Abstract
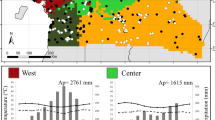
We analyzed the flowering phenodynamics of 43 Asteraceae species co-occurring in natural populations of Chaco Serrano forests in central Argentina. We explored the potential influence of factors such as photoperiod and climate (variations in temperature, rainfall, and frost), animal-plant interactions (richness of floral visitors, frequency of visits), some plant attributes (plant growth form, seed dispersal mechanism), and evolutionary relationships among species on flowering phenodynamics. Cluster Analysis (CA) and Principal Component Analysis (PCA) were the multivariate statistical methods used to analyze emerging patterns associated with these co-occurring species. Null-model analyses were used to evaluate whether flowering times are aggregated, segregated, or random. Results showed that flowering phenology was significantly correlated with the seasonal variation in temperature, photoperiod, rainfall, and frost. The multivariate statistical methods separated all the species in three groups: 1) species with short flowering time, large plant floral display, high frequency of visits by a large number of species of floral visitors, anemochorous fruits, and shrubby growth form, with a tendency to a segregated flowering pattern; 2) species with long flowering time, small plant floral display, low frequency of visits by few insect species, anemochorous fruits, and herbaceous growth form; and 3) species with long flowering time, small plant floral display, intermediate values for frequency of visits and number of species of floral visitors, seed dispersal mechanisms other than anemochory, and herbaceous growth form. In addition, all but one species belonging to early-branching tribes (tribes phylogenetically close to the root of the Asteraceae tree) were grouped together and clustered in the same region of the two-dimensional PCA ordination. All species belonging to the late-branching tribes (Asteroideae subfamily tribes) included in group 1 were separated from the other Asteroideae species in the PCA. In conclusion, it seems that climatic factors restrict the phenological period of most species, and that plant attributes and taxonomic membership are strongly related to flowering phenodynamics in this group of Asteraceae studied.




Similar content being viewed by others
References
Abouheif, E. (1999). A method for testing the assumption of phylogenetic independence in comparative data. Evolutionary Ecology Research, 1, 895–909.
Ackerly, D. D. (2000). Taxon sampling, correlated evolution, and independent contrasts. Evolution, 54, 1480–1492.
Agren, G. I., & Fagerström, T. (1980). Increased or decreased separation of flowering times? The joint effect of competition for space and pollination in plants. Oikos, 35, 161–164.
Armbruster, W. S. (1995). The origins and detection of plant community structure: reproductive versus vegetative processes. Folia geobotanica & phytotaxonomica, 30, 483–497.
Armbruster, W. S., & McGuire, D. (1991). Experimental assessment of reproductive interactions between sympatric Aster and Erigeron (Asteraceae) in interior Alaska. American Journal of Botany, 78, 1449–1457.
Ashton, P. S., Givnish, T. J., & Appanah, S. (1988). Staggered flowering in the Dipterocarpaceae: New insights into floral induction and the evolution of mast fruiting in the aseasonal tropics. The American Naturalist, 132, 44–66.
Baskin, J., Baskin, C., & Leck, M. (1993). After ripening pattern during cold stratification of achenes of ten perennial Asteraceae from eastern North America, and evolutionary implication. Plant Species Biology, 8, 61–65.
Bawa, K. S. (1983). Patterns of flowering in tropical plants. In C. E. Jones & R. J. Little (Eds.), Handbook of experimental pollination biology (pp. 394–410). New York: Scientific and Academic Editions.
Bertiller, M. B., Beeskow, A. M., & Coronato, F. (1991). Seasonal environmental variation and plant phenology in arid Patagonia (Argentina). Journal of Arid Environments, 21, 1–11.
Bolmgren, K., & Lönnberg, K. (2005). Herbarium data reveal an association between fleshy fruit type and earlier flowering time. International Journal of Plant Sciences, 166, 663–670.
Bolmgren, K., Eriksson, O., & Linder, H. P. (2003). Constrasting flowering phenology and species richness in abiotically and biotically pollinated angiosperms. Evolution, 57, 2001–2011.
Borchert, R. (1996). Phenology and flowering periodicity of Neotropical dry forest species: evidence from herbarium collections. Journal of Tropical Ecology, 12, 65–80.
Bremer, K. (1994). Asteraceae: Cladistics & classification. Portland: Timber.
Brody, A. K. (1997). Effects of pollinators, herbivores, and seed predators on flowering phenology. Ecology, 78, 1624–1631.
Cole, B. J. (1981). Overlap, regularity, and flowering phenologies. The American Naturalist, 117, 993–997.
