Abstract
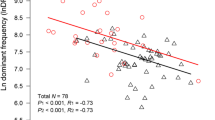
Environmental noise can be an important selective force modulating signal evolution in species with acoustic communication. Many anuran species breed alongside streams; hence, the sound produced by the flowing water is an important source of noise for acoustic communication. Since calling is physiologically very expensive in anurans, and communication is essential for reproduction, we expected adaptations that reduce environmental masking effects and allow acoustic communication in streamside breeders. This basic assumption of the acoustic adaptation hypothesis has not been yet evaluated at a large phylogenetic scale. We combined ahistorical and phylogenetic methods to test whether anuran species that breed alongside streams call at higher frequencies than species that breed away from streams. We compiled primary and secondary data on body size, breeding habitat, and the dominant frequency of the advertisement call for 110 species; 40 of them breed alongside streams and 70 away from streams. Call frequency was slightly higher and body size was significantly smaller in streamside breeding species. After controlling for the effects of body size and phylogenetic signal, only differences in body size persisted between species breeding at both kinds of habitats. Our data suggest that habitat filtering rather than acoustic adaptation explains the high call frequency of stream breeders. Species with large body size, pleiotropically constrained to utter low-frequency calls, would have succeeded less often in establishing viable populations alongside streams, due to the masking effect of low-frequency noise. Thus, small species calling at relatively high frequencies would be more common there. Although our data do not preclude adaptations to noisy habitats in some anuran species, they do not provide support for the acoustic adaptation hypothesis at a wider phylogenetic scale.



Similar content being viewed by others
References
Amézquita A, Hödl W, Lima AP, Castellanos L, Erdtmann L, De Araujo MC (2006) Masking interference and the evolution of the acoustic communication system in the Amazonian dendrobatid frog Allobates femoralis. Evolution 60:1874–1887
Amézquita A, Lima AP, Jehle R, Castellanos L, Ramos O, Crawford AJ, Gasser H, Hödl W (2009) Calls, colours, shape, and genes: a multi-trait approach to the study of geographic variation in the Amazonian frog Allobates femoralis. Biol J Linn Soc 98:826–838
Arch VS, Grafe TU, Narins PM (2008) Ultrasonic signalling by a Bornean frog. Biol Lett 4:19–22
Bee MA, Swanson EM (2007) Auditory masking of anuran advertisement calls by road traffic noise. Anim Behav 74:1765–1776
Bernal MH, Montealegre DP, Páez CA (2004) Estudio de la vocalización de trece especies de anuros del municipio de Ibagué, Colombia. Rev Acad Col Cienc Exact Fís Nat 28:385–390
Bernal XE, Guarnizo C, Lüddecke H (2005) Geographic variation in advertisement call and genetic structure of Colostethus palmatus (Anura, Dendrobatidae) from the Colombian Andes. Herpetologica 61:395–408
Blomberg SP, Garland T Jr, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745
Boeckle M, Preininger D, Hödl W (2009) Communication in noisy environments I: acoustic signals of Stautorois latopalmatus Boulenger 1887. Herpetologica 65:154–165
Bosch J, De la Riva I, Marquez R (1996) The calling behaviour of Lysapsus limellus and Pseudis paradoxa (Amphibia: Anura: Pseudidae). Folia Zool 45:49–55
Bosch J, De la Riva I (2004) Are frog calls modulated by the environment? An analysis with anuran species from Bolivia. Can J Zool 82:880–888
Bourne GR, Collins AC, Holder AM, McCarthy CL (2001) Vocal communication and reproductive behavior of the frog Colostethus beebei in Guyana. J Herpetol 35:272–281
Brown CH, Waser PM (1984) Hearing and communication in blue monkeys (Cercopithecus mitis). Anim Behav 32:66–75
