categorized as 0 (no ACB activity), 1–2 (low to moderate activity), and 3 or more (severe activity).
Other assessments
For the present analysis, the potential confounders were selected based on theoretical knowledge from previous epidemiological studies (Han et al. 2008; Koyama et al. 2014; Lau et al. 2010; Shah et al. 2013; Sönnerstam et al. 2018; Trevisan et al. 2021; Uusvaara et al. 2013) and empirical criteria (p-value ≤ 0.05 in univariate analysis). We considered sociodemographic information including age (classified as 65–69, 70–74, 75–79, 80–84, and 85 +), sex, education (categorized as university, high school, middle school, primary school or less), and living arrangement (living alone vs not living alone). Health status variables included depressive symptoms as assessed by the 20-item Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff 1977), waist circumference (in centimeters, as a proxy for central obesity), and cardio-metabolic disorders. The cardio-metabolic variable was created by combining self-reported dyslipidemia, hypertension, diabetes, myocardial infarction, stroke, coronary heart disease, peripheral vascular disease, or heart failure. The variable was then categorized as none, one, two, three and four conditions present simultaneously. Lifestyle variables included smoking habits (classified as never and former or current smoker) and the frequency of leisure time activities as assessed by the Cognitive Reserve Index Questionnaire (CRIq) (Nucci et al. 2012). Leisure time activities were first grouped into mental, social, and physical and then categorized into tertiles of engagement as previously described (Perdixi et al. 2022).
Statistical analysis
Characteristics of the study participants were described using mean (standard deviation—SD) or median (interquartile range, IQR) for continuous variables and frequency (%) for categorical variables. The dependent variables were (i) MCI and (ii) domain-specific cognitive tests (memory, executive function, language, and visuospatial ability). The independent variables were PP, DDIs, and ACB score, considered as both continuous and categorical variables. The association between the dependent and independent variables was examined using binary logistic regression, and odds ratios (OR) with 95% confidence intervals (CI) were estimated. Two sets of models were run, the first including age, sex and education, and the second also including living arrangements, leisure activities, cardio-metabolic disorders, waist circumference, smoking habits and depressive symptoms. A sensitivity analysis was performed after adjustment for the ADL scale to check whether the functional status might affect the results. To account for the potential confounding of co-medications and drug interactions in the assessment of AC effects, we performed a further sensitivity analysis by simultaneously including ACB, PP and potential DDIs in the final model. Sex-stratified analysis was performed. All the analyses were performed with IBM SPSS Statistics for Windows version 25.0 (IBM Corp., Armonk, NY, USA). A two-tailed p value ≤ 0.05 was considered statistically significant.
Results
The 636 participants who met the inclusion criteria were aged 73.17 (SD 6.04) years, 57.9% were women (n = 368), 64.1% had completed at least high school, 23% did live alone, 46.5% had never smoked, 32.2% were highly engaged in leisure activities, 30.2% had at least one cardio-metabolic disorders, and 24.2% had MCI. Regarding medications, 27.2% were exposed to PP (continuous score range 0–14), 19% to cumulative ACB (range 0–5) and 42.3% to potential DDIs (range 0–21). Compared to men, women were less educated, more likely to live alone, had never smoked, had a lower waist circumference, had more depressive symptoms, were less exposed to PP but more exposed to severe ACB. There were no differences in age categories, leisure time activities, occurrence of cardio-metabolic disorders, MCI, and exposure to potential DDIs (Table 1).
The highest level of PP was found in the 85 + age group (48.3%) (Fig. 1a). There was a sex-related difference in all age groups, with men using more drugs than women. In particular, the difference was significant in the 75–79 age group, where 49.2% of men were exposed to PP compared with 34.7% of women; and in the 85 + age group where 64.7% of men and 25% of women were exposed to PP. We also observed the highest level of potential DDIs in the 85 + age group (72.4%) with a significant sex difference (88.2% of men were exposed to potential DDIs compared to 50% of women) (Fig. 1b).
On average, each participant used 3.2 different therapeutic substances simultaneously (3.5 in men and 3 in women), ranging from 2.2 substances (2 in men and 2.2 in women) in the 65–69 years age group and 4.8 substances (5.5 in men and 3.9 in women) in the 85 + age group (data not shown).
As shown in Supplementary figure 1, the top 5 therapeutic categories (ATC, grouped up to the 2nd level) used by participants included drugs acting on the renin–angiotensin–aldosterone system (39.0%), lipid-modifying substances (34.8%), antithrombotics (30.5%), drugs for acid-related disorders (24.8%) and beta-blockers (22.1%), with women taking fewer antithrombotics, urological agents, drugs for diabetes, cardiac therapy, anti-gout drugs and more psycholeptics, thyroid therapy, and osteoporosis drugs than men.
Supplementary table 1 shows the top 20 most frequent potential DDIs (ATC 5th level), the first 5 being acetylsalicylic acid, levothyroxine sodium, pantoprazole, atorvastatin calcium and bisoprolol.
Supplementary table 2 lists the chemical substances based on their predicted AC effect (clinically relevant to cognition) in the analyzed participants. Drugs with moderate AC effects included amantadine and carbamazepine, whereas drugs with marked AC effects included amitriptyline, clomipramine, paroxetine, olanzapine, quetiapine, solifenacin, and tolterodine.