Dafni, A. (1992). Pollination ecology. A practical approach. Oxford: Oxford University Press.
Diekmann, M. (1996). Relationship between flowering phenology of perennial herbs and meteorological data in deciduous forests of Sweden. Canadian Journal of Botany, 74, 528–537.
Digby, P. G. N., & Kempton, R. A. (1996). Multivariate analysis of ecological communities. London: Chapman and Hall.
Faegri, K., & van der Pijl, L. (1966). The principles of pollination ecology. Oxford: Pergamon.
Feinsinger, P. (1987). Effects of plant species on each other’s pollination: Is community structure influenced? Trends in Ecology & Evolution, 2, 123–126.
Felsenstein, J. (1985). Phylogenies and the comparative method. The American Naturalist, 125, 1–15.
Felsenstein, J. (1988). Phylogenies and quantitative characters. Annual Review of Ecology and Systematics, 19, 445–471.
Fenner, M. (1998). The phenology of growth and reproduction in plants. Perspectives in Plant Ecology, 1, 78–91.
Fitter, A. H., Fitter, R. S. R., Harris, I. T. B., & Williamson, M. H. (1995). Relationships between first flowering date and temperature in the flora of a locality in central England. Functional Ecology, 9, 55–60.
Fleming, T. H. (1985). Coexistence of five sympatric Piper (Piperaceae) species in a tropical dry forest. Ecology, 66, 688–700.
Fleming, T. H., & Partridge, B. L. (1984). On the analysis of phenological overlap. Oecologia, 62, 344–350.
Freckleton, R. P. (2000). Phylogenetic tests of ecological and evolutionary hypotheses: checking for phylogenetic independence. Functional Ecology, 14, 129–134.
Friedel, M. H., Nelson, D. J., Sparrow, A. D., Kinloch, J. E., & Maconochie, J. R. (1994). Flowering and fruiting of arid zone species of Acacia in central Australia. Journal of Arid Environments, 27, 221–239.
Gotelli, N. J., & Entsminger, G. L. (2001). EcoSim: Null models software for ecology. Version 6. Burlington, VT: Acquired Intelligence Inc. & Kesey-Bear. http://homepages.together.net/-gentsmin/ecosim.htm. Accessed 15 March 2002.
Gotelli, N. J., & Graves, G. R. (1996). Null models in ecology. Washington, D.C.: Smithsonian Institution.
Gross, R. S., & Werner, P. A. (1983). Relationships among flowering phenology, insect visitors, and seed-set of individuals: experimental studies on four co-occurring species of goldenrod (Solidago: Compositae). Ecological Monographs, 53, 95–117.
Hamann, A. (2004). Flowering and fruiting phenology of a Philippine submontane rain forest: climatic factors as proximate and ultimate causes. Journal of Ecology, 92, 24–31.
Harvey, P. H., & Pagel, M. (1991). The comparative method in evolutionary biology. Oxford: Oxford University Press.
Inouye, D. W. (2000). The ecological and evolutionary significance of frost in the context of climate change. Ecology Letters, 3, 457–463.
Jennings, W. B. (2001). Comparative flowering phenology of plants in the western Mojave desert. Madrono, 48, 162–171.
Johnson, S. D. (1992). Climatic and phylogenetic determinants of flowering seasonality in the Cape flora. Journal of Ecology, 81, 567–572.
Kochmer, J. P., & Handel, S. N. (1986). Constraints and competition in the evolution of flowering phenology. Ecological Monographs, 56, 303–325.
Lane, M. A. (1996). Pollination biology of compositae. In P. D. S. Caligari & D. J. N. Hind (Eds.), Compositae: Biology & utilization. Proceedings of the International Compositae Conference, Kew, 1994 (pp. 61–80). Kew: Royal Botanic Gardens.
LeBuhn, G. (1997). Timing is everything: New perspectives on floral phenology. Trends in Ecology & Evolution, 12, 4–5.
Levin, D. A., & Anderson, W. W. (1970). Competition for pollinators between simultaneously flowering species. The American Naturalist, 104, 455–467.
Lieberman, D. (1982). Seasonality and phenology in a dry tropical forest in Ghana. Journal of Ecology, 70, 791–806.
Lönnberg, K. (2004). Flowering phenology and distribution in fleshy fruited plants. Plant Ecology, 9, 1–25.
Losos, J. B. (1999). Uncertainty in the reconstruction of ancestral character states and limitations on the use of phylogenetic comparative methods. Animal Behaviour, 58, 1319–1324.
Losos, J. B. (2000). Ecological character displacement and the study of adaptation. Proceedings of the National Academy of Sciences/Biology, 97, 5693–5695.
