Abstract
The remarkable diversity of ecological niches that birds have evolved to inhabit have resulted in their status as model organisms to study how the same morphological features can adapt to different environments. Not least of these features is the avian foot, which has diversified into a wide range of forms suited to several different ecological functions. In this investigation, we examine how a potential trade-off between two such functions is expressed in the foot morphology of the Australian avifauna; namely, the impact that specialising for either walking or grasping has on the proportions of the phalanges. The lengths of the body, foot, third digit and its phalanges, and the hallux were recorded from 106 preserved skins belonging to 22 species. Our analysis of these data shows that this functional specialisation presents a similar morphological gradient in Australian birds as has been previously observed in American species, with a few unique exceptions. Generally, species that are reliant on the foot to grasp (e.g., perching and gripping prey) display greater distal phalanx and hallux lengths than species that are specialised for walking or wading. However, the terrestrial Megapodes of Australia demonstrate a more intermediate morphology, potentially as a result of the unique mound construction behaviour occurring in this clade. These findings have relevance not only for use in identifying the ecology of cryptic or extinct species from morphology, but also for determining future evolutionary changes in different avian groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Birds have a reputation as being amongst the most exemplary taxa with which to explore the field of evolutionary biology. Comprised of approximately 11,000 extant species (Widrig and Field 2022) and inhabiting all continents, modern birds (Neornithes) are one of the most speciose and diverse of all tetrapod groups (Lovette and Fitzpatrick 2016). As such, this group provides the means to observe how adaptation can drive evolution in many directions to occupy a vast range of ecological niches, from ostriches to hummingbirds. Whilst much research regarding the evolution of bird morphology (shape and size) has focused on the development of flight and radiation of beak forms in this group (e.g., Darwin 1845; Marsh 1880; Vazquez 1992; Bright et al. 2016; and see Ostrom 1979), there is also value in examining the unique diversity of the avian hind limb (Raikow and Bledsoe 2000; Zeffer et al. 2003; Abourachid and Höfling 2012; Kavanagh et al. 2013; Backus et al. 2015).
The diversity of foot morphologies observed across the avian phylogeny are commonly designated to five categories (Fig. 1) that are differentiated by the direction and degree of fusion in the toes, of which there are generally four (though reduction can occur) (Bock and Miller 1959, but see Raikow 1985). The ancestral condition is anisodactyly, as observed in Archaeopteryx and other ancestral Aves (Raikow 1982), whereas the development of alternative foot morphologies such as zygodactyly in parrots and woodpeckers, heterodactyly in trogons, and pamprodactyly in swifts, are functional specialisations in part to perching and locomoting on vertical substrates (Bock and Miller 1959; Swierczewski and Raikow 1981; Collins 1983; Ksepka et al. 2019). Several evolutionary pathways exist in birds, resulting in both disparate and convergent forms adapted to perform various ecological functions; from the enlargement of a rear-facing toe to create a grasping, ‘pincer-like’ foot (Abourachid et al. 2017), to the webbed, paddle-like feet of aquatic birds (Provini et al. 2012a).
In addition to the direction of the toes, specialisation of the foot can occur through changes in configuration of the individual phalanges (bones of the toes). The general phalangeal formula of bird feet is the same as found in ancestral amniotes, albeit lacking digit V; 2–3–4–5 from digits I through IV (Fedak and Hall 2004). Exceptions are found most notably amongst Coraciiformes (kingfishers, rollers, etc.) and Trochilidae (hummingbirds) (Maurer and Raikow 1981, in Botelho et al. 2015).
The lengths of the phalanges in relation to one another can be used to make inferences regarding the ecological uses of the foot; Fisher (1946) is amongst the earliest researchers to remark upon arboreal species tending towards shorter proximal phalanges and longer distal phalanges, and that the opposite pattern exists in cursorial/terrestrial species. This proximo-distal length variation, observed in several consequent studies (chiefly Hopson 2001; Kambic 2008; Abourachid et al. 2017), occurs in a way that suggests a functional trade-off may exist between use of the foot for grasping motions—such as perching in arboreal environments, climbing vertical substrates, capturing prey items, and manipulating objects like fruit—and use of the foot primarily for terrestrial locomotion (i.e., walking, wading, or running) (Kavanagh et al. 2013). The length of the hallux (or other rear-facing toe such as in zygodactylous birds) has also been shown to be strongly indicative of this behavioural gradient, with greater length generally indicating a preference for grasping and a corresponding reduction or loss in cursorial species (Raikow 1985; Feduccia 1999; Abourachid et al. 2017; Falk et al. 2021). In such studies, intermediate phenotypes tend to indicate generalist species, resulting in a linear gradient of phalanx proportions that is also suggestive of a trade-off between competing functions (Kavanagh et al. 2013). The comparative study of Abourachid et al. (2017) is particularly notable as the authors demonstrate that foot morphology is adaptive and relates to orientation of the substrate—i.e., whether birds are moving mostly on horizontal (ground) or vertical substrates (tree trunks and cliffs), and that convergent evolution of a specialist grasping foot has evolved in separate lineages.
