Abstract
Both clinical depression and subthreshold depressive symptoms have been associated with alterations in cortical thickness. Studies have yielded conflicting results regarding whether cortical thinning or cortical thickening best characterize the depressive state. Also unclear is whether cortical thickness differences are lateralized. This study examined the relationship between depressive symptom dimensions and cortical thickness asymmetry in cingulate and orbitofrontal regions. Fifty-four community-dwelling adults between the ages of 18 and 81 years received a 3-Tesla magnetic resonance imaging scan and completed the Center for Epidemiologic Studies Depression Scale (CES-D). Cortical thickness values were extracted for the rostral anterior cingulate, caudal anterior cingulate, posterior cingulate, isthmus cingulate, and orbitofrontal cortex. An asymmetry index was calculated for each region. Data were analyzed using separate general linear models for each region, in which the CES-D somatic symptoms, negative affect, and anhedonia subscale scores predicted the asymmetry indices, controlling for age and sex. Higher scores on the anhedonia subscale were associated with right-sided asymmetry in orbitofrontal thickness, whereas higher somatic symptom subscale scores predicted greater left-sided asymmetry in posterior cingulate thickness. Follow-up analyses showed the orbitofrontal effect was specific to the medial, not the lateral, orbitofrontal cortex. These results suggest asymmetries in cortical thickness are apparent at even subthreshold levels of depressive symptoms, as all but five participants were below the CES-D cutoff for clinical depression, and that the relationship varies for different symptom dimensions of depression. Understanding brain asymmetries across the range of depressive symptom severity is important for informing targeted depression treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Volumetric changes in depressed individuals are well documented, particularly in frontolimbic regions of the brain (Arnone et al., 2016; Dotson et al., 2009). Fewer studies focus on depression-related changes in cortical thickness, which, together with surface area, comprises gray matter volume (Winkler et al., 2010). Individual differences in brain volumes may be more closely related to surface area rather than cortical thickness (Reuter et al., 2010; Winkler et al., 2010), while cortical thickness is thought to be more closely related to features of the cortex, such as the organization of cortical layers, synaptic connections, and the size, number, and density of cell bodies in the neurons (Kabani et al., 2001). Cortical thickness is considered a more sensitive marker of disease state in depression and has been proposed as a phenotype for depression (Peterson & Weissman, 2011).
Studies of cortical thickness in depression have yielded conflicting results, with both reductions and increases in thickness reported for regions including the orbitofrontal, prefrontal and cingulate cortices, parahippocampal and other temporal regions, as well as the insula (Grieve et al., 2013; Jarnum et al., 2011; Lim et al., 2012; van Eijndhoven et al., 2013). It has been speculated that during the early course of a depressive disorder, increased cortical thickness occurs as a compensatory response to inflammation, whereas a more prolonged illness course eventually leads to decreased cortical thickness (Rajkowska & Miguel-Hidalgo, 2007; Yang et al., 2015).
Consistent with this hypothesis, Yang and colleagues (Yang et al., 2015) found that compared with controls, people with major depressive disorder (MDD) had greater cortical thickness in the medial orbitofrontal cortex (OFC) and inferior parietal gyrus; however, a longer duration of illness was associated with a thinner medial OFC. Indirect evidence also comes from our previous work examining subthreshold depressive symptoms, which often are a prodrome for a major depressive episode (see Lee et al., 2019 for a meta-analysis). We showed that older adults with higher depressive symptoms, but without clinical depression, have greater cortical thickness in the isthmus cingulate and precuneus (Szymkowicz et al., 2016).
Structural and functional neuroimaging studies suggest possible lateralization in the neurobiology of depression. According to the valence hypothesis (Davidson & Irwin, 1999; Wittling & Roschmann, 1993), positive emotions are associated with the left hemisphere and negative emotions with the right hemisphere. Consistent with this hypothesis, the approach-withdrawal model proposes that depression is characterized by a disturbance in the normal balance of left frontal activity underlying reactions to positive stimuli, and right frontal activity underlying reactions to negative stimuli (Coan & Allen, 2003; Davidson, 1998). Functional imaging studies have shown greater reductions in left compared with right frontal activity at rest as a function of clinical depression or depressive symptoms (Bruder et al., 2017; Thibodeau et al., 2006). However, results have not been consistent, and some studies report null findings (Quinn et al., 2014; Reid et al., 1998). There also is evidence of lateralized effects of depression on cortical thickness. A review of this literature concluded that the right medial OFC is thinner in individuals with MDD compared with controls (Zhang et al., 2018). A more recent study found an effect of depression on thickness in the inferior temporal gyrus and caudal anterior cingulate that was specific to the right hemisphere (Schmaal et al., 2017).
Such studies typically make conclusions about asymmetry based on finding significant effects in one hemisphere but not the other. This approach does not shed light on the left and right hemispheres in relation to each other. Recent studies have used a different approach, calculating an asymmetry index (AI) that allows for a statistical analysis of differences in the relative size of left and right hemisphere regions using a scale that ranges from −100 to 100 (Kawasaki et al., 2008; Kurth et al., 2015; Postema et al., 2019; Shah et al., 2019). Unlike traditional approaches, the continuous nature of the AI provides a quantitative measure of the degree of right-left asymmetry, which allows for a more nuanced understanding of hemispheric differences in relation to an outcome, such as depression or depressive symptoms. Using this index, one study found depression-related differences in cortical thickness asymmetry in the caudal middle frontal, superior frontal, and rostral middle frontal cortices (Zuo et al., 2019). An ENIGMA consortium analysis found asymmetry differences in the caudal anterior cingulate, pars opercularis, and superior temporal gyrus in depressed adults older than 21 years of age, although none of these effects survived correction for multiple comparisons (de Kovel et al., 2019).