Madeira, J. A., & Fernandes, G. W. (1999). Reproductive phenology of sympatric taxa of Chamaecrista (Leguminosae) in Serra do Cipó, Brazil. Journal of Tropical Ecology, 15, 463–479.
Mani, M. S., & Saravanan, J. M. (1999). Pollination ecology and evolution in compositae (Asteraceae). Enfield: Science.
Marco, D. E., & Páez, S. A. (2002). Phenology and phylogeny of animal-dispersed plants in a Dry Chaco forest (Argentina). Journal of Arid Environments, 52, 1–16.
Marques, M. C. M., Roper, J. J., & Baggio Salvalaggio, A. P. (2004). Phenological patterns among plant life-forms in a subtropical forest in southern Brazil. Plant Ecology, 173, 203–213.
Milton, K. (1991). Leaf change and fruit production in six neotropical Moraceae species. Journal of Ecology, 79, 1–26.
Murali, K. S., & Sukumar, R. (1994). Reproductive phenology of a tropical dry forest in Mudumalai, southern India. Journal of Ecology, 82, 759–767.
Oberrath, R., & Böhning-Gaese, K. (2002). Phenological adaptation of ant-dispersed plants to seasonal variation in ant activity. Ecology, 83, 1412–1420.
Ollerton, J., & Lack, A. J. (1992). Flowering phenology: An example of relaxation of natural selection? Trends in Ecology & Evolution, 7, 274–276.
Pagel, M. (1992). A method for the analysis of comparative data. Journal of Theoretical Biology, 156, 431–442.
Pagel, M., & Harvey, P. H. (1992). On solving the correct problem: wishing does not make it so. Journal of Theoretical Biology, 156, 425–430.
Panero, J. L., & Crozier, B. S. (2008). Asteraceae. Sunflowers, daisies. Version 04 April 2008. http://tolweb.org/Asteraceae/20780/2008.04.04. Accessed 20 October 2010.
Panero, J. L., & Funk, V. A. (2008). The value of sampling anomalous taxa in phylogenetic studies: Major clades of the Asteraceae revealed. Molecular Phylogenetics and Evolution, 47, 757–782.
Pianka, E. R. (1986). Ecology and natural history of desert lizards. Princeton: Princeton University Press.
Pico, F. X., & Retana, J. (2000). Temporal variation in the female components of reproductive success over the extended flowering season of a Mediterranean perennial herb. Oikos, 89, 485–492.
Pleasants, J. M. (1980). Competition for bumblebee pollinators in Rocky Mountain plant communities. Ecology, 61, 1446–1459.
Pleasants, J. M. (1990). Null-model tests for competitive displacement: The fallacy of not focusing on the whole community. Ecology, 7, 1078–1084.
Poole, R. W., & Rathcke, B. J. (1979). Regularity, randomness and aggregation in flowering phenologies. Science, 203, 470–471.
Price, T. (1997). Correlated evolution and independent contrasts. Philosophical Transactions of the Royal Society of London, B, 352, 519–529.
Rathcke, B. J. (1984). Patterns of flowering phenologies: Testability and causal inference using a random model. In D. R. Strong, D. Simberloff, L. G. Abele, & A. B. Thistle (Eds.), Ecological communities: Conceptual issues and the evidence (pp. 383–393). Princeton: Princeton University Press.
Rathcke, B. (1988a). Flowering phenologies in a shrub community: Competition and constraints. Journal of Ecology, 76, 975–994.
Rathcke, B. (1988b). Interactions for pollination among coflowering shrubs. Ecology, 69, 446–457.
Rathcke, B., & Lacey, E. P. (1985). Phenological patterns of terrestrial plants. Annual Review of Ecology and Systematics, 16, 179–214.
Sakai, S. (2001). Phenological diversity in tropical forests. Population Ecology, 43, 77–86.
Sakai, S. (2002). General flowering in lowland mixed dipterocarp forests of South-east Asia. Biological Journal of the Linnean Society, 75, 233–247.
Schemske, D. W., Willson, M. F., Melampy, M. N., Miller, L. J., Verner, L., Schemske, K. M., et al. (1978). Flowering ecology of some spring woodland herbs. Ecology, 59, 351–366.
Seghieri, J., Floret, C., & Pontanier, R. (1995). Plant phenology in relation to water availability: Herbaceous and woody species in the savannas of northern Cameroon. Journal of Tropical Ecology, 11, 237–254.
Silvertown, J., Dodd, M., & Gowing, D. (2001). Phylogeny and the niche structure of meadow plant communities. Journal of Ecology, 89, 428–435.