Brown JL, Twomey E, Amézquita A, Barbosa De Souza M, Caldwell JP, Lötters S, Von May R, Melo-Sampaio PR, Mejía-Vargas D, Perez-Peña P, Pepper M, Poelman EH, Sanchez-Rodriguez M, Summers K (2011) A taxonomic revision of the Neotropical poison frog genus Ranitomeya (Amphibia: Dendrobatidae). Zootaxa 3083:1–120
Brumm H (2004) The impact of environmental noise on song amplitude in a territorial bird. J Anim Ecol 73:434–440
Brumm H, Slabbekoorn H (2005) Acoustic communication in noise. Adv Study Behav 35:151–209
Brumm H, Slater PJB (2006) Ambient noise, motor fatigue, and serial redundancy in chaffinch song. Behav Ecol Sociobiol 60:475–481
Brumm H, Voss K, Koellmer I, Todt D (2004) Acoustic communication in noise: regulation of call characteristics in a New World monkey. J Exp Biol 207:443–448
Capellini I, Nunn CL, McNamara P, Preston BT, Barton RA (2008) Energetic constraints, not predation, influence the evolution of sleep patterning in mammals. Funct Ecol 22:847–853
Cocroft RB, Ryan MJ (1995) Patterns of advertisement call evolution in toads and chorus frogs. Anim Behav 49:283–303
Cocroft RB, McDiarmid RW, Jaslow AP, Ruíz-Carranza PM (1990) Vocalizations of eight species of Atelopus (Anura: Bufonidae) with comments on communication in the genus. Copeia 1990:631–643
Coloma LA (1995) Ecuadorian frogs of the genus Colostethus (Anura: Dendrobatidae). Misc Publ Mus Nat Hist Univ Kansas 87:1–72
Davies NB, Halliday TR (1979) Competitive mate searching in male common toads, Bufo bufo. Anim Behav 27:1253–1267
Diaz-Uriarte R, Garland T Jr (1996) Testing hypotheses of correlated evolution using phylogenetically independent contrasts: sensitivity to deviations from Brownian motion. Syst Biol 45:27–47
Drewry GE, Rand AS (1983) Characteristics of an acoustic community: Puerto Rican frogs of the genus Eleutherodactylus. Copeia 1983:941–953
Dubois A, Martens J (1984) A case of possible vocal convergence between frogs and a bird in Himalayan torrents. J Ornithol 125:455–463
Duellman WE (1978) The biology of a Ecuatorian herpetofaunal in amazon Ecuador. Univ Kansas Mus Nat Hist Misc Publ 65:1–352
Duellman WE (2001) The Hylid frogs of middle America. Natural History Museum of the University of Kansas, Ithaca
Duellman WE (2005) Cusco Amazonico. The lives of amphibians and reptiles in an Amazonian rainforest. Comstock Publishing Associates, a division of Cornell University Press, Ithaca
Duellman WE, Trueb L (1986) Biology of amphibians. McGraw Hill Book Co, New York
Erdtmann L, Amézquita A (2009) Differential evolution of advertisement call traits in dart-poison frogs (Anura: Dendrobatidae). Ethology 115:801–811
Faivovich J, Haddad CFB, Garcia PCA, Frost DR, Campbell JA, Wheeler WC (2005) Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bull Am Mus Nat Hist 294:1–240
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Feng AS, Narins PM, Xu C-H, Lin W-Y, Yu Z-L, Qiu Q, Xu ZM, Shen JX (2006) Ultrasonic communication in frogs. Nature 440:333–336
Forrest TG (1994) From sender to receiver: propagation and environmental effects on acoustic signals. Am Zool 34:644–654
Francis CD, Ortega CP, Cruz A (2009) Noise pollution changes avian communities and species interactions. Curr Biol 19:1415–1419
Francis DC, Ortega CP, Cruz A (2011) Noise pollution filters bird communities based on vocal frequency. PLoS ONE 6:1–8
Francis CD, Kleist NJ, Ortega CP, Cruz A (2012) Noise pollution alters ecological services: enhanced pollination and disrupted seed dispersal. Proc R Soc B Biol Sci 279:2727–2735
Frost JS, Platz JE (1983) Comparative assessment of modes of reproductive isolation among four species of leopard frogs (Rana pipiens complex). Evolution 37:66–78
Frost DR, Grant T, Faivovich J, Bain RH, Haas A, De Haddad CFB, Sa RO, Channing A, Wilkinson M, Donnellan SC, Raxworthy CJ, Campbell JA, Blotto BL, Moler P, Drewes RC, Nussbaum RA, Lynch JD, Green DM, Wheeler WC (2006) The amphibian tree of life. Bull Am Mus Nat Hist 297:1–370
Galeotti P, Rubolini D, Dunn PO, Fasola M (2003) Colour polymorphism in birds: causes and functions. J Evolut Biol 16:635–646
Garland T Jr, Harvey PH, Ives AR (1992) Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol 41:18–32