Compared to those not exposed, the occurrence of MCI was significantly higher in those exposed to PP (21.4% vs 31.8%, p-value = 0.006), in those exposed to severe ACB (21.9% vs 42.3%, p-value = 0.012), and in those exposed to potential DDIs (19.9% vs 30.1%, p-value = 0.030) (Fig. 2).
Table 2 shows the results of the logistic regression model, using the cognitive status as the outcome in the whole sample. We did not find a statistically significant association between cognition, PP and potential DDIs, but we found that those exposed to severe ACB had a high probability of having MCI compared to those not exposed people, considering the model adjusted for age, sex, and education (model 1, OR 3.07, 95%CI 1.27–7.44) and the multivariate model (model 2, OR 3.34, 95%CI 1.35–8.25). Each unit increase in the continuous ACB score was associated with a 1.32 fold increase in the odds of MCI in the multivariate model (OR 1.32, 95%CI 1.04–1.67). Further analysis by including the ADL scale in the model did not alter the associations examined (Additional file 2: Table 3). To exclude that co-medications and drug interactions might confound the effect of anticholinergics on cognitive status, we simultaneously included ACB, PP and potential DDIs in the final model, but the association between ACB and MCI did not change substantially (OR 3.27, 95%CI 1.30–8.24) (data not shown).
We also analyzed the association between exposure to ACB and individual cognitive domains (Table 3) controlling for confounders and we found that people exposed to severe ACB had a higher adjusted OR of having impaired cognitive executive function (OR 4.45, 95%CI 1.72–11.49). For each unit increase, the odds increased by 1.44 times (OR 1.44, 95%CI 1.12–1.19). No associations were observed with memory, language and visuospatial ability. Looking at the tests assessing executive function, severe ACB was statistically significantly associated with FAB (OR 4.20, 95%CI 1.54–11.43) and the two tests assessing phonemic (OR 8.26, 95%CI 1.29–53.08) and semantic fluency (17.22, 95%CI 1.78–166.81). For each unit increase in ACB score, the odds of impaired FAB, phonological and semantic fluency increased by 1.38, 1.71 and 2.34 times, respectively (Table 4).
Table 5 shows the association between ACB score and cognitive outcomes stratified by sex. We found that women exposed to severe ACB had a statistically significant higher adjusted OR of having MCI (OR 2.90, 95% 1.03–8.15). Severe ACB was significantly associated with impaired memory domain in men (OR 11.12, 95% 1.17–105.38) and with executive function in women (OR 4.71, 95%CI 1.57–14.16).
Discussion
The present study provides a comprehensive picture of medication use in an older sample of men and women living in the community in northern Italy. It also explores the role of PP, potential DDIs and ACB on cognitive outcomes.
Regarding drug use, we found that 27.2% of the study population used at least 5 different therapeutic substances on a daily basis. This percentage increases with age category, ranging from 12.4% in individuals aged 65–69 to 48.3% in those aged 85 + . The prevalence of PP reported in the literature varies widely. A study carried out in the general population of northern Italy using the administrative data reported a prevalence of co-prescription of 31.7% in people aged ≥ 65 years (Valent 2019). A prevalence survey in 17 European countries reported that between 26.3% and 39.9% of the population aged ≥ 65 years reported taking at least five different medicines on a typical day, including prescribed medicines. In Italy, the reported prevalence was 32.9%, increasing with age category from 26.4% in subjects aged 65–74 years to 45.1% in those aged ≥ 85 years, and slightly higher in men (33.2%) than in women (32.5%) (Midão et al. 2018). We found a sex difference in all age groups, with men being more exposed to PP than women (32.8% vs 23.1%), especially in the 70–74 and 85 + age groups. In addition, on average, each participant reported using 3.2 different therapeutic substances simultaneously (range: 2.2–4.8 in the 65–69 and 85 + age groups, respectively). A similar trend was observed in the 2021 report of the Italian Medicines Agency (AIFA) (National Report on Medicines Use in Italy—Year 2021, s.d.). As the report provides a picture of pharmaceutical care in the local and hospital settings rather than in the community, the prevalence of PP was higher than in our study (66.6% vs 27.2%); however, it also showed the same lowest drug use in the 65–69 age group and the highest use in the 85 + age group. In addition, similar to our data, both sexes showed a progressive increase with age in the number of different substances used. Furthermore, in line with the AIFA report (National Report on Medicines Use in Italy—Year 2021, s.d.), the most commonly self-reported drugs in our cohort were antihypertensives, lipid-modifying agents, antithrombotics and drugs for acid-related disorders, with some expected sex differences.