Smith-Ramírez, C., & Armesto, J. J. (1994). Flowering and fruiting patterns in the temperate rainforest of Chiloé, Chile—ecological and climatic constraints. Journal of Ecology, 82, 353–365.
Smith-Ramírez, C., Armesto, J. J., & Figueroa, J. (1998). Flowering, fruiting, and seed germination in Chilean rain forest Myrtaceae: Ecological and phylogenetic constraints. Plant Ecology, 136, 119–131.
Sokal, R. R., & Rohlf, F. J. (1995). Biometry. The principles and practice of statistics in biological research (3rd ed.). New York: W. H. Freeman and Co.
SPSS Inc. (1992). SPSS for windows: Professional statistics, release 5. Chicago: SPSS Inc.
SPSS Inc. (1999). SPSS Base 10.0. Chicago: SPSS.
Stiles, F. G. (1975). Ecology, flowering phenology, and hummingbird pollination of some Costa Rican Heliconia species. Ecology, 56, 285–301.
Stiles, F. G. (1977). Coadapted competitors: The flowering seasons of hummingbird-pollinated plants in a tropical forest. Science, 198, 1177–1178.
Tabachnick, B. G., & Fidell, L. S. (1996). Using multivariate statistics (3rd ed.). New York: Harper Collins College.
Torres, C. (2000). Pollen size evolution: Correlation between pollen volume and pistil length in Asteraceae. Sexual Plant Reproduction, 12, 365–370.
Torres, C., & Galetto, L. (2002). Are nectar sugar composition and corolla tube length related to the diversity of insects that visit Asteraceae flowers? Plant Biology, 4, 360–366.
Torres, C., & Galetto, L. (2007). Style morphological diversity of some Asteraceae species from Argentina: Systematic and functional implications. Journal of Plant Research, 120, 359–364.
Torres, C., & Galetto, L. (2008). Visitantes florales y sistema reproductivo en Asteraceas de Argentina Central: Grado de dependencia entre las plantas y sus polinizadores. Acta botanica Venezuelica, 31, 473–494.
Waser, N. (1983). Competition for pollination and floral character differences among sympatric plant species: a review of evidence. In C. S. Jones & R. J. Little (Eds.), Handbook of experimental pollination biology (pp. 277–293). New York: Van Nostrand-Reinhold.
Waser, N. M., Chittka, L., Price, M. V., Williams, N. M., & Ollerton, J. (1996). Generalization in pollination systems, and why it matters. Ecology, 77, 1043–1060.
Westoby, M., Leishman, M., & Lord, J. (1995). Further remarks on phylogenetic correction. Journal of Ecology, 83, 727–729.
Wheelwright, N. T. (1985). Competition for dispersers, and the timing of flowering and fruiting in a guild of tropical trees. Oikos, 44, 465–477.
Wright, S. J., & Calderon, O. (1995). Phylogenetic patterns among tropical flowering phenologies. Journal of Ecology, 83, 937–948.
Wright, S. J., & van Schaik, C. P. (1994). Light and the phenology of tropical trees. The American Naturalist, 143, 192–199.
Yeboah Gyan, K., & Woodell, S. R. J. (1987). Flowering phenology, flower colour and mode of reproduction of Prunus spinosa L. (Blackthorn); Rosa canina L. (Dog Rose); and Rubus fruticosus L. (Bramble) in Oxfordshire, England. Functional Ecology, 1, 261–268.
Zuloaga, F. O., & Morrone, O. (1999). Catálogo de las plantas vasculares de la República Argentina II. Monographs in Systematic Botany from the Missouri Botanical Garden, 74. St. Louis: Missouri Botanical Garden.
Acknowledgements
We sincerely thank two anonymous reviewers, Olaf Bininda-Emonds, and Sasa Stefanovic for constructive criticisms, suggestions and encouragement, Nick Waser, Diego P. Vázquez, and Marcelo A. Aizen for useful evaluation and detailed comments on early versions of the manuscript, Claudio Sosa for insect identifications, Nicolás Soria for improvements to the figures, and Laura Bruno for careful text editing. The study was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción Científica y Tecnológica, Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba, and Agencia Córdoba Ciencia. Thanks are due the Academia Nacional de Ciencias Exactas, Físicas y Naturales and to CONICET for fellowships to the first author. LG and CT are members of Carrera del Investigador from CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torres, C., Galetto, L. Flowering phenology of co-occurring Asteraceae: a matter of climate, ecological interactions, plant attributes or of evolutionary relationships among species?. Org Divers Evol 11, 9–19 (2011). https://doi.org/10.1007/s13127-011-0038-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-011-0038-2