Building on previous literature, the current study used the AI to examine the relationship between dimensions of depressive symptoms (somatic symptoms, negative affect, and anhedonia) and asymmetry in cortical thickness in a community sample with primarily subthreshold depressive symptoms. Growing literature shows that different components, or symptom dimensions, of depression have distinct associations, including with cognitive functioning, brain structure and function, etiological contributors, and clinical outcomes (Brailean et al., 2016; Dotson & McClintock, 2020; Kappelmann et al., 2020; McLaren et al., 2017; Murri et al., 2018). This is in line with the National Institute of Mental Health’s Research Domain Criteria (RDoC) framework, which emphasizes research on dimensions of behavior that cut across disorders (Insel et al., 2010). Within the RDoC framework, neuroimaging studies are one of multiple units of analysis that are used to link behavioral dimensions with underlying neurobiological mechanisms that are thought to underlie those dimensions, independent of specific diagnostic categories. For example, anhedonia and somatic symptoms are symptom dimensions of depression that are also relevant to schizophrenia and anxiety disorders. Thus, by identifying neurobiological correlates of symptom dimensions within depression, we also can enhance our understanding of other disorders.
Structural neuroimaging studies of depressive symptom dimensions are limited, but the existing studies show unique structural correlates of different symptom dimensions (Bogoian et al., 2020; Lener et al., 2016; McLaren et al., 2016; Tully et al., 2017). The current study adds to this growing literature by using a more sensitive structural measure—cortical thickness—and a more sensitive measure of hemispheric differences—the AI. We focused on cingulate subregions and the OFC based on our previous work showing distinct relationships between dimensions of subthreshold depression and cingulate volumes (McLaren et al., 2016), and recent reviews that identified the OFC as showing consistent depression-related differences in cortical thickness (Suh et al., 2019; Zhang et al., 2018). We predicted that higher depressive symptoms would be associated with greater asymmetry. Based on our previous volumetric findings and the role of the OFC in reward processing (Hoflich et al., 2019; McLaren et al., 2016), we specifically expected anhedonia to be associated with orbitofrontal and anterior cingulate asymmetry, while somatic symptoms and negative affect were expected to be associated with asymmetry in the posterior and isthmus cingulate, regions associated with arousal states, internally directed cognition, and affective flattening. Given inconsistent laterality effects in the depression literature, we did not have specific predictions about the direction of asymmetry in our regions of interest (ROIs).
Methods and Materials
Participants
This study included a community-dwelling sample of 54 participants between the ages of 18 and 81 years. Demographic data are presented in Table 1. Participants were required to have at least 9 years of education, to be right-handed, native-English speakers, and to score above the suggested dementia cutoff of 30 on the Telephone Interview for Cognitive Status (Brandt et al., 1988)—a cognitive screening measure. Potential participants were excluded based on the following criteria: self-reported major medical conditions (e.g., stroke, cancer), history of head trauma, current antiepileptic or antipsychotic use, language comprehension difficulties or learning disorder, and magnetic resonance imaging (MRI) contraindications. This study was approved by the local institutional review board, and all participants gave written and verbal informed consent.
Depressive Symptom Assessment
Participants completed the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977), a 20-item self-report measure of depressive symptoms experienced during the preceding week. The CES-D has been well validated in both young and older adults (Caracciolo & Giaquinto, 2002; Cosco et al., 2017). Meta-analysis of the factor structure of the CES-D revealed three factors based on 14 of the 20 items: somatic symptoms, negative affect, and anhedonia. Participants in the sample had mostly subthreshold depressive symptoms based on the CES-D cutoff of 16 that is generally recommended for identifying clinical depression (Weissman et al., 1977). Five participants scored ≥16.
MRI Data Acquisition
All participants received a 3-Tesla structural MRI scan in a Phillips (Amsterdam, Netherlands) scanner located at the University of Florida’s McKnight Brain Institute. A high resolution, T1-weighted turbo field echo anatomical scan was collected using a standard 8-channel head radio-frequency coil and the following parameters: TR = 28.1 ms, TE = 2.7 ms, 170 slices acquired in a sagittal orientation, flip angle = 8 degrees, 1-mm cubic resolution.
Cortical Thickness Measurement
Cortical thickness was extracted using Freesurfer version 5.3, which has standard procedures for automated parcellation of both cortical and subcortical brain structures that are documented and freely available for download (http://surfer.nmr.mgh.harvard.edu/). Automated cortical thickness measures have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Kuperberg et al., 2003; Salat et al., 2004).
The procedure includes motion correction, removing non-brain tissue (Segonne et al., 2004), applying a Talairach transformation, segmenting the subcortical structures (Fischl et al., 2002; Fischl, Salat, et al., 2004a), correcting intensity inhomogeneity (Sled et al., 1998), tessellating the gray/white matter boundary, and topology correction (Fischl et al., 2001; Segonne et al., 2007). Surface deformation followed intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid (CSF) borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale et al., 1999; Dale & Sereno, 1993; Fischl & Dale, 2000). Then, the cortex was parcellated into discrete regions with respect to gyral and sulcal structure (Desikan et al., 2006; Fischl, van der Kouwe, et al., 2004b). Using intensity and continuity information in segmentation and deformation procedures, cortical thickness was calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl & Dale, 2000). As a quality control measure, we calculated interclass correlation coefficients (ICCs) for manual adjustments in cortical thickness segmentation. ICC between raters ranged from good to excellent (.644 to .903) across ROIs in the current study (Cicchetti, 1994).
