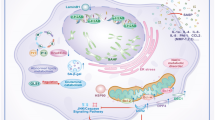
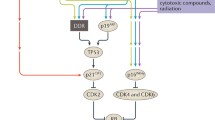
Abstract
Somatic cellular senescence (SCS) describes the limited ability of cells to divide. Normally, SCS is associated with physiological aging, but evidence suggests that it may play a role in disease progression, even in young patients. Stressors such as acute injury or chronic inflammation may induce SCS, which in turn exhausts organ regenerative potential. This review summarizes what is known about SCS in the kidney with aging and disease. As most patients with chronic kidney disease (CKD) also develop cardiovascular complications, a second focus of this review deals with the role of SCS in cardiovascular disease. Also, as SCS seems to accelerate CKD and cardiovascular disease progression, developing strategies for new treatment options that overcome SCS or protect a patient from it represents an exciting challenge.




Similar content being viewed by others
Abbreviations
- ACE:
-
angiotensin-converting enzyme
- ANRIL:
-
antisense noncoding RNA in the INK4 locus
- ApoE:
-
apolipoprotein E
- ARF:
-
alternate open reading frame
- ATM:
-
ataxia telangiectasia mutated
- ATR:
-
ataxia telangiectasia mutated and Rad3-related
- CIP1:
-
cyclin-dependent kinase-interacting protein 1
- CKD:
-
chronic kidney disease
- CRP:
-
C-reactive protein
- eNOS:
-
endothelial nitric oxide synthase
- HDM2:
-
human double minute 2
- HUS:
-
hemolytic uremic syndrome
- INK4:
-
inhibitor of cyclin-dependent kinase 4
- KIP:
-
cyclin-dependent kinase-inhibitory protein
- MAPK:
-
mitogen-activated protein kinase
- MDM2:
-
mouse double minute 2
- mTOR:
-
mammalian target of rapamycin
- NO:
-
nitric oxide
- PBMC:
-
peripheral blood mononuclear cell
- PIKK:
-
phosphatidylinositol 3 kinase-like kinases
- Rb:
-
retinoblastoma protein
- S6K1:
-
ribosomal protein s6 kinase 1
- SA-β-GAL:
-
senescence-associated-β-galactosidase
- SCS:
-
somatic cellular senescence
- SNP:
-
single nucleotide polymorphism
- SOD2:
-
partial mitochondrial superoxide dismutase
- STASIS:
-
stimulation and stress-induced senescence-like arrest
- WAF1:
-
wild-type p53-activated fragment 1
References
Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F (2002) Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106:100–105
Schmitt R, Cantley LG (2008) The impact of aging on kidney repair. Am J Physiol Renal Physiol 294:F1265–F1272
Chkhotua A, Shohat M, Tobar A, Magal N, Kaganovski E, Shapira Z, Yussim A (2002) Replicative senescence in organ transplantation-mechanisms and significance. Transpl Immunol 9:165–171
Westhoff JH, Schildhorn C, Jacobi C, Homme M, Hartner A, Braun H, Kryzer C, Wang C, Von ZT, Kranzlin B, Gretz N, Melk A (2010) Telomere shortening reduces regenerative capacity after acute kidney injury. J Am Soc Nephrol 21:327–336
Halloran PF, Melk A, Barth C (1999) Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol 10:167–181
Melk A, Schmidt BM, Braun H, Vongwiwatana A, Urmson J, Zhu LF, Rayner D, Halloran PF (2009) Effects of donor age and cell senescence on kidney allograft survival. Am J Transplant 9:114–123
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu C-P, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352
Deng Y, Chan SS, Chang S (2008) Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer 8:450–458
Meek DW (2009) Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer 9:714–723
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Chen JH, Stoeber K, Kingsbury S, Ozanne SE, Williams GH, Hales CN (2004) Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. J Biol Chem 279:49439–49446
Wright WE, Shay JW (2000) Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat Med 6:849–851
Wright WE, Shay JW (2002) Historical claims and current interpretations of replicative aging. Nat Biotechnol 20:682–688
Weinberg RA (1995) The retinoblastoma protein and cell cycle control. Cell 81:323–330
Serrano M, Lee HW, Chin L, CordonCardo C, Beach D, DePinho RA (1996) Role of the INK4a locus in tumor suppression and cell mortality. Cell 85:27–37
Ramirez RD, Morales CP, Herbert BS, Rohde JM, Passons C, Shay JW, Wright WE (2001) Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev 15:398–403
Carnero A, Hudson JD, Price CM, Beach DH (2000) p16(INK4A) and p19(ARF) act in overlapping pathways in cellular immortalization. Nat Cell Biol 2:148–155
Krimpenfort P, Quon KC, Mooi WJ, Loonstra A, Berns A (2001) Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413:83–86