Garland JRT, Dickerman WA, Janis MC, Jones JA (1993) Phylogenetical analysis of covariance by computer simulation. Syst Biol 42:265–292
Gerhardt HC (1978) Acoustic properties used in call recognition by frogs and toads. In: Fritzsch B, Ryan MJ, Wilczynsky W, Hetherington TE, Walkowiak W (eds) The evolution of amphibian auditory system. Wiley Interscience, Austin, pp 455–483
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. The University of Chicago Press, Chicago
Gómez JP, Bravo GA, Brumfield RT, Tello JG, Cadena CD (2010) A phylogenetic approach to disentangling the role of competition and habitat filtering in community assembly of Neotropical forest birds. J Anim Ecol 79:1181–1192
Goosem M, Hoskin C, Dawe G (2007) Nocturnal noise levels and edge impacts on amphibian habitats adjacent to Kuranda range road. Report to the marine and tropical sciences research facility. Reef and Rainforest Research Centre Limited, Cairns. In (ed) James Cook University, North Queensland, Australia, pp 76
Grafe TH, Preininger D, Sztatecsny M, Kasah R, Dehling JM, Proksch S, Höld W (2012) Multimodal communication in a noisy environment: a case study of the bornean rock frog Staurois parvus. PLoS ONE 7:1–8
Grafen A (1989) The phylogenetic regression. Phil Trans R Soc B Biol Sci 326:119–157
Grant T, Rodriguez LO (2001) Two new species of frogs of the genus Colostethus (Dendrobatidae) from Peru and a redescription of C. trilineatus (Boulenger, 1883). Am Mus Novit 3355:1–24
Grant T, Frost DR, Caldwell JP, Gagliardo RON, Haddad CFB, Kok PJR, Means DB, Noonan BP, Schargel WE, Wheeler WC (2006) Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae). Bull Am Mus Nat Hist 299:1–262
Greding EJ Jr (1976) Call of the tropical American frog Rana palmipes Spix (Amphibia, Anura, Ranidae). J Herpetol 10:263–264
Guayasamín JM, Castroviejo-Fisher S, Ayarzaguena J, Trueb L, Vila C (2008) Phylogenetic relationships of glassfrogs (Centrolenidae) based on mitochondrial and nuclear genes. Mol Phylogenet Evol 48:574–595
Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W (2008) GEIGER: investigating evolutionary radiations. Bioinformatics 24:129–131
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, Oxford
Hödl W (1977) Call differences and calling site segregation in anuran species from central Amazonian floating meadows. Oecologia 28:351–363
Hödl W, Amézquita A (2001) Visual signaling in anuran amphibians. In: Ryan MJ (ed) Anuran communication. Smithsonian Institution Press, Washington, pp 121–141
Ibáñez R, Smith EM (1995) Systematic status of Colostethus flotador and C. nubicola (Anura: Dendrobatidae) in Panamá. Copeia 1995:446–456
Ibáñez RD, Rand SA, Jaramillo CA (1999) Los Anfibios del monumento natural Barro Colorado, Parque Nacional Soberanía y áreas adyacentes. Panamá. Editorial Mizrachi and Pujol, S.A., Panamá
Jacobson SK (1985) Reproductive behavior and male mating success in two species of glass frogs (Centrolenidae). Herpetologica 41:396–404
Juncá FA (1996) Parental care and egg mortality in Colostethus stepheni. J Herpetol 30:292–294
Juncá FA (1998) Reproductive biology of Colostethus stepheni and Colostethus marchesianus (Dendrobatidae), with the description of a new anuran mating behavior. Herpetologica 54:377–387
Jungfer KH (1985) Beitrag zur Kenntnis von Dendrobates speciosus O. Schmidt, 1857 (Salientia: Dendrobatidae). Salamandra 21:263–280
Jungfer KH (1989) Pfeilgiftfrösche der gattung Epipedobates mit rot granuliertem rücken aun dem Oriente von Ecuador und Perú. Salamandra 25:81–98
Jungfer KH, Weygoldt P (1994) The reproductive biology of the leaf frog Phyllomedusa lemur Boulenger, 1882, and a comparison with other members of the Phyllomedusinae. Revue française d’aquariologie 21:57–64
Kime NM, Turner WR, Ryan MJ (2000) The transmission of advertisement calls in Central American frogs. Behav Ecol 11:71–83
La Marca E (1994) Taxonomy of the frogs of the genus Mannophryne (Amphibia: Anura: Dendrobatidae). Publ Asoc Amigos Doñana 4:1–75