Statistical Analysis
To quantify differences between cortical thickness in the left and right hemispheres, an asymmetry index (AI) was calculated for ROIs in the OFC (medial + lateral OFC), rostral anterior cingulate, caudal anterior cingulate, posterior cingulate, and isthmus cingulate. The following formula was applied, consistent with prior studies (de Kovel et al., 2019; Zuo et al., 2019): AI = (left – right) × 100 / (left + right). Values for the AI can range from −100 to +100, representing complete right- or left-lateralized asymmetry, respectively.
Statistical analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC) using the PROC GLM procedure. CES-D somatic symptoms, negative affect, and anhedonia scores were the predictors, thus allowing us to identify the effect of each symptom dimension while controlling for the other subscales. In this way, we can interpret effects as the association of each dimension of depression with cortical thickness asymmetry, controlling for the relationship of other symptom dimensions with thickness asymmetry in that region. Correlations between subscales ranged from 0.40 to 0.74. The variance inflation factors were all ≤2.55, suggesting that multicollinearity was not a significant issue (Hair, 2010). Models controlled for age and sex. Separate models were conducted for the five ROIs. All variables besides sex were continuous measures in the models. Statistical significance was set at α = 0.05. Effect sizes are reported as partial eta squared (ηp2).
For comparison with the existing literature, parallel models were conducted with left and right cortical thickness in our ROIs as the dependent variables.
Results
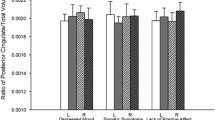
Table 2 and Fig. 1 summarize results of the regression analyses. Higher scores on the anhedonia subscale were associated with greater right-sided asymmetry in orbitofrontal thickness [F(5, 48) = 8.07, p = 0.006, ηp2 = 0.144]. For the somatic symptoms subscale, higher scores were associated with greater left-sided asymmetry in the posterior cingulate [F(5, 48) = 5.03, p = 0.029, ηp2 = 0.095]. None of the CES-D subscales were associated with thickness asymmetry in the rostral or caudal anterior cingulate. The Shapiro-Wilk test indicated that the residuals were normally distributed.
Least-square means showing the asymmetry index for each region of interest by Center for Epidemiologic Studies Depression Scale (CES-D) subscales scores. CES-D subscale scores were continuous measures in all analyses. The depressive symptom group are based on tertiles of CES-D scores within the study sample and are for graphical purposes only. Error bars represent standard error. *p < 0.05,**p < 0.01
To follow up on the significant effect of anhedonia on orbitofrontal asymmetry, separate models were run for the medial and lateral OFC. The association remained significant for medial [F(5, 48) = 6.35, p = 0.015, ηp2 = 0.117] but not lateral [F(5, 48) = 2.01, p = 0.163, ηp2 = 0.040] orbitofrontal asymmetry.
None of the effects were significant when left and right ROIs were examined separately.
Discussion
Our hypotheses were largely confirmed. We found significant asymmetry in cortical thickness related to depressive symptoms. Specifically, higher scores on the anhedonia subscale were associated with right > left asymmetry in the OFC, and higher somatic symptoms of depression were associated with left > right asymmetry in the posterior cingulate. Contrary to our hypothesis, the negative affect subscale was not associated with cortical thickness asymmetry. Our findings support the growing evidence that different aspects of depressive symptomatology have distinct neural underpinnings.
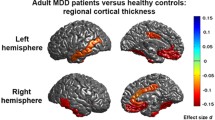
In our sample, participants with higher scores on the anhedonia subscale tended to have greater cortical thickness in the right compared to left OFC, in contrast to those with lower scores, who showed a left > right asymmetry. It is not surprising that asymmetry in the OFC was specifically associated with anhedonia, considering the neurobiology of anhedonia. The OFC is a component of the reward system, the frontosubcortical network of brain regions that subserves the anticipation and experience of pleasure (Bray et al., 2010; Padoa-Schioppa, 2007). Anhedonia has a strong role in reward processing, and OFC activity is decreased in anhedonic individuals with MDD (Keedwell et al., 2005). Follow-up analyses showed that our finding was driven specifically by asymmetry in the medial OFC. This is consistent with findings of increased thickness in the right medial OFC in clinical depression (Qiu et al., 2014; Yang et al., 2015), although other research has shown the same for the left lateral OFC (Grieve et al., 2013). Nonetheless, a meta-analysis (Suh et al., 2019) showed bilateral thinning as opposed to thickening of the OFC in clinical depression.
The differences in findings across studies likely relates at least in part to differences in the severity and chronicity of depression. Our study is unique in its focus on a community sample with primarily subthreshold depressive symptoms, which could be a prodrome to more significant clinical depression in some individuals (Cuijpers & Smit, 2004; Tuithof et al., 2018). Similar to our results, the right medial OFC has been found to be thicker in people at risk for MDD (Peterson et al., 2009) and in first-episode treatment-naïve MDD (Qiu et al., 2014), while a longer duration of illness has been associated with thinning in the same region (Yang et al., 2015). Together, these findings are in line with the hypothesis that increased cortical thickness occurs as a compensatory mechanism in early stages of depression, but cortical thinning will eventually be observed after more prolonged illness (Rajkowska & Miguel-Hidalgo, 2007; Yang et al., 2015). However, it should be noted that due to the cross-sectional nature of our study, we are unable to definitively characterize subthreshold symptoms in our sample as prodromal. While prior studies do suggest that subthreshold symptoms can be a prodrome to major depression (Fava & Tossani, 2007; Lee et al., 2019), not everyone with subthreshold symptoms goes on to develop clinical depression. Moreover, subthreshold symptoms can be part of a residual phase of depression (Ji, 2012). Longitudinal studies will help to clarify these possibilities.
