Abstract
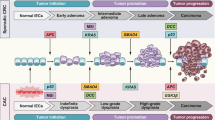
Ulcerative colitis and Crohn's disease (together known as Inflammatory Bowel Disease or IBD) are both associated with increased risk for colorectal cancer. Although it is conventional to emphasise differences between IBD-associated and sporadic colon cancer, such as a lower rate of Adenomatosis Polyposis Coli mutations and earlier p53 mutations, IBD-associated cancer has a similar dysplasia-cancer sequence to sporadic colon cancer, similar frequencies of major chromosomal abnormalities and of microsatellite instability and similar glycosylation changes. This suggests that IBD-associated colon cancer and sporadic colon cancer might have similar pathogenic mechanisms. Because the normal colon is arguably in a continual state of low-grade inflammation in response to its microbial flora, it is reasonable to suggest that both IBD-associated and sporadic colon cancer may be the consequence of bacteria-induced inflammation. We have speculated that the glycosylation changes might result in recruitment to the mucosa of bacterial and dietary lectins that might otherwise pass harmlessly though the gut lumen. These could then lead to increased inflammation and/or proliferation and thence to ulceration or cancer. The glycosylation changes include increased expression of onco-fetal carbohydrates, such as the galactose-terminated Thomsen-Friedenreich antigen (Galβ1,3GalNAcα-), increased sialylation of terminal structures and reduced sulphation. These changes cannot readily be explained by alterations in glycosyltransferase activity but similar changes can be induced in vitro by alkalinisation of the Golgi lumen. Consequences of these changes may be relevant not only for cell-surface glycoconjugates but also for intracellular glycoconjugates.
Similar content being viewed by others
References
Shanahan F, Inflammatory bowel disease: Immunodiagnostics, immunotherapeutics, and ecotherapeutics, Gastroenterology 120, 622–35 (2001).
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH, A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease, Nature 411, 603–6 (2001).
Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G, Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease, Nature 411, 599–603 (2001).
Ekbom A, Helmick C, Zack M, Adami HO, Increased risk of largebowel cancer in Crohn's disease with colonic involvement, Lancet 336, 357–9 (1990).
Collier PE, Turowski P, Diamond DL, Small intestinal adenocarcinoma complicating regional ileitis, Cancer 55, 516–21 (1985).
Askling J, Dickman PW, Karlen P, Brostrom O, Lapidus A, Lofberg R, Ekbom A, Colorectal cancer rates among first-degree relatives of patients with inflammatory bowel disease: A population-based cohort study, Lancet 357, 262–6 (2001).
Rhodes JM, Yu LG, Glycosylation and disease, In Encyclopaedia of Life Sciences, http://www.els.net. (Nature Publishing Group, London, 2000.)
Boland CR, Deshmukh GD, The carbohydrate composition of mucin in colonic cancer, Gastroenterology 98, 1170–7 (1990).
Campbell BJ, Hounsell E, Finnie IA, Rhodes JM, Direct demonstration of increased expression of Thomsen-Friedenreich antigen (Galβ1-3GalNAc) by mucus in colon cancer and inflammatory bowel disease, J Clin Invest 95, 571–6 (1995).
Karlen P, Young E, Brostrom O, Lofberg R, Tribukait B, Ost K, Bodian C, Itzkowitz S, Sialyl-Tn antigen as a marker of colon cancer risk in ulcerative colitis: Relation to dyplasia and DNA aneuploidy, Gastroenterology 115, 1395–404 (1998).
Itzkowitz SH, Marshall A, Kornbluth A, Harpaz N, McHugh JB, Ahnen D, Sachar DB, Sialosyl-Tn antigen: Initial report of a new marker of malignant progression in long-standing ulcerative colitis, Gastroenterology 109, 490–7 (1995).
Raouf AH, Tsai HH, Parker N, Hoffman J, Walker RJ, Rhodes JM, Sulphation of colonic and rectal mucin in inflammatory bowel disease: Reduced sulphation of rectal mucus in ulcerative colitis, Clin Sci 83, 623–6 (1992).
Corfield AP, Myerscough N, Bradfield N, Corfield Cdo A, Gough M, Clamp JR, Durdey P, Warren BF, Bartolo DC, King KR, Williams JM, Colonic mucins in ulcerative colitis: Evidence for loss of sulfation, Glycoconj J 13, 809–22 (1996).