Although much literature exists describing the evolution and complex anatomy of grasping, both in birds (e.g., Collins 1983; Backus et al. 2015; Tsang and McDonald 2019; Dickinson et al. 2022) and other lineages (Sustaita et al. 2013 and references therein), few have remarked upon the potential biomechanical bases for the apparent trade-off in phalanx proportion between grasping and walking birds. It has been suggested that elongate distal phalanges confer a more powerful grip in grasping appendages (Trinkaus and Villemeur 1991; Gianechini et al. 2020), but discussion of the converse pattern is scarcer still. Given that walking birds from perching clades still display shortened distal phalanges compared to their relatives (Kavanagh et al. 2013), it is entirely possible that this arrangement provides some advantage in adapting to a non-grasping lifestyle. Kinematic evidence has suggested there is no apparent disadvantage in the presence of an elongate hallux in birds when walking at speed (Middleton 2003), a finding that is at odds with the general trend of digital reduction in cursorially-adapted species (Smith and Maynard 1956; Coombs 1978). Regardless, a conclusive discussion of the reason behind this adaptational trend is beyond the scope of this investigation; we seek primarily to assess its presence in a new geographical setting.
In this study, we examine how this form-function relationship in foot shape in birds is expressed across the avian assemblage of the Australian continent. Australia is home to around 900 bird species, 45% of which are endemic to the region (Clarke and Dolby 2014). Likewise, there is ample evidence to suggest Australasian (through Gondwanan) origins of parrots and songbirds (Psittacopasserae), the latter of which—at approximately 6,000 species strong—comprise around 60% of known avian species (Edwards and Boles 2002; Wright et al. 2008; Oliveros et al. 2019). The biota of Australia is often represented in scientific literature as either exemplarily convergent with, or primitive to, taxa from other continents (Clarke and Dolby 2014), but it is in truth home to a range of uniquely well-adapted taxa that can provide a healthy complement to knowledge derived from studies of exclusively American or European assemblages. Thusly we aim to strengthen understanding of the trade-offs associated with grasping and walking locomotion demonstrated with a unique assemblage of avian species.
We selected a range of species displaying many of the toe arrangements and different locomotor styles that occur in Australian birds in order to assess the relationships between foot morphology and ecological habits across the Australian avifauna. As such, we examine many species with the same morphological and ecological types as Abourachid et al. (2017), as well as several unique Australian species, primarily to further investigate the potential trade-off in distal phalanx lengthening from walking to grasping birds. To this end, we aim to provide further evidence of form-function relationships in a unique assemblage of avian species, as well as to describe any pertinent exceptions in the Australian avifauna that provide an opportunity to better understand the processes underlying their diversity. Therefore, we strengthen the understanding of the trade-offs associated with grasping and terrestrial locomotion and highlight the contribution of the foot to the impressive ecological adaptability of the Aves.
Materials and methods
A total of 22 species were examined in this investigation. This species sampling was selected to mirror and extend the exemplary range of ecologies included in Abourachid et al. (2017) using Australian native species. Figure 2 illustrates the phylogenetic relationships of these species alongside their respective ecotypes, which are generalised into five ranked categories based on the predominant lifestyle of each bird. Although the majority of species will engage in both terrestrial locomotion and grasping to a certain extent, and both functions encompass a range of motions with different potential morphological impacts, previous work has shown a consistent association between foot morphology and the broad categories described below. This method of quantifying ecology was adapted from the methods used by Kambic (2008) to rank the prevalence of grasping motions in the lifestyle of birds based on descriptions of their behaviour in the literature; birds identified as ‘strongly arboreal/grasping’ were described as being almost never observed on the ground, and ‘weakly arboreal/grasping’ were described as rarely or sometimes being seen on the ground (such as when capturing prey spotted from a perch). Conversely, birds identified as ‘strongly non-arboreal/cursorial’ are those described as never being observed in trees and that run rather than taking flight when disturbed, whilst ‘weakly non-arboreal/cursorial’ birds are those that preferentially inhabit terrestrial or aquatic habitats but may roost or nest in trees. Birds considered to have a ‘mix of grasping and terrestrial’ lifestyles are described as being equally likely to be seen in trees as on the ground, and as spending equal amounts of time foraging on both substrates (Kambic 2008). Data on these behaviours were taken from personal observations, publicly available footage, and the literature cited herein (with particular reference to del Hoyo et al. 2004).
Diagram showing the phylogenetic relationships, morphologies, and ecotypes of the study species. Gross foot morphology is indicated by shape (O = anisodactyl, ◊ = zygodactyl, Δ = syndactyl, □ = heterodactyl). The primary ecology of each bird is represented by a red-blue colour gradient from species that most commonly use the foot for grasping or arboreal locomotion to species that do not display these behaviours. Finally, species that frequently make use of the foot for additional functions such as swimming are indicated by non-gradated colours. Phylogenetic tree pruned from Claramunt and Cracraft (2015)
One notable morphology that was not examined in this investigation was the zygodactyl Piciformes, of which Australia does not have a native population. Heterodactyl trogons similarly are not native to Australia, however measurements were taken from museum specimens of Trogon viridis, the species used by Abourachid et al. (2017), to assess the heterodactyl morphotype in a novel analysis whilst providing a point of reference to this previous investigation.