We also found a relationship between higher somatic symptoms of depression and asymmetry in the posterior cingulate, reflecting greater cortical thickness in the left compared to right hemisphere. Similarly, earlier work showed thickness in the left posterior cingulate was increased in first-episode medication-naïve major depression (van Eijndhoven et al., 2013). Taken with reports of orbitofrontal thinning in depressed non-remitters (Jarnum et al., 2011), this once again supports the possibility that structural changes shift from cortical thickening to cortical thinning after prolonged illness.
The link between the somatic symptoms subscale and the posterior cingulate is consistent with previous literature, as is the trend-level association of somatic symptoms with the isthmus cingulate (p = 0.09). The posterior cingulate is a highly connected region (Hagmann et al., 2008) that is a central component of the default mode network (Buckner & DiNicola, 2019). This region has a number of affective and cognitive roles, some of which align with the symptoms measured by the somatic symptoms subscale. For example, items such as ‘my sleep was restless’ and ‘I had trouble keeping my mind on what I was doing’ are consistent with the posterior cingulate’s role in sleep and insomnia (Li et al., 2018; Vogt & Laureys, 2005; Wu et al., 2020), and regulating the focus of attention (Gusnard et al., 2001; Hahn et al., 2007; Hampson et al., 2006). The isthmus cingulate, which connects the posterior cingulate to the parahippocampal gyrus, also is part of the default mode network. It has been implicated in memory and pain processing (Nielsen et al., 2005), as well as mood symptoms (Whitford et al., 2014). Thus, the specific associations we observed between dimensions of depressive symptoms and cortical thickness asymmetry are in line with the known roles of each region.
We did not find a relationship between the negative affect subscale and asymmetry in any of our ROIs, contrary to our hypotheses and to numerous studies linking depressive symptoms with structural abnormalities in the cingulate cortex, as well as the OFC (Schmaal et al., 2017; Wise et al., 2017). For example, we previously found that negative mood symptoms were associated with larger volumes in the left posterior cingulate and smaller volumes in the right isthmus cingulate (McLaren et al., 2016). However, the previous findings were in an older sample (aged 51 years or older) and the study focused on separate analysis of left and right hemisphere volumes rather than an AI of cortical thickness. Given the limited structural imaging studies of depressive symptom dimensions, the reasons for the null findings for the negative affect subscale are unclear. It is possible that the cingulate cortex and OFC have more of a role in anhedonia and somatic symptoms of depression rather than sad mood per se. However, given the dearth of research using the AI to examine the relationship between symptom dimensions of depression and cortical thickness, null results should be taken with caution.
In addition, our findings should be considered within the context of study limitations, including the relatively small sample size, homogenous sample, reliability of the CES-D subscales, and lack of information about psychiatric comorbidities for the full sample. The reliability of questionnaire subscales is particularly important to consider in studies that examine symptom dimensions of depression using measures such as the CES-D, as poor subscale reliability can limit our confidence that we are measuring the symptom dimension of interest. Nonetheless, this study contributes to a fairly small but growing literature. Though preliminary, our findings suggest that the relative size of left and right cingulate and orbitofrontal thickness might be a marker of subthreshold depressive symptoms. Considering that we did not find significant effects when examining left and right ROIs separately, results also suggest that the relative hemispheric difference, measured by the AI, is more sensitive than examining each hemisphere in isolation. Moreover, the study highlights distinct associations between symptom dimensions of depression and cortical thickness. While replication and extension of our findings is critical, these findings suggest that our understanding of the neurobiological basis of depression will be enhanced by considering symptom presentation and subtle differences in structural asymmetry.
Neuroimaging studies have shown both structural and functional asymmetries in clinical depression (Bruder et al., 2017; Thibodeau et al., 2006; Zhang et al., 2018), but much less is known about lateralization differences in the neurobiology of subthreshold depression. Subthreshold symptoms are a risk factor for the development of major depression, and are associated with cognitive, brain changes, and functional deficits, even at a subclinical level (Dotson et al., 2008; Li et al., 2017; Ludvigsson et al., 2019; Tuithof et al., 2018; Zhang et al., 2020). More work is needed to identify brain asymmetries across the depression spectrum. There is preliminary evidence that hemispheric asymmetry is related to clinical outcome after stimulation antidepressant treatments such as repetitive transcranial magnetic stimulation and electroconvulsive therapy (Downar et al., 2014; Wade et al., 2017). As such, understanding brain asymmetries related to different symptom dimensions of depression might inform targeted treatment for depression based on symptom profile. Studies in larger samples that can examine possible demographic moderators such as age, race, and sex also will provide a valuable contribution to this area of research.
References
Arnone, D., Job, D., Selvaraj, S., Abe, O., Amico, F., Cheng, Y., . . . McIntosh, A. M. (2016). Computational meta-analysis of statistical parametric maps in major depression. Human Brain Mapping, 37(4), 1393-1404. https://doi.org/10.1002/hbm.23108
Bogoian, H. R., King, T. Z., Turner, J. A., Semmel, E. S., & Dotson, V. M. (2020). Linking depressive symptom dimensions to cerebellar subregion volumes in later life. Translational Psychiatry, 10(1), 201. https://doi.org/10.1038/s41398-020-00883-6
Brailean, A., Comijs, H. C., Aartsen, M. J., Prince, M., Prina, A. M., Beekman, A., & Huisman, M. (2016). Late-life depression symptom dimensions and cognitive functioning in the Longitudinal Aging Study Amsterdam (LASA). Journal of Affective Disorders, 201, 171-178. https://doi.org/10.1016/j.jad.2016.05.027
Brandt, J., Spencer, M., & Folstein, M. (1988). The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, and Behavioral Neurology, 1(2), 111-117.