As highlighted by previous studies, PP is associated with a higher risk of potential DDIs. A very recent meta-analysis by Hughes et al. (2023) reported a prevalence of potential DDIs ranging from 0.8 to 90.6% in the older community dwellers. In Italy, in particular, the prevalence reported in three studies of older community dwellers ranged from 19.3% (Burato et al. 2020), 26.4% (Tragni et al. 2013), and 45.0% (Trevisan et al. 2021). In our study, we found that 42.3% were exposed to potential DDIs, with no significant differences between the sexes (44.4% men and 40.8% women) particularly in the 85 + age group. According to the AIFA report (National Report on Medicines Use in Italy—Year 2021, s.d.), we found that the five most common potential DDIs are drugs commonly used in the older population, such as non-steroidal anti-inflammatory drugs (NSAIDs), thyroid hormones, drugs for acid-related disorders, lipid-modifying agents, and beta-blockers.
Regarding the use of AC drugs, in our sample drugs with moderate AC effects include amantadine (antiparkinsonian) and carbamazepine (anticonvulsant or antiepileptic), whereas drugs with marked AC effects that are clinically relevant to cognition include antidepressants (amitriptyline, clomipramine, paroxetine), antipsychotics (olanzapine, quetiapine), and antimuscarinics (solifenacin, tolterodine). This is consistent with the AIFA report (National Report on Medicines Use in Italy—Year 2021, s.d.), which indicates that the therapeutic category of central nervous system drugs appears to have a greater AC effect and includes antiepileptics, antipsychotics, antiparkinsons, and tricyclic antidepressants.
In line with the prevalence in the general population (Sachdev et al. 2015), 24.2% of our sample met the criteria for MCI. Looking at the associations between medication use and MCI, we did not find a statistically significant association with PP and potential DDIs, but we did observe that people exposed to severe ACB had a 3.3 fold increased odds of having MCI. The association was independent of potential confounders, co-medications and potential DDIs. We also found that people exposed to severe ACB were more likely to have impaired cognitive executive function, and we observed greater impairment in skills related to frontal activity measured by the FAB, such as motor and strategic planning and interference control, and in the ability to access the lexicon with both phonemic (phonemic verbal fluency) and semantic (semantic verbal fluency) cues.
Our results are consistent with previous observational evidence showing a consistent association between AC medications use and risk of cognitive decline or dementia in cognitively healthy older adults (Taylor-Rowan et al. 2021) and risk of dementia progression (Trevisan et al. 2021) or poor outcomes, such as mortality, in older adults with pre-existing cognitive problems.
For example, a study of 896 community-dwelling older American Catholic clergy without dementia at baseline, assessed with the MMSE and a neuropsychological battery, reported that people who started AC medication had a steeper annual decline in cognitive function over a median follow-up of 10 years (Shah et al. 2013). Another study of 13,065 community-dwelling Brazilian participants, showed a significant association of ACB with poor memory (CERAD test) and executive function (TMT-B test) in participants aged less than 65 years suggesting that executive function may be altered before other cognitive domains (Dos Santos et al. 2022). Ziad et al. demonstrated a negative cross-sectional association between total cumulative exposure to AC drugs and cognitive performance in 34,267 individuals aged 45–70 years; in particular, the association was moderate for executive function (Digit Symbol Substitution Test, TMT-A and TMT-B) and less pronounced for episodic memory (immediate and delayed free recall) (Ziad et al. 2018). A cross-sectional study conducted in Finland of 400 home-dwelling individuals aged 75–90 years without major clinical dementia but with a history of stable atherosclerotic disease found that drugs with AC properties were associated with lower verbal fluency and naming scores (Uusvaara et al. 2013). Han et al. also showed that cumulative AC exposure from multiple medications over 1-year impaired verbal memory and executive function (using IADL as a proxy) in 544 community-dwelling men aged 65 years and older with diagnosed hypertension (Han et al. 2008). Kyoama et al. found that higher ACB scores were associated with poorer cognitive performance in verbal fluency and immediate and delayed word recall in a 5-year longitudinal follow-up study of 1429 older participants (Koyama et al. 2014). The potential biological basis for the reduced cognitive function associated with the use of medications with moderate or high AC effects was investigated through the functional and structural changes in the human brain, in a longitudinal study of two cohorts of cognitively normal older adults, the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and the Indiana Memory and Aging Study (IMAS). The study showed that AC drugs are associated with poorer cognition (particularly in immediate memory recall and executive function measured by the TMT-B test), brain hypometabolism, whole brain and temporal lobe atrophy, and an increased risk of clinical conversion to cognitive impairment (Risacher et al. 2016). Indeed, the authors speculate that increased brain atrophy and reduced brain function may be related to the central effects of AC drugs on cholinergic pathways in the brain. In a more recent study performed in a population-based cohort of non-demented adults aged from 20 to 80 years, Kilimann et al. reported an inverse association between the ACB and the hippocampal volume (Kilimann et al. 2022).
AC drugs block the binding of the neurotransmitter acetylcholine to cholinergic receptors within the cholinergic system and inhibit its activity at both central and peripheral nervous system synapses. Acetylcholine is a neurotransmitter that plays an important role in many functions of the nervous system and, in the brain, in learning, memory and attention (Klinkenberg et al. 2011; Taylor-Rowan et al. 2022). By interfering with the cholinergic system, together with potential inflammatory, or vascular pathways, AC drugs are thought to affect short and long-term cognitive function, with a greater AC exposure leading to greater impairment (Sanghavi et al. 2022; Singh et al. 2013). In addition, older adults may be more susceptible to the effects of AC medications due to increased permeability of the blood–brain barrier and decreased acetylcholine-induced transmission within the central nervous system (Reiter et al. 2021), as well as age-related changes in drug metabolism and excretion (Trenaman et al. 2021), so greater caution is required when prescribing AC medications in this population.