The skin specimens used in this investigation were sourced from the Ornithology collection of the South Australian Museum. A detailed list of the specimens used is provided in Supplementary Table S1. A total of 106 specimens were measured; five of each species, with the exception of the superb lyrebird (Menura novohollandiae) and white-tailed trogon (T. viridis), of which only three adult specimens each were available.
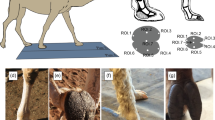
The collection of eight linear measures in this study was performed by one author (EMM) using Mitutoyo ABSOLUTE Digimatic callipers. The callipers were used to measure phalanx proportion externally from the dorsal surface of the foot of each preserved specimen as per Fig. 3. Curved surfaces such as talons were measured through use of a waxed thread held against the dorsal surface of the structure. As this method relies upon external morphology rather than skeletal data as in Abourachid et al. (2017), the measurements may be considered as the ‘functional lengths’ of each digit or phalanx. These functional lengths are taken from the visible ‘segments’ of the intact toes rather than isolated phalanx bones (see Fig. 3), and thusly should reflect how the lengths of the underlying skeletal morphology translate into and interact with the external environment. The functional lengths of the ungual phalanx, hallux, and third digit were considered to be inclusive of the claw sheath length, as this is the aspect of the ungual phalanx that interfaces with the external environment in each case. The morphology of the claw sheath, although more plastic than the length of the ungual phalanx bone, has been shown to provide a similar level of predictive power regarding the functional uses of the foot (Thomson and Motani 2023).
The measurement scheme used in this investigation, demonstrated on the red wattlebird. a in lateral view; length of digit I (pink) and phalanges 1 (purple), 2 (blue), 3 (cyan), and 4 (green) of digit III. b in dorsal view; digit III phalanges (colours as per a). c in lateral view; length of digit III (red), and the foot (red + yellow). d in ventral view; length of body from crown (dorsal of eye) to base of tail (immediately behind legs). The functional length of the phalanges was recorded from digit III only, as Hopson (2001) identifies the phalanx proportions of this digit as being most representative of the pattern of interest
One caveat in the methodology of this study lies in the measurements being taken from the dorsal surface of the foot; as the plantar surface of the foot is the aspect that interfaces with the substrate, its morphological features are in many ways correlated with ecological use (e.g., Höfling and Abourachid 2021). However, the dry preservation methods of the specimens used, as well as the inconsistencies presented by features such as webbing and enlarged tubercules, make the lengths of the plantar surface prohibitively difficult to accurately measure using the methodology outlined herein. In this context, the dorsal surface provides more reliable measurements, and—should the results prove meaningful—demonstrate a convenient method to generate large datasets of this type.
The length of each specimen from the crown to the base of the tail was taken as a proxy for body size to be used in measurement standardisation. This process prevents differences in overall body size from obscuring patterns in the proportions of the feet between orders. This standardisation was performed using log-shape ratio correction (Mosimann and James 1979), wherein a geometric mean is calculated by dividing the product of all variables by the number of total variables. Standardisation is then performed by dividing each measurement by this geometric mean and applying a logarithmic transformation. This process is the preferred standardisation technique for linear morphometric data (Jungers et al. 1995).
Statistical analyses and visualisations were implemented in the R statistical environment (ver. 4.1.0; R Core Team 2021), with packages and functions detailed for each analysis. The log-shape ratio data were evaluated through principal components analysis (PCA), implemented with the function ‘prcomp’ in the R package stats (R Core Team 2021). This ordination method reduces data dimensionality by generating orthogonal axes of variance from the measurement matrix, thereby allowing for analysis and visualisation of multivariate trends in 2-dimensional graphs. In this investigation, PCA was used to assess the morphological similarity of each species based on their ecotype. The phylogenetic tree in Fig. 1 was projected into the PCA scatterplots by estimating the position of internal nodes using maximum likelihood to create a phylomorphospace that visualises whether morphological similarity is due to shared ancestry or convergent evolution. This was implemented with the ‘phylomorphospace’ function in the phytools package (Revell 2012).
In an evolutionary context, it is appropriate to assess traits with consideration of the shared ancestry of species, as similarities in morphology may be caused by phylogenetic closeness rather than adaptation (Felsenstein 1985). Tests for phylogenetic signal on the main axes of variation in the data were performed using the K statistic (Blomberg et al. 2003), implemented in the ‘phylosig’ function in phytools package (Revell 2012). The impact of ecotype on morphology in a phylogenetic context was tested using a multivariate phylogenetic generalised least squares (PGLS) analysis (Adams 2014) via the ‘procD.pgls’ function in the geomorph package (Baken et al. 2021; Adams et al. 2022).
Results
Morphological trends in foot shape with locomotory ecotype among Australian species are shown in Fig. 4a. The phylomorphospace illustrates where each of the species sampled lie in relation to one another with regards to phalanx proportion, and how this relates to foot morphology, ecotype, and function. PC1 (73.6% of variance) is primarily representative of the length of the hallux, which is proportionately smaller to the negative end and proportionally larger to the positive end. Species at the negative side of PC1 mainly represent ecotypes that primarily locomote through walking or swimming and are rarely or never seen using the feet to grasp or perch, whereas those to the positive side of PC1 are primarily of birds that favour arboreal environments and/or use the feet for grasping behaviours. Many species that spend time both on the ground and in arboreal settings lie centrally on PC1, which includes all zygodactylous birds sampled in this investigation. PC2 (9.8%) describes the degree of proximo-distal length gradient present in the phalanges. Species with comparatively longer distal and shorter proximal phalanges are placed to the positive end of PC2, whereas species exhibiting a converse pattern populate the negative end of PC2. The specific changes in length of each variable that impact both PC1 and PC2 are shown in Fig. 4b.