Bray, S., Shimojo, S., & O'Doherty, J. P. (2010). Human medial orbitofrontal cortex is recruited during experience of imagined and real rewards. Journal of Neurophysiology, 103(5), 2506-2512. https://doi.org/10.1152/jn.01030.2009
Bruder, G. E., Stewart, J. W., & McGrath, P. J. (2017). Right brain, left brain in depressive disorders: Clinical and theoretical implications of behavioral, electrophysiological and neuroimaging findings. Neuroscience and Biobehavioral Reviews, 78, 178-191. https://doi.org/10.1016/j.neubiorev.2017.04.021
Buckner, R. L., & DiNicola, L. M. (2019). The brain's default network: updated anatomy, physiology and evolving insights. Nature Reviews. Neuroscience, 20(10), 593-608. https://doi.org/10.1038/s41583-019-0212-7
Caracciolo, B., & Giaquinto, S. (2002). Criterion validity of the center for epidemiological studies depression (CES-D) scale in a sample of rehabilitation inpatients. Journal of Rehabilitation Medicine, 34(5), 221-225. https://doi.org/10.1080/165019702760279215
Cicchetti, D. V. (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment, 6(4), 284-290. https://doi.org/10.1037/1040-3590.6.4.284
Coan, J. A., & Allen, J. J. (2003). Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology, 40(1), 106-114. https://doi.org/10.1111/1469-8986.00011
Cosco, T. D., Prina, M., Stubbs, B., & Wu, Y. T. (2017). Reliability and Validity of the Center for Epidemiologic Studies Depression Scale in a Population-Based Cohort of Middle-Aged U.S. Adults. Journal of Nursing Measurement, 25(3), 476-485. https://doi.org/10.1891/1061-3749.25.3.476
Cuijpers, P., & Smit, F. (2004). Subthreshold depression as a risk indicator for major depressive disorder: a systematic review of prospective studies. Acta Psychiatrica Scandinavica, 109(5), 325-331. https://doi.org/10.1111/j.1600-0447.2004.00301.x
Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179-194. https://doi.org/10.1006/nimg.1998.0395
Dale, A. M., & Sereno, M. I. (1993). Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. Journal of Cognitive Neuroscience, 5(2), 162-176. https://doi.org/10.1162/jocn.1993.5.2.162
Davidson, R. J. (1998). Affective Style and Affective Disorders: Perspectives from Affective Neuroscience. Cognition and Emotion, 12(3), 307-330. https://doi.org/10.1080/026999398379628
Davidson, R. J., & Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3(1), 11-21. https://doi.org/10.1016/s1364-6613(98)01265-0
de Kovel, C. G. F., Aftanas, L., Aleman, A., Alexander-Bloch, A. F., Baune, B. T., Brack, I., . . . Francks, C. (2019). No Alterations of Brain Structural Asymmetry in Major Depressive Disorder: An ENIGMA Consortium Analysis. The American Journal of Psychiatry, 176(12), 1039-1049. https://doi.org/10.1176/appi.ajp.2019.18101144
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., . . . Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968-980. https://doi.org/10.1016/j.neuroimage.2006.01.021
Dotson, V. M., Davatzikos, C., Kraut, M. A., & Resnick, S. M. (2009). Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. Journal of Psychiatry & Neuroscience, 34(5), 367-375. https://www.ncbi.nlm.nih.gov/pubmed/19721847
Dotson, V. M., & McClintock, S. M. (2020). Intersecting dimensions of cognitive and neuropsychiatric symptoms: Embarking on a new era of clinical neuropsychological research. Neuropsychology Review https://doi.org/10.1007/s11065-020-09461-5
Dotson, V. M., Resnick, S. M., & Zonderman, A. B. (2008). Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. The American Journal of Geriatric Psychiatry, 16(4), 318-330. https://doi.org/10.1097/JGP.0b013e3181662a9c
Downar, J., Geraci, J., Salomons, T. V., Dunlop, K., Wheeler, S., McAndrews, M. P., . . . Giacobbe, P. (2014). Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biological Psychiatry, 76(3), 176-185. https://doi.org/10.1016/j.biopsych.2013.10.026
Fava, G. A., & Tossani, E. (2007). Prodromal stage of major depression. Early Intervention in Psychiatry, 1(1), 9-18. https://doi.org/10.1111/j.1751-7893.2007.00005.x
Fischl, B., & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050-11055. https://doi.org/10.1073/pnas.200033797
Fischl, B., Liu, A., & Dale, A. M. (2001). Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging, 20(1), 70-80. https://doi.org/10.1109/42.906426
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., . . . Dale, A. M. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341-355. http://www.ncbi.nlm.nih.gov/pubmed/11832223
Fischl, B., Salat, D. H., van der Kouwe, A. J., Makris, N., Segonne, F., Quinn, B. T., & Dale, A. M. (2004a). Sequence-independent segmentation of magnetic resonance images. Neuroimage, 23 Suppl 1, S69-84. https://doi.org/10.1016/j.neuroimage.2004.07.016
Fischl, B., van der Kouwe, A., Destrieux, C., Halgren, E., Segonne, F., Salat, D. H., . . . Dale, A. M. (2004b). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11-22. http://www.ncbi.nlm.nih.gov/pubmed/14654453
Grieve, S. M., Korgaonkar, M. S., Koslow, S. H., Gordon, E., & Williams, L. M. (2013). Widespread reductions in gray matter volume in depression. Neuroimage Clin, 3, 332-339. https://doi.org/10.1016/j.nicl.2013.08.016
Gusnard, D. A., Raichle, M. E., & Raichle, M. E. (2001). Searching for a baseline: functional imaging and the resting human brain. Nature Reviews. Neuroscience, 2(10), 685-694. https://doi.org/10.1038/35094500
Hagmann, P., Cammoun, L., Gigandet, X., Meuli, R., Honey, C. J., Wedeen, V. J., & Sporns, O. (2008). Mapping the structural core of human cerebral cortex. PLoS Biology, 6(7), e159. https://doi.org/10.1371/journal.pbio.0060159
Hahn, B., Ross, T. J., & Stein, E. A. (2007). Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cerebral Cortex, 17(7), 1664-1671. https://doi.org/10.1093/cercor/bhl075
Hair, J. F. (2010). Multivariate data analysis (7th ed.). Prentice Hall.