Sex-stratified analysis showed that women were more likely to be exposed to severe ACB than their male counterparts (5.7% vs 1.9%), which may be partly because they take more psychoanalytic and psychoanaleptic medications. Women were statistically significantly more likely to have MCI when exposed to severe ACB, and to have poorer executive function. In men, we observed a non-significant increased likelihood, possibly due to the small number of exposed subjects. Severe ACB exposure was associated with impaired memory domain only in men. As previously reported, specific sex-related differences may potentiate the increased exposure to AC drugs by affecting drug metabolism and adsorption, especially in old age. Women have delayed gastric and colonic emptying, higher gastric pH, reduced catechol-O-methyltransferase activity (involved in the metabolism of catecholamine neurotransmitters) and glucuronidation (involved in drug metabolism), and reduced renal clearance, which may affect the absorption of AC drugs (Trenaman et al. 2021).
Strengths and limitations
Several limitations need to be considered. First, the cross-sectional nature of the study precludes causal inference for the association between ACB score and cognitive outcomes, so a potential reverse causation bias may have influenced our findings. Second, the data on medications use relied on self-report rather than direct ascertainment from medical/prescription records (with detailed information on duration, dose, etc.), which may have led to recall bias and exposure misclassification. However, given the exclusion of people with dementia at baseline, it is unlikely that participants would have reported taking medication that they were not actually taking. Third, drugs use was recorded according to the medication actually taken, without any information about past use (for example, a participant who had taken an AC medication for several years but stopped shortly before the baseline visit would not be recorded as a user). Fourth, although we controlled for several potential confounders, we cannot completely exclude the possibility of residual confounding due to unmeasured factors. Finally, mortality or hospitalization may be outcomes more closely related to polypharmacy or drug-drug interactions than cognitive outcomes analyzed cross-sectionally. Future studies investigating the longitudinal effect of PP and DDIs on the risk of hospitalization, rehospitalization and mortality are warranted. The present study also has several strengths. It provides a picture of drug use in a community-based sample, which is more representative of the general population than samples recruited in clinical settings. The study is also characterized by a detailed assessment of individual’s cognitive function and clinical and behavioral factors, using validated instruments, administered by trained staff, which reduces non-response and recall bias. In addition, the diagnosis of MCI was made by a multidisciplinary consensus expert team based on the uniform application of widely accepted criteria, using a combination of neuropsychological tests and clinical examination, rather than a single screening tool (such as the MMSE) or a single cognitive test, thus avoiding the risk of false negatives. Finally, we examined sex-differences in our analysis, which have been largely unexplored in the literature.
Conclusions
This study has shown that PP, potential DDIs and anticholinergics use are very common in the community-dwelling older men and women, and that ACB is associated with impaired cognitive function, particularly poor executive function. Further longitudinal studies with detailed assessment of medical/pharmacy records are warranted, as well as studies using both structural and functional brain imaging and biomarker measures to explore the underlying pathophysiological basis of these associations. Our findings have important policy implications and support clinical decision-making about prescribing drugs and AC medications to safely treat people, accounting for their age and sex. Indeed, healthcare professionals need to be aware of the effects of AC drugs and are strongly encouraged to try to reduce an individual’s ACB by de-prescribing commonly used anticholinergics to reduce the risk of developing long-term cognitive problems in cognitively healthy people, or to reduce the rate of cognitive decline and clinical outcomes in those with pre-existing cognitive impairment (Taylor-Rowan et al. 2022).