Phylomorphospace from PC1 and 2 of foot shape in Australian species A. Symbols and phylogeny as per Fig. 2, with abbreviated binomial nomenclature provided for ease of interpretation. Four species at the extremes of each axis as well as their associated foot morphology are illustrated. B: Loading scores of the variables on both PC axes, coloured by magnitude
There is significant phylogenetic signal in PC1 (K = 0.93, P = 0.004), whereas PC2 does not display significant phylogenetic signal (K = 0.34, P = 0.291). This indicates evolutionary history likely has a stronger impact on the length of the hallux compared to the lengths of the phalanges. The PGLS analysis of these data shows that ecotype is a significant predictor of foot shape (R2 = 0.39, F4,21 = 2.75, P = 0.015).
Discussion
While the evolution of the avian wing was an innovation that enabled birds to make use of an entirely novel environment, the feet have remained a crucial means of locomotion upon terrestrial, arboreal, and scansorial substrates, among other ecological functions (Sustaita et al. 2013). Specifically, the presence of an elongate rear-facing toe (typically the hallux) has been shown to be strongly indicative of behaviour, with greater length generally indicating a preference for grasping, perching, or climbing, and a corresponding reduction or loss in cursorial species (Raikow 1985; Middleton 2001; Abourachid et al. 2017). The results of this investigation indicate that a correlation between such ecological functions and phalanx length proportions is present in Australian avifauna, and lends more support to the notion of a universal relationship in form-function of the avian foot. As a general rule, birds that utilise their feet for perching or grasping prey (e.g., passerines and raptors) show phalanx proportions specialised to this function through elongation of the hallux, and a short-to-long proximodistal gradient in phalanx lengths. Conversely, walking and aquatic birds that are adapted for either swimming or locomoting on flat surfaces often display a reduction of the hallux and a long-to-short proximodistal phalanx length gradient (Feduccia 1999; Hopson 2001; Kambic 2008; Abourachid et al. 2017; Höfling and Abourachid 2021). In the results of this investigation, this is most clearly illustrated by the two Columbiformes sampled; in Fig. 4, the arboreal superb fruit dove (Ptilinopus superbus) diverges notably from its closest relative in the group, the exclusively terrestrial spinifex pigeon (Geophaps plumifera). The differences in morphology between these species occur in the way predicted by the trends described above.
As such, our results are largely consistent with those of Abourachid et al. (2017), who identified this morphological trend at a broad evolutionary scale amongst American avifauna. This suggests that this adaptational pattern has a solid ecological basis across different avian assemblages, with a gradient supporting the idea it could act as a functional trade-off, and further validates the inferences of researchers such as Hopson (2001) and Kambic (2008) into the locomotor modes of extinct theropods. Additionally, we confirm that it is possible to verify this pattern from external dorsal foot morphology as well as skeletal material, allowing for a much more accessible method of data collection. Despite this sample displaying the same overall trends in phalanx proportion as are established elsewhere in the literature, there are several points of interest that warrant individual examination.
This investigation sampled four of the five most common foot morphologies in birds. Amongst these morphologies, the greatest variation in phalanx proportion is seen in anisodactyl taxa, whilst zygodactyl, syndactyl, and heterodactyl birds occupy a more limited section of the phylomorphospace. It is possible this is an artefact of the greater number of anisodactyls assessed, however, the results of Abourachid et al. (2017) show a similar distribution of morphologies along the corresponding PC axis. This provides support that there may be a selective pressure (or lack thereof) driving non-anisodactyl taxa towards a generalised morphology in lieu of becoming more specialised walkers or perchers. Of the non-anisodactyl species and anisodactyl species lying centrally on PC1 (namely Podargus strigoides and Aegotheles cristatus), all but the Psittaciformes employ sallying (or perch-and-pounce) to capture prey (see Remsen and Robinson 1990). However, most have also been observed foraging on the ground—a behaviour requiring some form of terrestrial locomotion, be that walking or hopping (Skutch 1962; Webb 1989; Brigham et al. 1999; del Hoyo et al. 2004; Madani 2020). Whilst different forms of terrestrial locomotion exert different selective pressures (Provini and Höfling 2020), it is possible this mixed mode of foraging leads to an intermediate phenotype in general, as taxa to either end of the PC1 axis tend to utilise grasping or walking in their foraging styles to a greater extent (Temple-Smith 1969; Hopper and Burbidge 1978; Olsen et al. 1990; Heather and Robertson 2000; Saunders 2004; del Hoyo et al. 2004; Forshaw 2015). Considering the presence of other morphological distinctions in the limbs based on semi-aquatic habits (Provini et al. 2012b), it is interesting to note that many of the non-arboreal species sampled exhibit similar phalanx proportions despite additional behaviours such as swimming and paddling. Whether this morphology reflects a convergence on a non-grasping phenotype, or is adaptive to the different individual locomotor styles of the species sampled here is not clear, but is certainly worth further investigation.