Hampson, M., Driesen, N. R., Skudlarski, P., Gore, J. C., & Constable, R. T. (2006). Brain connectivity related to working memory performance. The Journal of Neuroscience, 26(51), 13338-13343. https://doi.org/10.1523/JNEUROSCI.3408-06.2006
Hoflich, A., Michenthaler, P., Kasper, S., & Lanzenberger, R. (2019). Circuit Mechanisms of Reward, Anhedonia, and Depression. The International Journal of Neuropsychopharmacology, 22(2), 105-118. https://doi.org/10.1093/ijnp/pyy081
Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D. S., Quinn, K., . . . Wang, P. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American Journal of Psychiatry, 167(7), 748-751. https://doi.org/10.1176/appi.ajp.2010.09091379
Jarnum, H., Eskildsen, S. F., Steffensen, E. G., Lundbye-Christensen, S., Simonsen, C. W., Thomsen, I. S., . . . Larsson, E. M. (2011). Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatrica Scandinavica, 124(6), 435-446. https://doi.org/10.1111/j.1600-0447.2011.01766.x
Ji, J. (2012). Distinguishing subclinical (subthreshold) depression from the residual symptoms of major depression. Shanghai Archives of Psychiatry, 24(5), 288-289. https://doi.org/10.3969/j.issn.1002-0829.2012.05.007
Kabani, N., Le Goualher, G., MacDonald, D., & Evans, A. C. (2001). Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage, 13(2), 375-380. https://doi.org/10.1006/nimg.2000.0652
Kappelmann, N., Arloth, J., Georgakis, M. K., Czamara, D., Rost, N., Ligthart, S., . . . Binder, E. B. (2020). Dissecting the association between inflammation, metabolic dysregulation, and specific depressive symptoms: A genetic correlation and 2-sample Mendelian randomization study. JAMA Psychiatry https://doi.org/10.1001/jamapsychiatry.2020.3436
Kawasaki, Y., Suzuki, M., Takahashi, T., Nohara, S., McGuire, P. K., Seto, H., & Kurachi, M. (2008). Anomalous cerebral asymmetry in patients with schizophrenia demonstrated by voxel-based morphometry. Biological Psychiatry, 63(8), 793-800. https://doi.org/10.1016/j.biopsych.2007.08.008
Keedwell, P. A., Andrew, C., Williams, S. C., Brammer, M. J., & Phillips, M. L. (2005). The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry, 58(11), 843-853. https://doi.org/10.1016/j.biopsych.2005.05.019
Kuperberg, G. R., Broome, M. R., McGuire, P. K., David, A. S., Eddy, M., Ozawa, F., . . . Fischl, B. (2003). Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry, 60(9), 878-888. https://doi.org/10.1001/archpsyc.60.9.878
Kurth, F., Gaser, C., & Luders, E. (2015). A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM). Nature Protocols, 10(2), 293-304. https://doi.org/10.1038/nprot.2015.014
Lee, Y. Y., Stockings, E. A., Harris, M. G., Doi, S. A. R., Page, I. S., Davidson, S. K., & Barendregt, J. J. (2019). The risk of developing major depression among individuals with subthreshold depression: a systematic review and meta-analysis of longitudinal cohort studies. Psychological Medicine, 49(1), 92-102. https://doi.org/10.1017/S0033291718000557
Lener, M. S., Kundu, P., Wong, E., Dewilde, K. E., Tang, C. Y., Balchandani, P., & Murrough, J. W. (2016). Cortical abnormalities and association with symptom dimensions across the depressive spectrum. Journal of Affective Disorders, 190, 529-536. https://doi.org/10.1016/j.jad.2015.10.027
Li, G., Zhang, X., Zhang, J., Wang, E., Zhang, H., & Li, Y. (2018). Magnetic resonance study on the brain structure and resting-state brain functional connectivity in primary insomnia patients. Medicine (Baltimore), 97(34), e11944. https://doi.org/10.1097/MD.0000000000011944
Li, J., Wang, Z., Hwang, J., Zhao, B., Yang, X., Xin, S., . . . Kong, J. (2017). Anatomical brain difference of subthreshold depression in young and middle-aged individuals. Neuroimage Clinical, 14, 546-551. https://doi.org/10.1016/j.nicl.2017.02.022
Lim, H. K., Jung, W. S., Ahn, K. J., Won, W. Y., Hahn, C., Lee, S. Y., . . . Lee, C. U. (2012). Regional cortical thickness and subcortical volume changes are associated with cognitive impairments in the drug-naive patients with late-onset depression. Neuropsychopharmacology, 37(3), 838-849. https://doi.org/10.1038/npp.2011.264
Ludvigsson, M., Marcusson, J., Wressle, E., & Milberg, A. (2019). Morbidity and mortality in very old individuals with subsyndromal depression: an 8-year prospective study. International Psychogeriatrics, 31(11), 1569-1579. https://doi.org/10.1017/S1041610219001480
McLaren, M. E., Szymkowicz, S. M., O'Shea, A., Woods, A. J., Anton, S. D., & Dotson, V. M. (2016). Dimensions of depressive symptoms and cingulate volumes in older adults. Translational Psychiatry, 6, e788. https://doi.org/10.1038/tp.2016.49
McLaren, M. E., Szymkowicz, S. M., O'Shea, A., Woods, A. J., Anton, S. D., & Dotson, V. M. (2017). Vertex-wise examination of depressive symptom dimensions and brain volumes in older adults. Psychiatry Research: Neuroimaging, 260, 70-75. https://doi.org/10.1016/j.pscychresns.2016.12.008