Wei W, Hemmer RM, Sedivy JM (2001) Role of p14(ARF) in replicative and induced senescence of human fibroblasts. Mol Cell Biol 21:6748–6757
Ben-Porath I, Weinberg RA (2005) The signals and pathways activating cellular senescence. Int J Biochem Cell Biol 37:961–976
Kurz DJ, Decary S, Hong Y, Erusalimsky JD (2000) Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113:3613–3622
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92:9363–9367
Itahana K, Campisi J, Dimri GP (2007) Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol 371:21–31
Terman A, Gustafsson B, Brunk UT (2007) Autophagy, organelles and ageing. J Pathol 211:134–143
Narita M, Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA, Myers MP, Lowe SW (2006) A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell 126:503–514
Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW (2003) Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113:703–716
Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J (2003) Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 22:4212–4222
Melk A, Ramassar V, Helms LMH, Moore R, Rayner D, Solez K, Halloran PF (2000) Telomere shortening in kidneys with age. J Am Soc Nephrol 11:444–453
Ferlicot S, Durrbach A, Ba N, Desvaux D, Bedossa P, Paradis V (2003) The role of replicative senescence in chronic allograft nephropathy. Hum Pathol 34:924–928
Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BMW, Halloran PF (2003) Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int 63:2134–2143
Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF (2004) Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65:510–520
Chkhotua AB, Gabusi E, Altimari A, D'Errico A, Yakubovich M, Vienken J, Stefoni S, Chieco P, Yussim A, Grigioni WF (2003) Increased expression of p16(INK4a) and p27(Kip1) cyclin-dependent kinase inhibitor genes in aging human kidney and chronic allograft nephropathy. Am J Kidney Dis 41:1303–1313
Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al Regaiey K, Su L, Sharpless NE (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114:1299–1307
Sitte N, Merker K, Grune T, von Zglinicki T (2001) Lipofuscin accumulation in proliferating fibroblasts in vitro: an indicator of oxidative stress. Exp Gerontol 36:475–486
Christensen EI, Verroust PJ (2008) Interstitial fibrosis: tubular hypothesis versus glomerular hypothesis. Kidney Int 74:1233–1236
Strutz FM (2009) EMT and proteinuria as progression factors. Kidney Int 75:475–481
Nath KA (1992) Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20:1–17
Chkhotua AB, Schelzig H, Wiegand P, Grosse S, Reis S, Art M, Abendroth D (2005) Influence of ischaemia/reperfusion and LFA-1 inhibition on telomere lengths and CDKI genes in ex vivo haemoperfusion of primate kidneys. Transpl Int 17:692–698
Schelzig H, Chkhotua AB, Wiegand P, Grosse S, Reis S, Art M, Abendroth D (2003) Effect of ischemia/reperfusion on telomere length and CDKI genes expression in a concordant ex-vivo hemoperfusion model of primate kidneys. Ann Transplant 8:17–21
Hochegger K, Koppelstaetter C, Tagwerker A, Huber JM, Heininger D, Mayer G, Rosenkranz AR (2007) p21 and mTERT are novel markers for determining different ischemic time periods in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 292:F762–F768
Chkhotua AB, Abendroth D, Froeba G, Schelzig H (2006) Up-regulation of cell cycle regulatory genes after renal ischemia/reperfusion: differential expression of p16(INK4a), p21(WAF1/CIP1) and p27(Kip1) cyclin-dependent kinase inhibitor genes depending on reperfusion time. Transpl Int 19:72–77
Joosten SA, Van HV, Nolan CE, Borrias MC, Jardine AG, Shiels PG, Van Kooten C, Paul LC (2003) Telomere shortening and cellular senescence in a model of chronic renal allograft rejection. Am J Pathol 162:1305–1312
Westhoff JH, Hilgers KF, Stenbach MP, Hartner A, Klanke B, Amann K, Melk A (2008) Hypertension induces somatic cellular senescence in rat and human by induction of cell cycle inhibitor p16INK4a. Hypertension 52:123–129
Baumann M, Bartholome R, Peutz-Kootstra CJ, Smits JF, Struijker-Boudier HA (2008) Sustained tubulo-interstitial protection in SHRs by transient losartan treatment: an effect of decelerated aging? Am J Hypertens 21:177–182
Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, Vaziri ND (2007) Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol 102:255–260
Blasco MA, Lee H-W, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25–34