Lamb T (1984) Amplexus displacement in the southern toad Bufo terrestris. Copeia 1984:1023–1025
Lee JC (1996) The amphibians and reptiles of the Yucatan Peninsula. Comstock Publishing Associates, Cornell University Press, Ithaca
Lescure J, Marty C (2000) Atlas des amphibiens de Guyane. Museum national d’histoire naturelle, Paris
Lima AP, Caldwell JP (2001) A new Amazonian species of Colostethus with sky blue digits. Herpetologica 57:180–189
Lima PA, Magnusson WE, Menin M, Erdtman LK, Rodrigues DJ, Keller C, Höld W (2006) Guide to the frogs of Reserva Adolpho Ducke, central Amazonia. Instituto Nacional de Pesquisas da Amazônia–INPA, Manaus, Brazil
Lötters S (1996) The Neotropical toad genus Atelopus: checklist, biology, distribution. M. Vences & F. Glaw Verlags GbR, Köln
Lötters S, Reichle S, Jungfer KH (2003) Advertisement calls of Neotropical poison frogs (Amphibia: Dendrobatidae) of the genera Colostethus, Dendrobates and Epipedobates, with notes on dendrobatid call classification. J Nat Hist 37:1899–1911
Lötters S, Jungfer KH, Henkel FW, Schmidt W (2007) Poison frogs: biology, species and captive husbandry. Chimaira, Frankfurt
Lougheed SC, Austin JD, Bogart JP, Boag PT, Chek AA (2006) Multi-character perspectives on the evolution of intraspecific differentiation in a neotropical hylid frog. BMC Evol Biol 6:1–16
Love EK, Bee MA (2010) An experimental test of noise-dependent voice amplitude regulation in Cope’s grey treefrog, Hyla chrysoscelis. Anim Behav 80:509–515
Lüddecke H (1999) Behavioral aspects of the reproductive biology of the Andean frog Colostethus palmatus (Amphibia: Dendrobatidae). Rev Acad Col Cienc Exact Fís Nat 23:303–316
Lüddecke H (2002) Male and female responses to call playbacks in the Andean frog Colostethus subpunctatus. Amphibia-Reptilia 23:141–150
Luther DA, Wiley RH (2009) Production and perception of communicatory signals in a noisy environment. Biol Lett 5:183–187
Lyon RH (1973) Propagation of environmental noise. Science 179:1083–1090
Maire V, Gross N, Börger L, Proulx RI, Wirth C, et al (2012) Habitat filtering and niche differentiation jointly explain species relative abundance within grassland communities along fertility and disturbance gradients. New Phytol 196: 497–509
Martin WF (1972) Evolution of vocalization in the genus Bufo. In: Blair WF (ed) Evolution in the genus Bufo. University of Texas Press, Austin, pp 279–309
Martins EP (2000) Adaptation and the comparative method. Trends Ecol Evol 15:296–299
Martins EP, Hansen TF (1996) The statistical analysis of interspecific data: a review and evaluation of phylogenetic comparative methods. In: Martins EP (ed) Phylogenies and the comparative method in animal behavior. Oxford University Press, New York, pp 22–75
Martins IA, Jim J (2003) Bioacoustic analysis of advertisement call in Hyla nana and Hyla sanborni (Anura, Hylidae) in Botucatu, Sao Paulo, Brazil. Braz J Biol 63:507–516
Martins EP, Diniz JAF, Housworth EA (2002) Adaptive constraints and the phylogenetic comparative method: a computer simulation test. Evolution 56:1–13
Mendelson JR III, Campbell JA (1999) The taxonomic status of populations referred to Hyla chaneque (Anura: Hylidae) in southern Mexico, with the description of a new treefrog from Oaxaca. J Herpetol 33:80–86
Menéndez-Guerrero P (2001) Ecología trófica de la comunidad de anuros del Parque Nacional Yasuní en la amazonía ecuatoriana. Pontificia Universidad Católica del Ecuador, Thesis
Moen SD, Wiens JA (2009) Phylogenetic evidence for competitively driven divergence: body-size evolution in Caribbean treefrogs (Hylidae: Osteopilus). Evolution 63:195–214
Morales VR (1992) Dos especies nuevas de Dendrobates (Anura: Dendrobatidae) para Perú. Caribb J Sci 28:191–199
Myers CW (1982) Spotted poison frogs: descriptions of three new Dendrobates from western Amazonia, and resurrection of a lost species from ‘Chiriqui’. Am Mus Novit 2721:1–23
Myers CW, Daly JW (1976) Preliminary evaluation of skin toxins and vocalisations in taxonomic and evolutionary studies of poison-dart frogs (Dendrobatidae). Bull Am Mus Nat Hist 157:173–262