A notable exception to the broad pattern is the brown treecreeper (Climacteris picumnus), which has been observed foraging arboreally and on the ground in fairly equal amounts; the strong grasping morphology in this species is likely an adaptation to its largely scansorial lifestyle (Cooper 2000). Differences exist in the toe proportions of climbing birds depending on their scaling technique and overall foot morphology; species possessing stiff tail feathers to act as a brace (such as woodpeckers) exhibit shorter toes than those that must grip the substrate unassisted (such as nuthatches) (Feduccia 1973; Cartmill 1985). The Australian treecreepers do not utilise the tail in climbing, and (unlike nuthatches) climb directly upwards with the body and feet positioned parallel to the trunk of the tree. Orenstein (1977) notes that Australian treecreepers tend toward particularly elongate toes compared with other scansorial birds, potentially granting resistance to the specific gravitational forces that act upon this climbing method. Although the brown treecreeper is the only climbing bird sampled in this investigation, its position at the extreme end of PC1 (indicating an elongate hallux) aligns with the propositions of Leblanc et al. (2022) that the rear toe is more relevant in climbing morphotypes than previously thought. Indeed, Orenstein (1977) suggests from musculoskeletal observations that the hallux possesses the ability to act as a brace in the particular climbing style of the Australian treecreepers.
Additional behaviours using the foot may also explain the disparity between the two primarily-herbivorous Psittaciformes sampled; being larger, the sulphur-crested cockatoo (Cacatua galerita) favours feeding on large seeds, nuts, and bulbs that must be manipulated with the feet to a greater extent, rather than the small seeds consumed directly as in the cockatiel (Nymphicus hollandicus) (Brown and Magat 2011). This increased food-grasping behaviour may therefore contribute to the sulphur-crested cockatoo exhibiting a phalanx proportion more similar to perching birds than the cockatiel. In a similar way, the two raptorial birds exhibit a difference in the use of the foot to capture prey. Accipiters such as the grey goshawk (Accipiter novaehollandiae) kill using the feet, whereas falcons such as the Nankeen kestrel (Falco cenchroides)—whilst still seizing prey with the feet—kill using the beak. In examining this difference between the two groups, Sustaita (2008) found a biomechanical propensity for higher-velocity grasping in accipiters over falcons, a factor that is likely to be reflected by longer joint out levers—in this case, longer distal phalanges—providing a functional and biomechanical basis for the disparity between morphology in the raptors seen in this investigation.
The morphology of several iconic species of Australian ground-dwelling birds is also deserving of remark. The superb lyrebird (M. novaehollandiae), although a primarily terrestrial bird (del Hoyo et al. 2004), is observed on the right-hand side of the phylomorphospace with birds that favour perching and grasping. It is likely this is in part the result of the phylogenetic placement of the lyrebird as a basal passerine (perching bird), however, a comparison may be made to the other large, terrestrial birds in this sample. The Australian brushturkey (Alectura lathami) and malleefowl (Leipoa ocellata) are members of the family Megapodiidae, a group of Galliformes renowned for their use of environmental heat for egg incubation—in the case of these species, through building large mounds of plant litter and earth (Diamond 1983). Megapodes (alongside the Cracidae) are distinguished from other Galliformes in part by their hallux, which is more prehensile and positioned level with the other digits rather than higher on the tarsus (Hudson and Lanzillotti 1964). It is possible this increased length and dexterity of the hallux provides an increased capacity for stability as well as gripping and manoeuvring substrate during mound construction. Similarly, the nest of the lyrebird is often constructed on a platform of large, partly decayed sticks that are excavated from the leaf litter (Maisey et al. 2016). Although the lyrebird nest is largely constructed using the beak, it is possible that a grasping foot morphology may also be beneficial in the ground foraging used both in gathering nesting materials and digging for food (del Hoyo et al. 2004; Maisey et al. 2022).
These idiosyncrasies in terrestrial Australian birds present the question of other potential exceptions to established locomotor adaptations in the limbs of terrestrial birds worldwide. Although unique Australasian species such as the brown kiwi (Apteryx australis Shaw, 1813), northern cassowary (Casuarius unappendiculatus Blyth, 1860), and the kākāpō (Strigops habroptilus G.R. Gray, 1845)—as well as many other birds with unique evolutionary histories and foot functions—have been examined in terms of their placement along the adaptational gradient of this trade-off, this has predominantly been in service of identifying the palaeoecology of extinct theropods (Hopson 2001; Kambic 2008). Such research into the evolutionary past of birds is of great benefit in illuminating their present state of speciosity, as well as in identifying the lifestyles of avian taxa known only from fossils or museum specimens, but these data also have potential in discerning the evolutionary future of the group. Studies of adaptations to differing ecologies in the context of theories such as generalisation and specialisation, evolvability, and morphological integration (see Kavanagh et al. 2013; Orkney et al. 2021; Shatkovska and Ghazali 2020) may aid in assessing the capacity of different taxa to adapt to changing habitats. For example, it has been hypothesised in the past that the nature of the anisodactyl foot enables a degree of ecological flexibility that enables such birds to better cope with events such as aridification and habitat fragmentation (Collias 1997; Raikow and Bledsoe 2000). Examining further the factors contributing to the wider diversity of anisodactyl morphology when compared to other toe configurations through the lens of evolvability could enrich this discussion greatly. This is of particular relevance as current ecological trends present a growing environmental instability to which birds must adapt; for example, birds are showing rapid morphological responses in body size and shape to climate change (e.g., Ryding et al. 2021; Zheng et al. 2023). Thus, new perspectives into which foot morphologies provide the most capacity for evolutionary change may be key in identifying how these shifts are likely to impact avian fauna worldwide.