Murri, M. B., Ekkekakis, P., Menchetti, M., Neviani, F., Trevisani, F., Tedeschi, S., … Amore, M. (2018). Physical exercise for late-life depression: Effects on symptom dimensions and time course. Journal of Affective Disorders, 230, 65-70. https://doi.org/10.1016/j.jad.2018.01.004
Nielsen, F. A., Balslev, D., & Hansen, L. K. (2005). Mining the posterior cingulate: segregation between memory and pain components. NeuroImage, 27(3), 520-532. https://doi.org/10.1016/j.neuroimage.2005.04.034
Padoa-Schioppa, C. (2007). Orbitofrontal cortex and the computation of economic value. Annals of the New York Academy of Sciences, 1121, 232-253. https://doi.org/10.1196/annals.1401.011
Peterson, B. S., Warner, V., Bansal, R., Zhu, H., Hao, X., Liu, J., . . . Weissman, M. M. (2009). Cortical thinning in persons at increased familial risk for major depression. Proceedings of the National Academy of Sciences of the United States of America, 106(15), 6273-6278. https://doi.org/10.1073/pnas.0805311106
Peterson, B. S., & Weissman, M. M. (2011). A brain-based endophenotype for major depressive disorder. Annual Review of Medicine, 62, 461-474. https://doi.org/10.1146/annurev-med-010510-095632
Postema, M. C., van Rooij, D., Anagnostou, E., Arango, C., Auzias, G., Behrmann, M., . . . Francks, C. (2019). Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nature Communications, 10(1), 4958. https://doi.org/10.1038/s41467-019-13005-8
Qiu, L., Lui, S., Kuang, W., Huang, X., Li, J., Li, J., . . . Gong, Q. (2014). Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Translational Psychiatry, 4, e378. https://doi.org/10.1038/tp.2014.18
Quinn, C. R., Rennie, C. J., Harris, A. W., & Kemp, A. H. (2014). The impact of melancholia versus non-melancholia on resting-state, EEG alpha asymmetry: electrophysiological evidence for depression heterogeneity. Psychiatry Research, 215(3), 614-617. https://doi.org/10.1016/j.psychres.2013.12.049
Radloff, L. S. (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385-401. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=1979-10129-001&site=ehost-live
Rajkowska, G., & Miguel-Hidalgo, J. J. (2007). Gliogenesis and glial pathology in depression. CNS & Neurological Disorders Drug Targets, 6(3), 219-233. https://doi.org/10.2174/187152707780619326
Reid, S. A., Duke, L. M., & Allen, J. J. (1998). Resting frontal electroencephalographic asymmetry in depression: inconsistencies suggest the need to identify mediating factors. Psychophysiology, 35(4), 389-404. https://doi.org/10.1111/1469-8986.3540389
Reuter, M., Rosas, H. D., & Fischl, B. (2010). Highly accurate inverse consistent registration: a robust approach. Neuroimage, 53(4), 1181-1196. https://doi.org/10.1016/j.neuroimage.2010.07.020
Rosas, H. D., Liu, A. K., Hersch, S., Glessner, M., Ferrante, R. J., Salat, D. H., . . . Fischl, B. (2002). Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology, 58(5), 695-701. http://www.ncbi.nlm.nih.gov/pubmed/11889230
Salat, D. H., Buckner, R. L., Snyder, A. Z., Greve, D. N., Desikan, R. S., Busa, E., . . . Fischl, B. (2004). Thinning of the cerebral cortex in aging. Cerebral Cortex, 14(7), 721-730. https://doi.org/10.1093/cercor/bhh032
Schmaal, L., Hibar, D. P., Samann, P. G., Hall, G. B., Baune, B. T., Jahanshad, N., . . . Veltman, D. J. (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular Psychiatry, 22(6), 900-909. https://doi.org/10.1038/mp.2016.60
Segonne, F., Dale, A. M., Busa, E., Glessner, M., Salat, D., Hahn, H. K., & Fischl, B. (2004). A hybrid approach to the skull stripping problem in MRI. Neuroimage, 22(3), 1060-1075. https://doi.org/10.1016/j.neuroimage.2004.03.032
Segonne, F., Pacheco, J., & Fischl, B. (2007). Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging, 26(4), 518-529. https://doi.org/10.1109/TMI.2006.887364
Shah, P., Bassett, D. S., Wisse, L. E. M., Detre, J. A., Stein, J. M., Yushkevich, P. A., . . . Davis, K. A. (2019). Structural and functional asymmetry of medial temporal subregions in unilateral temporal lobe epilepsy: A 7T MRI study. Human Brain Mapping, 40(8), 2390-2398. https://doi.org/10.1002/hbm.24530
Sled, J. G., Zijdenbos, A. P., & Evans, A. C. (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging, 17(1), 87-97. https://doi.org/10.1109/42.668698
Suh, J. S., Schneider, M. A., Minuzzi, L., MacQueen, G. M., Strother, S. C., Kennedy, S. H., & Frey, B. N. (2019). Cortical thickness in major depressive disorder: A systematic review and meta-analysis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 88, 287-302. https://doi.org/10.1016/j.pnpbp.2018.08.008
Szymkowicz, S. M., McLaren, M. E., Kirton, J. W., O'Shea, A., Woods, A. J., Manini, T. M., .… Dotson, V. M. (2016). Depressive symptom severity is associated with increased cortical thickness in older adults. International Journal of Geriatric Psychiatry, 31(4), 325-333. https://doi.org/10.1002/gps.4324
Thibodeau, R., Jorgensen, R. S., & Kim, S. (2006). Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. Journal of Abnormal Psychology, 115(4), 715-729. https://doi.org/10.1037/0021-843X.115.4.715