Myers CW, Daly JW (1979) A name for the poison frog of Cordillera Azul, eastern Peru, with notes on its biology and skin toxins (Dendrobatidae). Am Mus Novit 2674:1–24
Myers CW, Duellman WE (1982) Anew species of Hyla from Cerro Colorado and other tree frog records and geographical notes from western Panama. Am Mus Novit 2752:1–32
Myers CW, Daly JW, Malkin B (1978) A dangerously toxic new frog (Phyllobates) used by Embera Indians of western Colombia with discussion of blowgun fabrication and dart poisoning. Bull Am Mus Nat Hist 161:309–365
Myers CW, Daly JW, Martinez V (1984) An arboreal poison frog (Dendrobates) from western Panama. Am Mus Novit 2783:1–20
Narins PM, Smith SL (1986) Clinal variation in anuran advertisement calls basis for acoustic isolation? Behav Ecol Sociobiol 19:135–142
Narins PM, Feng AS, Lin W, Schnitzler H-U, Denzinger A, Suthers RA, Xu C (2004) Old World frog and bird vocalizations contain prominent ultrasonic harmonics. J Acous Soc Am 115:910–913
Nevo E (1973) Adaptive variation in size of cricket frogs. Ecology 54:1271–1281
Ord TJ, Martins EP (2006) Tracing the origins of signal diversity in anole lizards: phylogenetic approaches to inferring the evolution of complex behaviour. Anim Behav 71:1411–1429
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Parris KM (2002) More bang for your buck: the effect of caller position, habitat and chorus noise on the efficiency of calling in the spring peeper. Ecol Model 156:213–224
Parris KM, Schneider A (2009) Impacts of traffic noise and traffic volume on birds of roadside habitats. Ecol Soc 14:29
Patricelli GL, Blickley JL (2006) Avian communication in urban noise: causes and consequences of vocal adjustment. Auk 123:639–649
Penna M, Solis R (1998) Frog call intensities and sound propagation in the South American temperate forest region. Behav Ecol Sociobiol 42:371–381
Platz JE, Frost JS (1984) Rana yavapaiensis, a new species of leopard frog (Rana pipiens complex). Copeia 1984:940–948
Platz JE, Mecham JS (1979) Rana chiracahuensis, a new species of leopard frog (Rana pipiens complex) from Arizona. Copeia 1979:383–390
Porter KR (1966) Mating calls of six Mexican and Central American toads (genus Bufo). Herpetologica 22:60–67
Pramuk JB (2006) Phylogeny of South American Bufo (Anura: Bufonidae) inferred from combined evidence. Zool J Linn Soc 146:407–452
Preininger D, Boeckle M, Hödl W (2007) Comparison of anuran acoustic communities of two habitat types in the Danum Valley Conservation Area, Sabah, Malaysia. Salamandra 43:129–138
Proppe DS, Sturdy CB, St Clair CC (2013) Anthropogenic noise decreases urban songbird diversity and may contribute to homogenization. Global Change Biol 19:1075–1084
Pyron RA, Wiens JA (2011) A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phylogenet Evol 61:543–583
Pytte CL, Rusch KM, Ficken MS (2003) Regulation of vocal amplitude by the blue-throated hummingbird, Lampornis clemenciae. Anim Behav 66:703–710
Rheindt FE (2003) The impact of roads on birds: does song frequency play a role in determining susceptibility to noise pollution? J Ornithol 144:295–306
Rodriguez LO, Duellman WE (1994) Guide to the frogs of the Iquitos region, Amazonian Perú. Univ Kansas Mus Nat Hist Spec Public 22:1–80
Rodriguez L, Myers CW (1993) A new poison frog from Manu National Park, southeastern Peru (Dendrobatidae, Epipedobates). Am Mus Novit 3068:1–15
Ryan MJ (1987) Constraints and patterns in the evolution of anuran acoustic communication. In: Fritzsch B, Ryan MJ, Wilczynsky W, Hetherington TE, Walkowiak W (eds) The evolution of amphibian auditory system. Wiley Interscience, Austin, pp 637–677
Ryan MJ, Brenowitz EA (1985) The role of body size phylogeny and ambient noise in the evolution of bird song. Am Nat 126:87–100
Ryan MJ, Wilczynski W (1991) Evolution of intraspecific variation in the advertisement call of a cricket frog (Acris crepitans Hylidae). Biol J Linn Soc 44:249–272
Santos JC, Coloma LA, Summers K, Caldwell JP, Ree R, Cannatella DC (2009) Amazonian amphibian diversity is primarily derived from Late Miocene Andean lineages. PLoS Biol 7:448–461