Filipe MI, Mucins in the human gastrointestinal epithelium: A review, Invest Cell Pathol 2, 195–216 (1979).
Probert CS, Warren BF, Perry T, Mackay EH, Mayberry JF, Corfield AP, South Asian and European colitics show characteristic differences in colonic mucus glycoprotein type and turnover, Gut 36, 696–702 (1995).
Kim YS, Yuan M, Itzkowitz SH, Sun Q, Kaizu T, Palekar A, Trump BF, Hakamori S, Expression of Le Y and extended Le Y blood group antigens in human malignant, premalignant and non-malignant colonic tissues, Cancer Res 46, 598–609 (1986).
Parker N, Tsai HH, Ryder SD, Raouf AH, Rhodes JM, Increased rate of sialylation of colonic mucin by cultured ulcerative colitis mucosal explants, Digestion 56, 52–6 (1995).
Chalifoux LV, Bronson RT, Colonic adenocarcinoma associated with chronic colitis in cotton top marmosets, Saguinus oedipus. Gastroenterology 80, 942–6 (1981).
Podolsky DK, Madara JL, King N, Sehgal P, Moore R, Winter HS, Colonic mucin composition in primates. Selective alterations associated with spontaneous colitis in the cotton-top tamarin, Gastroenterology 88, 20–5 (1985).
Raouf AH, Parker N, Iddon D, Ryder S, Langdon-Brown B, Milton JD, Walker R, Rhodes JM, Ion-exchange chromatography of puri-fied colonic mucus glycoproteins in inflammatory bowel disease: Absence of a selective subclass defect, Gut 32, 1139–46 (1991).
Moore R, King N, Alroy J, Differences in cellular glycoconjugates of quiescent, inflamed, and neoplastic colonic epithelium in colitis and cancer-prone tamarins, Am J Pathol 131, 484–9 (1988).
Boland CR, Clapp NK, Glycoconjugates in the colons of new world monkeys with spontaneous colitis. Association between in-flammation and neoplasia, Gastroenterology 92, 625–34 (1987).
Moore R, King N, Alroy J, Characterization of colonic cellular glycoconjugates in colitis and cancer-prone tamarins versus colitis and cancer-resistant primates, Am J Pathol 131, 477–83 (1988).
Singh R, Campbell BJ, Yu L-G, Fernig DG, Milton JD, Goodlad RA, FitzGerald AJ, Rhodes JM, Cell surface-expressed Thomsen-Friedenreich antigen in colon cancer is predominantly carried on high molecular weight splice variants of CD44, Glycobiology 11, 587–92 (2001).
Goupille C, Hallouin F, Meflah K, Le Pendu J, Increase of rat colon carcinoma cells tumorigenicity by α(1-2) fucosyltransferase gene transfection, Glycobiology 7, 221–9 (1997).
Camacho FI, Munoz C, Sanchez-Verde L, Saez AI, Alcantara M, Rodriguez R, CD44v6 expression in inflammatory bowel disease is associated with activity detected by endoscopy and pathological features, Histopathology 35, 144–9 (1999).
Martinez-Menarguez JA, Ballesta J, Aviles M, Madrid JF, Castells MT, Influence of sulphate groups in the binding of peanut agglutinin. Histochemical demonstration with light-and electronmicroscopy, Histochem J 24, 207–16 (1992).
Kuhns W, Jain RK, Matta KL, Paulsen H, Baker MA, Geyer R, Brockhausen I, Characterization of a novel mucin sulphotransferase activity synthesizing sulphated O-glycan core 1,3-sulphate-Gal beta 1-3GalNAc alpha-R, Glycobiology 5, 689–97 (1995).
Yang JM, Byrd JC, Siddiki BB, Chung YS, Okuno M, Sowa M, Kim YS, Matta KL, Brockhausen I, Alterations of O-glycan biosynthesis in human colon cancer tissues, Glycobiology 4, 873–84 (1994).
Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger EG, Warren G, Nilsson T, Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides, J Cell Sci 108, 1617–27 (1995).
Rottger S, White J, Wandall HH, Olivo J-C, Stark A, Bennett EP, Whitehouse C, Berger EG, Clausen H, Nilsson T, Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus, J Cell Sci 111, 45–60 (1998).