Abbreviations
- PP:
-
Polypharmacy
- DDIs:
-
Drug-drug interactions
- AChE:
-
Acetylcholinesterase inhibitors
- MCI:
-
Mild cognitive impairment
- AC:
-
Anticholinergic
- ACB:
-
Anticholinergic burden
- MMSE:
-
Mini-mental state examination
- NutBrain:
-
Nutrition, gut microbiota, and brain aging
- ADL:
-
Katz Index of Independence in activities of daily living
- IADL:
-
Instrumental activities of daily living scale
- FCSRT:
-
Free and cued selective reminding test
- ROCF:
-
Rey-Osterrieth complex figure test
- FAB:
-
Frontal assessment battery
- TMT:
-
Trail making test
- ATC:
-
Anatomical therapeutic chemical
- CPSS:
-
Computerized prescription support system
- IRCCS:
-
Scientific Institute for Research, Hospitalisation and Healthcare
- CES-D:
-
Center for epidemiologic studies depression scale
- CRIq:
-
Cognitive reserve index questionnaire
- SD:
-
Standard deviation
- ORs:
-
Odds ratios
- CIs:
-
Confidence intervals
- AIFA:
-
Italian Medicines Agency
References
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia J Alzheimer’s Assoc 7(3):270–279. https://doi.org/10.1016/j.jalz.2011.03.008
Anticholinergic burden: Considerations for older adults—Kouladjian O’Donnell—2017—Journal of Pharmacy Practice and Research—Wiley Online Library. (s.d.). Recuperato 10 ottobre 2023, da https://doi.org/10.1002/jppr.1303
Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, Forapani E, Russo A, Nichelli P (2005) The frontal assessment battery (FAB): normative values in an Italian population sample. Neurol Sci off J Ital Neurol Soc Ital Soc Clin Neurophysiol 26(2):108–116. https://doi.org/10.1007/s10072-005-0443-4
Burato S, Leonardi L, Antonazzo IC, Raschi E, Ajolfi C, Baraghini M, Chiarello A, Delmonte V, Di Castri L, Donati M, Fadda A, Fedele D, Ferretti A, Gabrielli L, Gobbi S, Lughi S, Mazzari M, Pieraccini F, Renzetti A, Poluzzi E (2020) Comparing the prevalence of polypharmacy and potential drug-drug interactions in nursing homes and in the community dwelling elderly of emilia romagna region. Front Pharmacol 11:624888. https://doi.org/10.3389/fphar.2020.624888
Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A (2002) Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci off J Ital Neurol Soc Ital Soc Clin Neurophysiol 22(6):443–447. https://doi.org/10.1007/s100720200003
Campbell N, Boustani M, Limbil T, Ott C, Fox C, Maidment I, Schubert CC, Munger S, Fick D, Miller D, Gulati R (2009) The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging 4:225–233. https://doi.org/10.2147/cia.s5358
Capitani E, Laiacona M (1988) Aging and psychometric diagnosis of intellectual impairment: some considerations on test scores and their use. Dev Neuropsychol DEVELOP NEUROPSYCHOL 4:325–330. https://doi.org/10.1080/87565648809540416
Capitani E, Laiacona M (1997) Composite neuropsychological batteries and demographic correction: Standardization based on equivalent scores, with a review of published data. The Italian Group for the Neuropsychological Study of Ageing. J Clin Exp Neuropsychol 19:795–809. https://doi.org/10.1080/01688639708403761
Carlesimo GA, Caltagirone C, Gainotti G (1996) The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the standardization of the mental deterioration battery. Eur Neurol 36(6):378–384. https://doi.org/10.1159/000117297
Cheng C-M, Chang W-H, Chiu Y-C, Sun Y, Lee H-J, Tang L-Y, Wang P-N, Chiu M-J, Yang C-H, Tsai S-J, Tsai C-F (2018) Association of polypharmacy with mild cognitive impairment and cognitive ability: a nationwide survey in Taiwan. J Clin Psychiatry 79(6):17m12043. https://doi.org/10.4088/JCP.17m12043
Chuang Y-F, Elango P, Gonzalez CE, Thambisetty M (2017) Midlife anticholinergic drug use, risk of Alzheimer’s disease, and brain atrophy in community-dwelling older adults. Alzheimer’s Dementia (n Y) 3(3):471–479. https://doi.org/10.1016/j.trci.2017.06.004
Corsonello A, Cozza A, D’Alia S, Onder G, Volpato S, Ruggiero C, Cherubini A, Di Rosa M, Fabbietti P, Lattanzio F (2019) The excess mortality risk associated with anticholinergic burden among older patients discharged from acute care hospital with depressive symptoms. Eur J Intern Med 61:69–74. https://doi.org/10.1016/j.ejim.2018.11.004
D’Alia S, Guarasci F, Bartucci L, Caloiero R, Guerrieri ML, Soraci L, Colombo D, Crescibene L, Onder G, Volpato S, Cherubini A, Ruggiero C, Corsonello A, Lattanzio F, Fabbietti P (2020) Hand grip strength may affect the association between anticholinergic burden and mortality among older patients discharged from hospital. Drugs Aging 37(6):447–455. https://doi.org/10.1007/s40266-020-00766-x
Dos Santos ANM, Junior GAAG, Benseñor IM, Goulart AC, Brunoni AR, Viana MC, Lotufo PA, Suemoto CK (2022) Anticholinergic burden and cognitive performance: cross-sectional results from the ELSA-Brasil study. Eur J Clin Pharmacol 78(9):1527–1534. https://doi.org/10.1007/s00228-022-03361-8