Data availability
All data generated and analysed during this study are included in the supplementary information file. Materials are housed in the Ornithology Collection of the South Australian Museum.
References
Abourachid A, Höfling E (2012) The legs: a key to bird evolutionary success. J Ornithol 153:193–198
Abourachid A, Fabre AC, Cornette R, Höfling E (2017) Foot shape in arboreal birds: two morphological patterns for the same pincer-like tool. J Anat 231:1–11
Adams DC (2014) A method for assessing phylogenetic least squares models for shape and other high-dimensional multivariate data. Evolution 68:2675–2688
Adams DC, Collyer ML, Kaliontzopoulou A, Baken EK (2022) Geomorph: Software for geometric morphometric analyses. R package version 4.0.4. https://cran.r-project.org/package=geomorph
Backus SB, Sustaita D, Odhner LU, Dollar AM (2015) Mechanical analysis of avian feet: multiarticular muscles in grasping and perching. R Soc Open Sci 2:140350
Baken EK, Collyer ML, Kaliontzopoulou A, Adams DC (2021) geomorph v4.0 and gmShiny: enhanced analytics and a new graphical interface for a comprehensive morphometric experience. Methods Ecol Evol 12:2355–2363
Blomberg S, Garland T Jr, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745
Bock WJ, Miller WD (1959) The scansorial foot of the woodpeckers, with comments on the evolution of perching and climbing feet in birds. Am Mus Novit 1931:1–45
Botelho JF, Smith-Paredes D, Vargas AO (2015) Altriciality and the evolution of toe orientation in birds. Evol Biol 42:502–510
Brigham RM, Geiser F, Gutsell RCA, Wiacek RS (1999) Foraging behaviour in relation to the lunar cycle by Australian owlet-nightjars Aegotheles cristatus. Emu 99:253–261
Bright JA, Marugán-Lobón J, Cobb SN, Rayfield EJ (2016) The shapes of bird beaks are highly controlled by nondietary factors. Proc Natl Acad Sci 113:5352–5357
Brown C, Magat M (2011) The evolution of lateralized foot use in parrots: a phylogenetic approach. Behav Ecol 22:1201–1208
Cartmill M (1985) Chapter 5. Climbing. In: Hildebrand, M, Bramble, DM, Liem, KF, Liem, KF Wake, DB (Eds.) Functional vertebrate morphology. (Harvard University Press: Cambrige)
Claramunt S, Cracraft J (2015) A new time tree reveals Earth history’s imprint on the evolution of modern birds. Science Advances 1
Clarke R, Dolby T (2014) Finding Australian Birds: A Field Guide to Birding Locations. Collingwood, CSIRO Publishing
Collias NE (1997) On the origin and evolution of nest building by passerine birds. The Condor 99:253–270
Collins CT (1983) A reinterpretation of pamprodactyly in swifts: a convergent grasping mechanism in vertebrates. Auk 100:735–737
Coombs Jr, WP (1978) Theoretical aspects of cursorial adaptations in dinosaurs. Q Rev Biol 53:393-418.
Cooper CB (2000) The behaviorial ecology and conservation of an Australian passerine, the brown treecreeper (Climacteris picumnus). PhD thesis, Virginia Polytechnic Institute and State University (USA)
Darwin CR (1845) Journal of researches into the natural history and geology of the countries visited during the voyage of H.M.S. Beagle round the world, under the Command of Capt. Fitz Roy, R.N. 2d ed. (John Murray: London)
Diamond J (1983) Egg incubation: The reproductive biology of mound-building birds. Nature 301:288–289
del Hoyo J, Elliott A, Christie DA (2004) Handbook of the birds of the world. Vol. 9. Cotingas to pipits and wagtails. (Lynx Edicions: Barcelona)
Dickinson E, Young MW, Kim CJ, Hadjiargyrou M, Granatosky MC (2022) The influence of substrate size upon pulling and gripping forces in parrots (Psittaciformes: Agapornis roseicollis). J Exp Biol 225(19):jeb244818
Edwards SV, Boles WE (2002) Out of Gondwana: the origin of passerine birds. Trends Ecol Evol 17:347–349
Falk AR, Lamsdell JC, Gong E (2021) Principal component analysis of avian hind limb and foot morphometrics and the relationship between ecology and phylogeny. Paleobiology 47:314–336
Fedak TJ, Hall BK (2004) Perspectives on hyperphalangy: patterns and processes. J Anat 204:151–163
Feduccia A (1973) Evolutionary trends in the Neotropical ovenbirds and woodhewers. Ornithol Monogr 13:i–69
Feduccia A (1999) The Origin and Evolution of Birds. London, Yale University Press
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Fisher HI (1946) Adaptations and comparative anatomy of the locomotor apparatus of New World Vultures. Am Midl Nat 35:545–727
Forshaw J (2015) Pigeons and doves in Australia. Clayton, CSIRO Publishing
Gianechini FA, Ercoli MD, Díaz-Martínez I (2020) Differential locomotor and predatory strategies of Gondwanan and derived Laurasian dromaeosaurids (Dinosauria, Theropoda, Paraves): Inferences from morphometric and comparative anatomical studies. J Anat 236:772–797