Zhang Y, Doranz B, Yankaskas JR, Engelhardt JF, Genotypic analysis of respiratory mucous sulphation defects in cystic fibrosis, J Clin Invest 96, 2997–3004 (1995).
Rhodes JM, Campbell BJ, Inflammation and colorectal cancer: Inflammatory bowel disease-associated cancer and sporadic cancer compared. Trends Mol Med 8, 10–6 (2002).
Glickman J, Croen K, Kelly S, Al-Awqati Q, Golgi membranes contain an electrogenic H+ pump in parallel to a chloride conductance, J Cell Biol 97, 1303–8 (1983).
Wu MM, Grabe M, Adams S, Tsien RY, Moore HP, Machen TE, Mechanisms of pH regulation in the regulated secretory pathway, J Biol Chem 276, 33027–35 (2001).
Bowman EJ, Siebers A, Altendorf K, Bafilomycins: A class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells, Proc Natl Acad Sci USA 85, 7972–6 (1988).
Campbell BJ, Rowe G, Leiper K, Rhodes JM, Increasing the intra-Golgi pH of cultured LS174T goblet-differentiatied cells mimics the decreased mucin sulphation and increased Thomsen-Friedenreich antigen (Galβ1-3GalNAcα-) expression seen in colon cancer, Glycobiology 11, 385–93 (2001).
Axelsson MAB, Karlsson NG, Steel DM, Ouwendiijk J, Nilsson T, Hansson GC, Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins, Glycobiology 11, 633–44 (2001).
Schindler M, Grabski S, Hoff E, Simon SM, Defective pH regulation of acidic compartments in human breast cancer cells (MCF-7) is normalized in adriamycin-resistant cells (MCF-7adr), Biochemistry 35, 2811–7 (1996).
Poschet JF, Boucher JC, Tatterson L, Skidmore J, Van Dyke RW, Deretic V, Molecular basis for defective glycosylation and Pseudomonas pathogenesis in cystic fibrosis lung, Proc Natl Acad Sci 98, 13972–7 (2001).
Campbell BJ, Oxley C, Singh R, Yu L-G, Rhodes JM, TNF-alpha decreases the sulphation of mucins and CD44 in human colonic epithelial cells: An effect which may explain the low mucosal sulphation seen in inflammatory bowel disease, Gastroenterology 118, A3836 (abstract) (2000).
Campbell BJ, Krishna Y, Rhodes JM, TNF-α causes reduced mucin synthesis and increased oncofetal carbohydrate expression by HT29-MTX cells, Gastroenterology 122, T1044 (abstract) (2002).
Kuninaka S, Yano T, Yokoyama H, Fukuyama Y, Terazaki Y, Uehara T, Kanematsu T, Asoh H, Ichinose Y, Direct influences of pro-inflammatory cytokines (IL-β, TNF-α, IL-6) on the proliferation and cell-surface antigen expression of cancer cells, Cytokine 12, 8–11 (2000).
Flieger D, Hoff AS, Sauerbruch T, Schmidt-Wolf IGH, Influence of cytokines, monoclonal antibodies and chemotherapeutic drugs on epithelial cell adhesion molecule (EpCAM) and Lewisy antigen expression, Clin Exp Immunol 123, 9–14 (2001).
Delmotte P, Degroote S, Lafitte JJ, Lamblin G, Perini JM, Roussel P, Tumor necrosis factor alpha increases the expression of glycosyltransferases and sulfotransferases responsible for the biosynthesis of sialylated and/or sulfated Lewis x epitopes in the human bronchial mucosa, J Biol Chem 277, 424–31 (2002).
Trejdosiewicz LK, Morton R, Yang Y, Banks RE, Selby PJ, Southgate J, Interleukin 4 and 13 upregulate expression of CD44 in human colonic epithelial cell-lines, Cytokine 10, 756–65 (1998).
Rosenberg WMC, MacDonald D, CD44 regulation and function in ulcerative colitis, Gut (suppl I) 48, A272 (abstract) (2001).
Wimmenauer S, Steiert A, Wolff-Vorbeck G, Xing B, Baier PK, Ruckauer KD, Kirste G, von Kleist S, Influence of cytokines on the expression of fas ligand and CD44 splice variants in colon carcinoma cells, Tumour Biol 20, 294–303 (1999).