Fabbietti P, Di Stefano G, Moresi R, Cassetta L, Di Rosa M, Fimognari F, Bambara V, Ruotolo G, Castagna A, Ruberto C, Lattanzio F, Corsonello A (2018a) Impact of potentially inappropriate medications and polypharmacy on 3-month readmission among older patients discharged from acute care hospital: a prospective study. Aging Clin Exp Res 30(8):977–984. https://doi.org/10.1007/s40520-017-0856-y
Fabbietti P, Ruggiero C, Sganga F, Fusco S, Mammarella F, Barbini N, Cassetta L, Onder G, Corsonello A, Lattanzio F, Di Rosa M (2018b) Effects of hyperpolypharmacy and potentially inappropriate medications (PIMs) on functional decline in older patients discharged from acute care hospitals. Arch Gerontol Geriatr 77:158–162. https://doi.org/10.1016/j.archger.2018.05.007
Frasson P, Ghiretti R, Catricalà E, Pomati S, Marcone A, Parisi L, Rossini PM, Cappa SF, Mariani C, Vanacore N, Clerici F (2011) Free and cued selective reminding test: an Italian normative study. Neurol Sci off J Ital Neurol Soc Ital Soc Clin Neurophysiol 32(6):1057–1062. https://doi.org/10.1007/s10072-011-0607-3
Ghibelli S, Marengoni A, Djade CD, Nobili A, Tettamanti M, Franchi C, Caccia S, Giovarruscio F, Remuzzi A, Pasina L (2013) Prevention of inappropriate prescribing in hospitalized older patients using a computerized prescription support system (INTERcheck(®)). Drugs Aging 30(10):821–828. https://doi.org/10.1007/s40266-013-0109-5
Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E (1996) Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci 17(4):305–309. https://doi.org/10.1007/BF01997792
Han L, Agostini JV, Allore HG (2008) Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. J Am Geriatr Soc 56(12):2203–2210. https://doi.org/10.1111/j.1532-5415.2008.02009.x
Hughes JE, Waldron C, Bennett KE, Cahir C (2023) Prevalence of drug-drug interactions in older community-dwelling individuals: a systematic review and meta-analysis. Drugs Aging 40(2):117–134. https://doi.org/10.1007/s40266-022-01001-5
Jyrkkä J, Enlund H, Lavikainen P, Sulkava R, Hartikainen S (2011) Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf 20(5):514–522. https://doi.org/10.1002/pds.2116
Katz S (1983) Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 31(12):721–727. https://doi.org/10.1111/j.1532-5415.1983.tb03391.x
Kilimann I, Wucherer D, Ittermann T, Völzke H, Bülow R, Hoffmann W, Grabe HJ, Wittfeld K, Teipel SJ (2022) Inverse association between the anticholinergic burden and hippocampus volume in a population-based cohort across the entire adult age range. GeroScience 44(3):1715–1726. https://doi.org/10.1007/s11357-021-00497-w
Klinkenberg I, Sambeth A, Blokland A (2011) Acetylcholine and attention. Behav Brain Res 221(2):430–442. https://doi.org/10.1016/j.bbr.2010.11.033
Koyama A, Steinman M, Ensrud K, Hillier TA, Yaffe K (2014) Long-term cognitive and functional effects of potentially inappropriate medications in older women. J Gerontol Ser A Biol Sci Med Sci 69(4):423–429. https://doi.org/10.1093/gerona/glt192
Lau DT, Mercaldo ND, Harris AT, Trittschuh E, Shega J, Weintraub S (2010) Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis Assoc Disord 24(1):56–63. https://doi.org/10.1097/WAD.0b013e31819d6ec9
Lawton MP, Brody EM (1969) Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9(3):179–186
López-Álvarez J, Sevilla-Llewellyn-Jones J, Agüera-Ortiz L (2019) Anticholinergic drugs in geriatric psychopharmacology. Front Neurosci 13:1309. https://doi.org/10.3389/fnins.2019.01309
Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE (2017) What is polypharmacy? A systematic review of definitions. BMC Geriatr 17:230. https://doi.org/10.1186/s12877-017-0621-2
Midão L, Giardini A, Menditto E, Kardas P, Costa E (2018) Polypharmacy prevalence among older adults based on the survey of health, ageing and retirement in Europe. Arch Gerontol Geriatr 78:213–220. https://doi.org/10.1016/j.archger.2018.06.018
Myint PK, Fox C, Kwok CS, Luben RN, Wareham NJ, Khaw K-T (2015) Total anticholinergic burden and risk of mortality and cardiovascular disease over 10 years in 21,636 middle-aged and older men and women of EPIC-Norfolk prospective population study. Age Ageing 44(2):219–225. https://doi.org/10.1093/ageing/afu185
National report on medicines use in Italy—Year 2021. (s.d.). Recuperato 10 ottobre 2023, da https://www.aifa.gov.it/en/-/l-uso-dei-farmaci-in-italia-rapporto-osmed-2021
Novelli G, Papagno C, Capitani E, Laiacona M, &, et al (1986) Tre test clinici di memoria verbale a lungo termine: Taratura su soggetti normali. [Three clinical tests for the assessment of verbal long-term memory function: norms from 320 normal subjects]. Arch Psicol Neurol Psichiatr 47(2):278–296
Nucci M, Mapelli D, Mondini S (2012) Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin Exp Res 24(3):218–226. https://doi.org/10.3275/7800
O'Donnell LK, Gnjidic D, Nahas R, Bell JS, Hilmer SN (2017) Anticholinergic burden: considerations for older adults. J Pharm Pract Res 47(1):67–77
Oyarzun-Gonzalez XA, Taylor KC, Myers SR, Muldoon SB, Baumgartner RN (2015) Cognitive decline and polypharmacy in an elderly population. J Am Geriatr Soc 63(2):397–399. https://doi.org/10.1111/jgs.13283