Heather BD, Robertson HA (2000) The field guide to the birds of New Zealand. (Viking\Penguin Books: Auckland)
Höfling E, Abourachid A (2021) The skin of birds’ feet: morphological adaptations of the plantar surface. J Morphol 282:88–97
Hopper S, Burbidge A (1978) Assortative pollination by red wattlebirds in a hybrid population of Anigozanthos Labill. (Haemodoraceae). Aust J Bot 26:335–350
Hopson JA (2001) Ecomorphology of avian and nonavian theropod phalangeal proportions: implications for the arboreal versus terrestrial origin of bird flight. In New perspectives on the origin and early evolution of birds. pp 211–235
Hudson GE, Lanzillotti PJ (1964) Muscles of the pectoral limb in Galliform birds. Am Midl Nat 71:1–113
Lovette IJ, Fitzpatrick JW (Eds.) (2016) Handbook of bird biology. (John Wiley & Sons: New Jersey)
Jungers WL, Falsetti AB, Wall CE (1995) Shape, relative size, and size-adjustments in morphometrics. Am J Phys Anthropol 38:137–161
Kambic RE (2008) Multivariate analysis of avian and non-avian theropod pedal phalanges. PhD thesis, Montana State University-Bozeman, College of Letters & Science (United States)
Kavanagh KD, Shoval O, Winslow BB, Alon U, Leary BP, Kan A, Tabin CJ (2013) Developmental bias in the evolution of phalanges. Proc Natl Acad Sci 110:18190–18195
Ksepka DT, Grande L, Mayr G (2019) Oldest finch-beaked birds reveal parallel ecological radiations in the earliest evolution of passerines. Curr Biol 29:657–663
Leblanc K, Pintore R, Galvão A, Heitz E, Provini P (2022) Foot adaptation to climbing in ovenbirds and woodcreepers (Furnariida). J Anat 242:607–626
Madani G (2020) Snake predation by the Tawny Frogmouth Podargus strigoides. Aust Field Ornithol 37:42–43
Maisey AC, Carter NT, Incoll JM, Bennett AF (2016) Environmental influences on variation in nest-characteristics in a long-term study population of the Superb Lyrebird, Menura novaehollandiae. Emu-Austral Ornithol 116:445–451
Maisey AC, Haslem A, Leonard SWJ, Bennett AF (2022) Differential effects of ecosystem engineering by the superb lyrebird Menura novaehollandiae and herbivory by large mammals on floristic regeneration and structure in wet eucalypt forests. Ecol Evol 12:e8956
Marsh OC (1880) Odontornithes: a Monograph on the Extinct Toothed Birds of North America: With Thirty-four Plates and Forty Woodcuts. Washington, DC, US Government Printing Office
Maurer DR, Raikow RJ (1981) Appendicular myology, phylogeny, and classification of the avian order Coraciiformes (including Trogoniformes). Annal Carnegie Museum 50:417–434
Middleton KM (2001) The morphological basis of hallucal orientation in extant birds. J Morphol 250:51–60
Middleton KM (2003) Morphology, evolution, and function of the avian hallux. PhD thesis, Brown University (United States)
Mosimann JE, James FC (1979) New statistical methods for allometry with application to Florida red-winged blackbirds. Evolution 33:444–459
Oliveros CH, Field DJ, Ksepka DT, Barker FK, Aleixo A, Andersen MJ, Alström P, Benz BW, Braun EL, Braun MJ, Bravo GA (2019) Earth history and the passerine superradiation. Proceed Nat Acad Sci 116(16):7916–7925
Olsen P, Debus S, Czechura G, Mooney N (1990) Comparative feeding ecology of the grey goshawk Accipiter novaehollandiae and brown goshawk Accipiter fasciatus. Aust Bird Watcher 13:178–192
Orenstein RI (1977) Morphological adaptations for bark foraging in the Australian treecreepers (Aves: Climacteridae). PhD thesis, University of Michigan (USA)
Orkney A, Bjarnason A, Tronrud BC, Benson RBJ (2021) Patterns of skeletal integration in birds reveal that adaptation of element shapes enables coordinated evolution between anatomical modules. Nat Ecol Evol 5:1250–1258
Ostrom JH (1979) Bird Flight: How Did It Begin? Did birds begin to fly from the trees down or from the ground up? Reexamination of Archaeopteryx adds plausibility to an up from the ground origin of avian flight. Am Sci 67:46–56
Remsen JV, Robinson SK (1990) A classification scheme for foraging behavior of birds in terrestrial habitats. Stud Avian Biol 13:144–160
Provini P, Höfling E (2020) To hop or not to hop? The answer is in the bird trees. Syst Biol 69:962–972
Provini P, Goupil P, Hugel V, Abourachid A (2012a) Walking, paddling, waddling: 3d kinematics anatidae locomotion (Callonetta leucophrys). J Exp Zool A 317:275–282
Provini P, Simonis C, Abourachid A (2012b) Functional implications of the intertarsal joint shape in a terrestrial (Coturnix coturnix) versus a semi-aquatic bird (Callonetta leucophrys). J Zool 290:12–18
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Raikow RJ (1982) Monophyly of the Passeriformes: test of a phylogenetic hypothesis. Auk 99:431–445