Jobin C, Sartor RB, The IκB/NF-κB system: A key determinant of mucosal inflammation and protection, Am J Physiol 278, C451–62 (2000).
Neurath MF, Fuss I, Schurmann G, Pettersson S, Arnold K, Muller-Lobeck H, Strober W, Herfarth C, Buschenfelde KH, Cytokine gene transcription by NF-κB family members in patients with inflammatory bowel disease, Ann NY Acad Sci 859, 149–59 (1998).
Agoff SN, Brentnall TA, Crispin DA, Taylor SL, Raaka S, Haggitt RC, Reed MW, Afonina IA, Rabinovitch PS, Stevens AC, Feng Z, Bronner MP, The role of cyclooxygenase 2 in ulcerative colitisassociated neoplasia, Am J Pathol 157, 737–45 (2000).
Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D, Enterocolitis and colon cancer in interleukin 10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses, J Clin Invest 98, 1010–20 (1996).
Rennick DM, Fort MM, Lessons from genetically engineered animal models. XII. IL-10 deficient [IL-10(—/—)] mice and intestinal inflammation, Am J Physiol 278, G829–33 (2000).
Okada F, Kawaguchi T, Habelhah H, Kobayashi T, Tazawa H, Takeichi N, Kitagawa T, Hosokawa M, Conversion of human colonic adenoma cells to adenocarcinoma cells through inflammation in nude mice, Lab Invest 80, 1617–28 (2000).
Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J, Colorectal cancer prevention in ulcerative colitis: A case-control study, Aliment Pharmacol Ther 14, 145–53 (2000).
Ryder SD, Jacyna MR, Levi AJ, Rizzi PM, Rhodes JM, Eating peanuts increases rectal proliferation in individuals with mucosal expression of peanut lectin receptor, Gastroenterology 114, 44–9 (1998).
Rhodes JM, Unifying hypothesis for inflammatory bowel disease and related colon cancer: Sticking the pieces together with sugar, Lancet 347, 40–4 (1996).
Evans RC, Fear S, Ashby D, Hackett A, Williams E, van der Vliet M, Dunstan FDJ, Rhodes JM, Diet and colorectal cancer: An investigation of the lectin/galactose hypothesis, Gastroenterology 122, 1784–92 (2002).
Yu LG, Jansson B, Fernig DG, Milton JD, Smith JA, Gerasimenko OV, Jones M, Rhodes JM, Stimulation of proliferation in human colon cancer cells by human monoclonal antibodies against the TF antigen (Galactose β 1-3 N-Acetyl-galactosamine), Int J Cancer 73, 424–31 (1997).
Yu LG, Fernig DG, Smith JA, Milton JD, Rhodes JM, Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin, Cancer Res 53, 4627–32 (1993).
Yu LG, Fernig DG, White MRH, Spiller DG, Appleton P, Evans RC, Grierson I, Smith JA, Davies H, Gerasimenko OV, Petersen O, Milton JD, Rhodes JM, Edible mushroom (Agaricus bisporus) lectin, which reversibly inhibits epithelial cell proliferation, blocks NLS-dependent nuclear protein import, J Biol Chem 274, 4890–9 (1999).
Yu LG, Andrews N, Weldon M, Gerasimenko OV, Campbell BJ, Singh R, Grierson I, Petersen OH, Rhodes JM, An N-terminal truncated form of Orp150 is a cytoplasmic ligand for the antiproliferative mushroom Agaricus bisporus lectin and is required for NLS-dependent nuclear protein import, J Biol Chem 277, 24538–45 (2002).
Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, Lochs H, Mucosal flora in inflammatory bowel disease, Gastroenterology 122, 44–54 (2002).
Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF, Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease, Gastroenterology 115, 1405–13 (1998).
Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A, Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease, Infect Immun 67, 4499–509 (1999).
Swidsinski A, Khilkin M, Kerjaschki D, Schreiber S, Ortner M, Weber J, Lochs H, Association between intraepithelial Escherichia coli and colorectal cancer, Gastroenterology 115, 281–6 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Campbell, B.J., Yu, LG. & Rhodes, J.M. Altered glycosylation in inflammatory bowel disease: A possible role in cancer development. Glycoconj J 18, 851–858 (2001). https://doi.org/10.1023/A:1022240107040
Issue Date:
DOI: https://doi.org/10.1023/A:1022240107040