Pasina L, Colzani L, Cortesi L, Tettamanti M, Zambon A, Nobili A, Mazzone A, Mazzola P, Annoni G, Bellelli G (2019) Relation between delirium and anticholinergic drug burden in a cohort of hospitalized older patients: an observational study. Drugs Aging 36(1):85–91. https://doi.org/10.1007/s40266-018-0612-9
Pasina L, Djade CD, Lucca U, Nobili A, Tettamanti M, Franchi C, Salerno F, Corrao S, Marengoni A, Iorio A, Marcucci M, Violi F, Mannucci PM (2013) Association of anticholinergic burden with cognitive and functional status in a cohort of hospitalized elderly: comparison of the anticholinergic cognitive burden scale and anticholinergic risk scale: results from the REPOSI study. Drugs Aging 30(2):103–112. https://doi.org/10.1007/s40266-012-0044-x
Perdixi E, Bernini S, Conti S, Jesuthasan N, Cotta Ramusino M, Costa A, Prinelli F (2022) Pre-existing mental health disorders and fear of COVID-19 pandemic: data from a phone survey in community-dwelling older adults recruited in the NutBrain study. Front Psych 13:995308. https://doi.org/10.3389/fpsyt.2022.995308
Pieper NT, Grossi CM, Chan W-Y, Loke YK, Savva GM, Haroulis C, Steel N, Fox C, Maidment ID, Arthur AJ, Myint PK, Smith TO, Robinson L, Matthews FE, Brayne C, Richardson K (2020) Anticholinergic drugs and incident dementia, mild cognitive impairment and cognitive decline: a meta-analysis. Age Ageing 49(6):939–947. https://doi.org/10.1093/ageing/afaa090
Prinelli F, Jesuthasan N, Severgnini M, Musicco M, Adorni F, Correa Leite ML, Crespi C, Bernini S (2020) Exploring the relationship between Nutrition, gUT microbiota, and BRain AgINg in community-dwelling seniors: the Italian NutBrain population-based cohort study protocol. BMC Geriatr 20(1):253. https://doi.org/10.1186/s12877-020-01652-2
Radloff LS (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1(3):385–401. https://doi.org/10.1177/014662167700100306
Rawle MJ, Cooper R, Kuh D, Richards M (2018) Associations between polypharmacy and cognitive and physical capability: a British birth cohort study. J Am Geriatr Soc 66(5):916–923. https://doi.org/10.1111/jgs.15317
Reiter L, Stenberg-Nilsen H, Økland HG (2021) Use of anticholinergic drugs in older patients. Tidsskrift for Den Norske Legeforening. https://tidsskriftet.no/en/2021/04/klinisk-oversikt/use-anticholinergic-drugs-older-patients
Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, Gao S, Boustani M, Crane PK, Petersen RC, Jack CR, Jagust WJ, Aisen PS, Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging Initiative (2016) Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 73(6):721–732. https://doi.org/10.1001/jamaneurol.2016.0580
Sachdev PS, Lipnicki DM, Kochan NA, Crawford JD, Thalamuthu A, Andrews G, Brayne C, Matthews FE, Stephan BCM, Lipton RB, Katz MJ, Ritchie K, Carrière I, Ancelin M-L, Lam LCW, Wong CHY, Fung AWT, Guaita A, Vaccaro R, Cohort Studies of Memory in an International Consortium (COSMIC) (2015) The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: the COSMIC collaboration. PLoS ONE 10(11):e0142388. https://doi.org/10.1371/journal.pone.0142388
Sanghavi R, Pana TA, Mamayusupova H, Maidment I, Fox C, Boekholdt SM, Mamas MA, Wareham NJ, Khaw K-T, Myint PK (2022) Higher anticholinergic burden from medications is associated with significant increase in markers of inflammation in the EPIC-Norfolk prospective population-based cohort study. Br J Clin Pharmacol 88(7):3297–3306. https://doi.org/10.1111/bcp.15261
Sartori G, Job R (1988) The oyster with four legs: a neuropsychological study on the interaction of visual and semantic information. Cogn Neuropsychol 5(1):105–132. https://doi.org/10.1080/02643298808252928
Sganga F, Vetrano DL, Volpato S, Cherubini A, Ruggiero C, Corsonello A, Fabbietti P, Lattanzio F, Bernabei R, Onder G (2014) Physical performance measures and polypharmacy among hospitalized older adults: results from the CRIME study. J Nutr Health Aging 18(6):616–621. https://doi.org/10.1007/s12603-014-0029-z
Shah RC, Janos AL, Kline JE, Yu L, Leurgans SE, Wilson RS, Wei P, Bennett DA, Heilman KM, Tsao JW (2013) Cognitive decline in older persons initiating anticholinergic medications. PLoS ONE 8(5):e64111. https://doi.org/10.1371/journal.pone.0064111
Singh S, Loke YK, Enright P, Furberg CD (2013) Pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Thorax 68(1):114–116. https://doi.org/10.1136/thoraxjnl-2011-201275
Sönnerstam E, Sjölander M, Lövheim H, Gustafsson M (2018) Clinically relevant drug-drug interactions among elderly people with dementia. Eur J Clin Pharmacol 74(10):1351–1360. https://doi.org/10.1007/s00228-018-2514-5
Taylor-Rowan M, Alharthi AA, Noel-Storr AH, Myint PK, Stewart C, McCleery J, Quinn TJ (2022) Anticholinergic deprescribing interventions for reducing risk of cognitive decline or dementia in older adults with and without prior cognitive impairment. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD015405