Raikow RJ, Bledsoe AH (2000) Phylogeny and evolution of the passerine birds. Bioscience 50:487–499
Raikow RJ (1985) Locomotor system. In form and function in birds. (Eds AS King, J McLelland.) Vol 3 (Academic Press: London)
Revell LJ (2012) phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223
Ryding S, Klaassen M, Tattersall GJ, Gardner JL, Symonds MRE (2021) Shape-shifting: changing animal morphologies as a response to climatic warming. Trends Ecol Evol 36:1036–1048
Saunders ASJ (2004) Comparative ecology of the noisy friarbird Philemon corniculatus (Latham 1790) and the Red Wattlebird Anthochaera carunculata (Shaw 1790) in Central Eastern New South Wales. PhD thesis, University of Western Sydney (Australia)
Shatkovska OV, Ghazali M (2020) Integration of skeletal traits in some passerines: impact (or the lack thereof) of body mass, phylogeny, diet and habitat. J Anat 236:274–287
Skutch AF (1962) Life history of the white-tailed trogon, Trogon viridis. Ibis 104:301–313
Smith JM, Savage RJ (1956) Some locomotory adaptations in mammals. Zool J Linn Soc 42:603–622
Sustaita D (2008) Musculoskeletal underpinnings to differences in killing behavior between North American accipiters (Falconiformes: Accipitridae) and falcons (Falconidae). J Morphol 269:283–301
Sustaita D, Pouydebat E, Manzano A, Abdala V, Hertel F, Herrel A (2013) Getting a grip on tetrapod grasping: form, function, and evolution. Biol Rev 88:380–405
Swierczewski EV, Raikow RJ (1981) Hind limb morphology, phylogeny, and classification of the Piciformes. Auk 98:466–480
Temple-Smith P (1969) Some aspects of the biology of the spurwinged plover—Vanellus miles novaehollandiae (Stephens, 1819). PhD thesis, University of Tasmania (Australia)
Thomson TJ, Motani R (2023) Morphological relationships between vertebrate claw unguals and sheaths and the functional morphology of these structures. J. Morphol 284: e21537
Trinkaus E, Villemeur I (1991) Mechanical advantages of the Neandertal thumb in flexion: a test of an hypothesis. Am J Phys Anthropol 84:249–260
Tsang LR, McDonald PG (2019) A comparative study of avian pes morphotypes, and the functional implications of Australian raptor pedal flexibility. Emu-Austral Ornithol 119:14–23
Vazquez RJ (1992) Functional osteology of the avian wrist and the evolution of flapping flight. J Morphol 211:259–268
Webb G (1989) A note on the diet of the Australian owlet-nightjar Aegotheles cristatus. Corella 13:90–91
Widrig K, Field DJ (2022) The evolution and fossil record of palaeognathous birds (neornithes: palaeognathae). Diversity. https://doi.org/10.3390/d14020105
Wright TF, Schirtzinger EE, Matsumoto T, Eberhard JR, Graves GR, Sanchez JJ, Capelli S, Müller H, Scharpegge J, Chambers GK, Fleischer RC (2008) A multilocus molecular phylogeny of the parrots (Psittaciformes): support for a Gondwanan origin during the cretaceous. Mol Biol Evol 25:2141–2156
Zeffer A, Johansson LC, Marmebro Å (2003) Functional correlation between habitat use and leg morphology in birds (Aves). Biol J Lin Soc 79:461–484
Zheng S, Hu J, Ma Z, Lindenmayer D, Liu J (2023) Increases in intraspecific body size variation are common among North American mammals and birds between 1880 and 2020. Nat Ecol Evol. https://doi.org/10.1038/s41559-022-01967-w
Acknowledgements
We thank Maya Penck of the South Australian Museum for access to the Ornithology collections, and Jeremy Austin for initial discussions on the evolution of the bird foot. We are also grateful to the editor Brian Weeks and two anonymous reviewers for providing feedback that greatly improved this manuscript and the discussion herein.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was supported by the University of Adelaide Summer Research Student Scholarship and University of Adelaide PhD Student Scholarship to EMM. ES was supported by a University of Adelaide Research fellowship and an Australian Research Council Future Fellowship (FT190100803).
Author information
Authors and Affiliations
Contributions
Conceived and designed by ES and EMM. Data collected by EMM and analysed by ES and EMM. Journal article written by EMM with review and comments from ES. Both authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
One author (Emma Sherratt) is presently an Assistant Editor for Evolutionary Ecology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martin, E.M., Sherratt, E. Grasping hold of functional trade-offs using the diversity of foot forms in Australian birds. Evol Ecol 37, 945–959 (2023). https://doi.org/10.1007/s10682-023-10261-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-023-10261-5