Tuithof, M., Ten Have, M., van Dorsselaer, S., Kleinjan, M., Beekman, A., & de Graaf, R. (2018). Course of subthreshold depression into a depressive disorder and its risk factors. Journal of Affective Disorders, 241, 206-215. https://doi.org/10.1016/j.jad.2018.08.010
Tully, P. J., Debette, S., Mazoyer, B., & Tzourio, C. (2017). White Matter Lesions are Associated with Specific Depressive Symptom Trajectories among Incident Depression and Dementia Populations: Three-City Dijon MRI Study. The American Journal of Geriatric Psychiatry, 25(12), 1311-1321. https://doi.org/10.1016/j.jagp.2017.06.003
van Eijndhoven, P., van Wingen, G., Katzenbauer, M., Groen, W., Tepest, R., Fernandez, G., . . . Tendolkar, I. (2013). Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. The American Journal of Psychiatry, 170(12), 1477-1486. https://doi.org/10.1176/appi.ajp.2013.12121504
Vogt, B. A., & Laureys, S. (2005). Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Progress in Brain Research, 150, 205-217. https://doi.org/10.1016/S0079-6123(05)50015-3
Wade, B. S. C., Sui, J., Hellemann, G., Leaver, A. M., Espinoza, R. T., Woods, R. P., . . . Narr, K. L. (2017). Inter and intra-hemispheric structural imaging markers predict depression relapse after electroconvulsive therapy: a multisite study. Translational Psychiatry, 7(12), 1270. https://doi.org/10.1038/s41398-017-0020-7
Weissman, M. M., Sholomskas, D., Pottenger, M., Prusoff, B. A., & Locke, B. Z. (1977). Assessing depressive symptoms in five psychiatric populations: a validation study. American Journal of Epidemiology, 106(3), 203-214. https://doi.org/10.1093/oxfordjournals.aje.a112455
Whitford, T. J., Lee, S. W., Oh, J. S., de Luis-Garcia, R., Savadjiev, P., Alvarado, J. L., … Shenton, M. E. (2014). Localized abnormalities in the cingulum bundle in patients with schizophrenia: a Diffusion Tensor tractography study. Neuroimage Clinical, 5, 93-99. https://doi.org/10.1016/j.nicl.2014.06.003
Winkler, A. M., Kochunov, P., Blangero, J., Almasy, L., Zilles, K., Fox, P. T., . . . Glahn, D. C. (2010). Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage, 53(3), 1135-1146. https://doi.org/10.1016/j.neuroimage.2009.12.028
Wise, T., Radua, J., Via, E., Cardoner, N., Abe, O., Adams, T. M., . . . Arnone, D. (2017). Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Molecular Psychiatry, 22(10), 1455-1463. https://doi.org/10.1038/mp.2016.72
Wittling, W., & Roschmann, R. (1993). Emotion-related hemisphere asymmetry: subjective emotional responses to laterally presented films. Cortex, 29(3), 431-448. https://doi.org/10.1016/s0010-9452(13)80252-3
Wu, Y., Zhuang, Y., & Qi, J. (2020). Explore structural and functional brain changes in insomnia disorder: A PRISMA-compliant whole brain ALE meta-analysis for multimodal MRI. Medicine (Baltimore), 99(14), e19151. https://doi.org/10.1097/MD.0000000000019151
Yang, X. H., Wang, Y., Huang, J., Zhu, C. Y., Liu, X. Q., Cheung, E. F., . . . Chan, R. C. (2015). Increased prefrontal and parietal cortical thickness does not correlate with anhedonia in patients with untreated first-episode major depressive disorders. Psychiatry Research, 234(1), 144-151. https://doi.org/10.1016/j.pscychresns.2015.09.014
Zhang, F. F., Peng, W., Sweeney, J. A., Jia, Z. Y., & Gong, Q. Y. (2018). Brain structure alterations in depression: Psychoradiological evidence. CNS Neuroscience & Therapeutics, 24(11), 994-1003. https://doi.org/10.1111/cns.12835
Zhang, T., Zhao, B., Shi, C., Nie, B., Liu, H., Yang, X., . . . Shan, B. (2020). Subthreshold depression may exist on a spectrum with major depressive disorder: Evidence from gray matter volume and morphological brain network. Journal of Affective Disorders, 266, 243-251. https://doi.org/10.1016/j.jad.2020.01.135
Zuo, Z., Ran, S., Wang, Y., Li, C., Han, Q., Tang, Q., . . . Li, H. (2019). Asymmetry in cortical thickness and subcortical volume in treatment-naive major depressive disorder. Neuroimage Clin, 21, 101614. https://doi.org/10.1016/j.nicl.2018.101614
Acknowledgments
This project was supported by an Age Related Memory Loss award from the McKnight Brain Research Foundation (VMD) and by the Health Resources and Service Administration (D40HP33346; HRB and ZT). AMG is supported by the Georgia State University Brains & Behavior graduate student fellowship. Neuroimaging was performed at the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility in the McKnight Brain Institute of the University of Florida, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida. The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Open Practices Statement
None of the data or materials for the experiments reported here is available, and none of the experiments was preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dotson, V.M., Taiwo, Z., Minto, L.R. et al. Orbitofrontal and Cingulate Thickness Asymmetry Associated with Depressive Symptom Dimensions. Cogn Affect Behav Neurosci 21, 1297–1305 (2021). https://doi.org/10.3758/s13415-021-00923-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-021-00923-8