Kown MH, Murata S, Jahncke CL, Mari C, Berry GJ, Lijkwan MA, Blankenberg FG, Strauss HW, Robbins RC (2002) Donor cardiac allografts from p53 knockout mice exhibit apoptosis-independent prolongation of survival. Transplant Proc 34:3274–3276
Melk A, Schmidt BMW, Vongwiwatana A, Rayner DC, Halloran PF (2005) Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am J Transplant 5:1375–1382
Sis B, Tasanarong A, Khoshjou F, Dadras F, Solez K, Halloran PF (2007) Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int 71:218–226
Szeto CC, Poon PY, Lai FM, Chow KM, Szeto CY, Li PK (2005) Chromosomal telomere shortening of kidney cells in IgA nephropathy by the measurement of DNA in urinary sediment. Clin Nephrol 64:337–342
Bergmann MW, Zelarayan L, Gehrke C (2008) Treatment-sensitive premature renal and heart senescence in hypertension. Hypertension 52:61–62
Koppelstaetter C, Schratzberger G, Perco P, Hofer J, Mark W, Ollinger R, Oberbauer R, Schwarz C, Mitterbauer C, Kainz A, Karkoszka H, Wiecek A, Mayer B, Mayer G (2008) Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation. Aging Cell 7:491–497
McGlynn LM, Stevenson K, Lamb K, Zino S, Brown M, Prina A, Kingsmore D, Shiels PG (2009) Cellular senescence in pretransplant renal biopsies predicts postoperative organ function. Aging Cell 8:45–51
El-Husseini A, Sabry A, Zahran A, Shoker A (2007) Can donor implantation renal biopsy predict long-term renal allograft outcome? Am J Nephrol 27:144–151
Mitsnefes MM (2008) Cardiovascular complications of pediatric chronic kidney disease. Pediatr Nephrol 23:27–39
Schiffrin EL, Lipman ML, Mann JF (2007) Chronic kidney disease: effects on the cardiovascular system. Circulation 116:85–97
Comi P, Chiaramonte R, Maier JA (1995) Senescence-dependent regulation of type 1 plasminogen activator inhibitor in human vascular endothelial cells. Exp Cell Res 219:304–308
Hoffmann J, Haendeler J, Aicher A, Rossig L, Vasa M, Zeiher AM, Dimmeler S (2001) Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res 89:709–715
Maier JA, Statuto M, Ragnotti G (1993) Senescence stimulates U937-endothelial cell interactions. Exp Cell Res 208:270–274
Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS (2001) eNOS activity is reduced in senescent human endothelial cells: Preservation by hTERT immortalization. Circ Res 89:793–798
Nakajima M, Hashimoto M, Wang F, Yamanaga K, Nakamura N, Uchida T, Yamanouchi K (1997) Aging decreases the production of PGI2 in rat aortic endothelial cells. Exp Gerontol 32:685–693
Sato I, Morita I, Kaji K, Ikeda M, Nagao M, Murota S (1993) Reduction of nitric oxide producing activity associated with in vitro aging in cultured human umbilical vein endothelial cell. Biochem Biophys Res Commun 195:1070–1076
van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF (2000) Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192:1731–1744
Chen J, Brodsky SV, Goligorsky DM, Hampel DJ, Li H, Gross SS, Goligorsky MS (2002) Glycated collagen I induces premature senescence-like phenotypic changes in endothelial cells. Circ Res 90:1290–1298
Fenton M, Barker S, Kurz DJ, Erusalimsky JD (2001) Cellular senescence after single and repeated balloon catheter denudations of rabbit carotid arteries. Arterioscler Thromb Vasc Biol 21:220–226
Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M, Ouchi Y (2008) Cilostazol inhibits oxidative stress-induced premature senescence via upregulation of Sirt1 in human endothelial cells. Arterioscler Thromb Vasc Biol 28:1634–1639
Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I (2006) Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 114:953–960
Khanna AK (2009) Enhanced susceptibility of cyclin kinase inhibitor p21 knockout mice to high fat diet induced atherosclerosis. J Biomed Sci 16:66
Imanishi T, Hano T, Nishio I (2005) Angiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stress. J Hypertens 23:97–104
Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P (2003) Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res 93:604–613
Ihling C, Menzel G, Wellens E, Monting JS, Schaefer HE, Zeiher AM (1997) Topographical association between the cyclin-dependent kinases inhibitor P21, p53 accumulation, and cellular proliferation in human atherosclerotic tissue. Arterioscler Thromb Vasc Biol 17:2218–2224
Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105:1541–1544
Minamino T, Miyauchi H, Yoshida T, Tateno K, Kunieda T, Komuro I (2004) Vascular cell senescence and vascular aging. J Mol Cell Cardiol 36:175–183
Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395
Erusalimsky JD, Kurz DJ (2005) Cellular senescence in vivo: its relevance in ageing and cardiovascular disease. Exp Gerontol 40:634–642
Nawrot TS, Staessen JA, Gardner JP, Aviv A (2004) Telomere length and possible link to X chromosome. Lancet 363:507–510
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM (2004) Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 101:17312–17315
Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A (2000) Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 36:195–200
Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH (2001) Telomere shortening in atherosclerosis. Lancet 358:472–473
Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A (2007) Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 165:14–21
Carrero JJ, Stenvinkel P, Fellstrom B, Qureshi AR, Lamb K, Heimburger O, Barany P, Radhakrishnan K, Lindholm B, Soveri I, Nordfors L, Shiels PG (2008) Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J Intern Med 263:302–312
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460:392–395
Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ (2009) Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326:140–144
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325:201–204
Kaeberlein M, Kapahi P (2009) Cell signaling. Aging is RSKy business. Science 326:55–56
Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK (2009) The TOR pathway comes of age. Biochim Biophys Acta 1790:1067–1074
Cox LS, Mattison JA (2009) Increasing longevity through caloric restriction or rapamycin feeding in mammals: common mechanisms for common outcomes? Aging Cell 8:607–613
Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV (2009) Rapamycin decelerates cellular senescence. Cell Cycle 8:1888–1895
Zhuo L, Cai G, Liu F, Fu B, Liu W, Hong Q, Ma Q, Peng Y, Wang J, Chen X (2009) Expression and mechanism of mammalian target of rapamycin in age-related renal cell senescence and organ aging. Mech Ageing Dev 130:700–708
Flechner SM (2008) Reviewing the evidence for de novo immunosuppression with sirolimus. Transplant Proc 40:S25–S28
Pontrelli P, Rossini M, Infante B, Stallone G, Schena A, Loverre A, Ursi M, Verrienti R, Maiorano A, Zaza G, Ranieri E, Gesualdo L, Ditonno P, Bettocchi C, Schena FP, Grandaliano G (2008) Rapamycin inhibits PAI-1 expression and reduces interstitial fibrosis and glomerulosclerosis in chronic allograft nephropathy. Transplant 85:125–134
Lo A, Egidi MF, Gaber LW, Amiri HS, Vera S, Nezakatgoo N, Gaber AO (2004) Comparison of sirolimus-based calcineurin inhibitor-sparing and calcineurin inhibitor-free regimens in cadaveric renal transplantation. Transplant 77:1228–1235
Kaeberlein M (2010) Resveratrol and rapamycin: are they anti-aging drugs? BioEssays 32:96–99
Jennings P, Koppelstaetter C, Aydin S, Abberger T, Wolf AM, Mayer G, Pfaller W (2007) Cyclosporine A induces senescence in renal tubular epithelial cells. Am J Physiol Renal Physiol 293:F831–F838
Lally C, Healy E, Ryan MP (1999) Cyclosporine A-induced cell cycle arrest and cell death in renal epithelial cells. Kidney Int 56:1254–1257
Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Moller K, Wigger M, Peruzzi L, Mehls O, Schaefer F (2009) Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361:1639–1650
Basso N, Paglia N, Stella I, de Cavanagh EM, Ferder L, del Rosario Lores AM, Inserra F (2005) Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept 128:247–252
Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ (2007) Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 369:107–114
Assmus B, Urbich C, Aicher A, Hofmann WK, Haendeler J, Rossig L, Spyridopoulos I, Zeiher AM, Dimmeler S (2003) HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ Res 92:1049–1055
Spyridopoulos I, Haendeler J, Urbich C, Brummendorf TH, Oh H, Schneider MD, Zeiher AM, Dimmeler S (2004) Statins enhance migratory capacity by upregulation of the telomere repeat-binding factor TRF2 in endothelial progenitor cells. Circulation 110:3136–3142
Baerlocher GM, Vulto I, de Jong G, Lansdorp PM (2006) Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc 1:2365–2376
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacobi, C., Hömme, M. & Melk, A. Is cellular senescence important in pediatric kidney disease?. Pediatr Nephrol 26, 2121–2131 (2011). https://doi.org/10.1007/s00467-010-1740-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1740-6