Savage JM (2002) The amphibians and reptiles of Costa Rica: a herpetofauna between two continents, between two seas. University of Chicago Press, Chicago
Schmidt W (1965) Some aspects of the water economies of nine species of amphibians. Ecology 46:261–269
Schwartz JJ (1987) The function of call alternation in anuran amphibians: a test of three hypotheses. Evolution 41:461–471
Señaris JC, Ayarzagüena J (2001) Una nueva especie de rana de cristal del género Hyalinobatrachium (Anura: Centrolenidae) del delta del río Orinoco, Venezuela. Rev Biol Trop 49:1083–1093
Sihler A, Sihler G (2006) AZDR.com Dendrobase Ranch. Available from http://www.azdr.com
Silverstone PA (1975) A revision of the poison: arrow frogs of the genus Dendrobates Wagler. Nat Hist Bull Angeles County Sci Bull 21:1–55
Slabbekoorn H (2013) Songs of the city: noise-dependent spectral plasticity in the acoustic phenotype of urban birds. Anim Behav 85:1089–1099
Slabbekoorn H, Smith TB (2002) Bird song, ecology and speciation. Philos Trans R Soc Lond B Biol Sci 357:493–503
Starrett PH, Savage JM (1973) The systematic status and distribution of Costa Rican glass-frogs genus Centrolenella (family Centrolenidae), with description of a new species. Bull South Calif Acad Sci 72:57–78
Sullivan BK (1982) Significance of size, temperature and call attributes to sexual selection in Bufo woodhousei australis. J Herpetol 16:103–106
Sullivan BK, Malmos KB (1994) Call variation in the Colorado River toad (Bufo alvarius): behavioral and phylogenetic implications. Herpetologica 50:146–156
Sun JWC, Narins PM (2005) Anthropogenic sounds differentially affect amphibian call rate. Biol Conserv 121:419–427
Wells KD (1980a) Behavioral ecology and social organization of a dendrobatid frog (Colostethus inguinalis). Behav Ecol Sociobiol 6:199–209
Wells KD (1980b) Social behavior and communication of a dendrobatid frog (Colostethus trinitatis). Herpetologica 36:189–199
Wells KD (2001) The energetics of calling frogs. In: Ryan MJ (ed) Anuran communication. Smithsonian Institution Press, Washington, pp 45–60
Wells KD (2007) Ecology and behavior of amphibians. The University of Chicago Press, Chicago
Wilczynski W, Ryan MJ (1999) Geographic variation in animal communication systems. In: Foster SA, Endler JA (eds) Geographic variation in behavior. Oxford University Press, New York, pp 234–261
Witte K, Farris HE, Ryan MJ, Wilczynski W (2005) How cricket frog females deal with a noisy world: habitat-related differences in auditory tuning. Behav Ecol 16:571–579
Zimmerman BL (1983) A comparison of structural features of calls of open and forest habitat frog species in the central Amazon. Herpetologica 39:235–246
Zimmerman E, Rahmann H (1987) Acoustic communication in the poison-arrow frog Phyllobates tricolor: advertisement calls and their effects on behavior and metabolic brain activity of recipients. J Comp Physiol A 160:693–702
Zweifel RC (1968) Effects of temperature, body size, and hybridization on mating calls of toads, Bufo a. americanus and Bufo woodhousii fowleri. Copeia 1968:269–285
Acknowledgments
This research was funded by the Faculty of Sciences, at the Universidad de los Andes, Bogotá. We also thank the Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnología “Francisco José de Caldas” COLCIENCIAS for supporting doctoral studies of FVS. Luis A Coloma, Enrique La Marca, Sandra M. Durán, Fernando Montealegre-Zapata, Olga L. Torres and Mauricio Rivera provided important comments and literatura references for this manuscript. Previous version of this manuscript was highly improved by commentaries by Peter M. Narins, two anonymous reviewers and J. A. Endler.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vargas-Salinas, F., Amézquita, A. Abiotic noise, call frequency and stream-breeding anuran assemblages. Evol Ecol 28, 341–359 (2014). https://doi.org/10.1007/s10682-013-9675-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-013-9675-6