Taylor-Rowan M, Edwards S, Noel-Storr AH, McCleery J, Myint PK, Soiza R, Stewart C, Loke YK, Quinn TJ (2021) Anticholinergic burden (prognostic factor) for prediction of dementia or cognitive decline in older adults with no known cognitive syndrome. Cochrane Database Syst Rev 5(5):CD013540. https://doi.org/10.1002/14651858.CD013540.pub2
Tragni E, Casula M, Pieri V, Favato G, Marcobelli A, Trotta MG, Catapano AL (2013) Prevalence of the prescription of potentially interacting drugs. PLoS ONE 8(10):e78827. https://doi.org/10.1371/journal.pone.0078827
Trenaman SC, Bowles SK, Andrew MK, Goralski K (2021) The role of sex, age and genetic polymorphisms of CYP enzymes on the pharmacokinetics of anticholinergic drugs. Pharmacol Res Perspect 9(3):e00775. https://doi.org/10.1002/prp2.775
Trevisan C, Limongi F, Siviero P, Noale M, Cignarella A, Manzato E, Sergi G, Maggi S (2021) Mild polypharmacy and MCI progression in older adults: the mediation effect of drug-drug interactions. Aging Clin Exp Res 33(1):49–56. https://doi.org/10.1007/s40520-019-01420-2
Uusvaara J, Pitkala KH, Kautiainen H, Tilvis RS, Strandberg TE (2013) Detailed cognitive function and use of drugs with anticholinergic properties in older people: a community-based cross-sectional study. Drugs Aging 30(3):177–182. https://doi.org/10.1007/s40266-013-0055-2
Valent F (2019) Polypharmacy in the general population of a Northern Italian area: analysis of administrative data. Annali Dell’istituto Superiore Di Sanita 55(3):233–239. https://doi.org/10.4415/ANN_19_03_06
Ziad A, Olekhnovitch R, Ruiz F, Berr C, Bégaud B, Goldberg M, Zins M, Mura T (2018) Anticholinergic drug use and cognitive performances in middle age: findings from the CONSTANCES cohort. J Neurol Neurosurg Psychiatry 89(10):1107–1115. https://doi.org/10.1136/jnnp-2018-318190
Acknowledgements
The authors would like to thank all the participants and their families for their taking part in the NutBrain study. Special thanks go to the communities of Bollate, Baranzate and Segrate for their cooperation and practical support. The authors also would like to thank the researchers, physicians, technicians, nurses and the administrative staff for their efforts, contributions, and support to the project. °NutBrain Study Group: Fulvio Adorni, Sara Bernini, Tania Camboni, Clarissa Consolandi, Silvia Conti, Maria Lea Correa Leite, Alfredo Costa, Matteo Cotta Ramusino, Nithiya Jesuthasan, Alfonso Mastropietro, Massimo Musicco, Orietta Pansarasa, Federica Prinelli, Elena Perdixi, Anna Pichiecchio, Giovanna Rizzo, Eveljn Scarian, Marco Severgnini, Elena Sinforiani.
Funding
Open access funding provided by Consiglio Nazionale Delle Ricerche (CNR) within the CRUI-CARE Agreement. The NutBrain Study is supported by a Grant of Ministero della Salute (Bando di Ricerca Finalizzata Giovani Ricercatori 2016, GR -2016-02361730). The study funders had no involvement in the design and conduct of the study, collection, management, analysis and interpretation of data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Contributions
EP formulated the research question, designed and conducted the study, helped in data acquisition and in data analysis, interpreted the findings, and wrote the first draft the manuscript. MCR, AC, SB, SC, NJ, MS formulated the research question, data acquisition, interpreted the findings, and revised the manuscript. FP supervised the study, formulated the research question, designed and conducted the study, acquired and analyzed the data, interpreted the findings, wrote and revised the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All participants provide a formal written informed consent in order to participate to the original study and in those individuals found to be without capacity to give full written informed consent, a caregiver or guardian is identified and their advice sought regarding participation. The study protocol and amendments have been reviewed and approved by the Medical Ethical Committee of Pavia, Italy. The planning, conduction and reporting of the studies were in line with the Declaration of Helsinki, as revised in 2013. Data were handled and stored following the European Union General Data Protection Regulation (EU GDPR) 2016/679, and data transfer was safeguarded by encrypting/decrypting and password protection.
Competing interest
The authors declare no competing interests.
Additional information
Responsible Editor: Matthias Kliegel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplementary Figure 1: Top ten therapeutic categories (ATC 2nd level) commonly used in men and women. *p-value ≤ 0.05
Additional file 2.
Supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perdixi, E., Cotta Ramusino, M., Costa, A. et al. Polypharmacy, drug-drug interactions, anticholinergic burden and cognitive outcomes: a snapshot from a community-dwelling sample of older men and women in northern Italy. Eur J Ageing 21, 11 (2024). https://doi.org/10.1007/s10433-024-00806-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s10433-024-00806-0