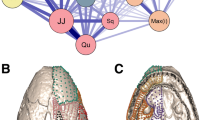
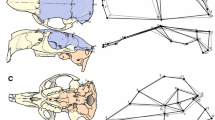
Abstract
Morphological integration refers to the modular structuring of inter-trait relationships in an organism, which could bias the direction and rate of morphological change, either constraining or facilitating evolution along certain dimensions of the morphospace. Therefore, the description of patterns and magnitudes of morphological integration and the analysis of their evolutionary consequences are central to understand the evolution of complex traits. Here we analyze morphological integration in the skull of several mammalian orders, addressing the following questions: are there common patterns of inter-trait relationships? Are these patterns compatible with hypotheses based on shared development and function? Do morphological integration patterns and magnitudes vary in the same way across groups? We digitized more than 3,500 specimens spanning 15 mammalian orders, estimated the correspondent pooled within-group correlation and variance/covariance matrices for 35 skull traits and compared those matrices among the orders. We also compared observed patterns of integration to theoretical expectations based on common development and function. Our results point to a largely shared pattern of inter-trait correlations, implying that mammalian skull diversity has been produced upon a common covariance structure that remained similar for at least 65 million years. Comparisons with a rodent genetic variance/covariance matrix suggest that this broad similarity extends also to the genetic factors underlying phenotypic variation. In contrast to the relative constancy of inter-trait correlation/covariance patterns, magnitudes varied markedly across groups. Several morphological modules hypothesized from shared development and function were detected in the mammalian taxa studied. Our data provide evidence that mammalian skull evolution can be viewed as a history of inter-module parcellation, with the modules themselves being more clearly marked in those lineages with lower overall magnitude of integration. The implication of these findings is that the main evolutionary trend in the mammalian skull was one of decreasing the constraints to evolution by promoting a more modular architecture.




Similar content being viewed by others
References
Ackermann, R. R. (2005). Ontogenetic integration of the hominoid face. Journal of Human Evolution, 48, 175–197. doi:10.1016/j.jhevol.2004.11.001.
Ackermann, R. R., & Cheverud, J. M. (2000). Phenotypic covariance structure in tamarins (genus Saguinus): A comparison of variation patterns using matrix correlation and common principal component analysis. American Journal of Physical Anthropology, 111, 489–501. doi :10.1002/(SICI)1096-8644(200004)111:4<489::AID-AJPA5>3.0.CO;2-U.
Ackermann, R. R., & Cheverud, J. M. (2004). Morphological integration in primate evolution. In M. Pigliucci & K. Preston (Eds.), Phenotypic integration: Studying the ecology and evolution of complex phenotypes (pp. 302–319). Oxford: Oxford University Press.
Almasy, L., & Blangero, J. (1998). Multipoint quantitative trait linkage analysis in general pedigrees. American Journal of Human Genetics, 62, 1198–1211. doi:10.1086/301844.
Asher, R. J. (2007). A database of morphological characters and a combined-data reanalysis of placental mammal phylogeny. BMC Evolutionary Biology, 7, 108. doi:10.1186/1471-2148-7-108.
Beck, R. M. D., Bininda-Emonds, O. R. P., Cardillo, M., Liu, F.-G. R., & Purvis, A. (2006). A higher-level MRP supertree of placental mammals. BMC Evolutionary Biology, 6, 93.
Begin, M., & Roff, D. A. (2005). From micro- to macroevolution through quantitative genetic variation: Positive evidence from field crickets. Journal of Evolutionary Biology, 58(10), 2287–2304.
Beldade, P., & Brakefield, P. M. (2003). Concerted evolution and developmental integration in modular butterfly wing patterns. Evolution & Development, 5, 169–179. doi:10.1046/j.1525-142X.2003.03025.x.
Berg, R. L. (1960). The ecological significance of correlation pleiades. Evolution; International Journal of Organic Evolution, 14(2), 171–180. doi:10.2307/2405824.
Chernoff, B., & Magwene, P. M. (1999). Morphological integration: 40 Years later. In E. C. Olson & R. L. Miller (Eds.), Morphological integration (pp. 316–360). Chicago: University of Chicago Press.
Cheverud, J. M. (1982). Phenotypic genetic and environmental morphological integration in the cranium. Evolution; International Journal of Organic Evolution, 36(3), 499–516. doi:10.2307/2408096.
Cheverud, J. M. (1984). Quantitative genetics and developmental constraints on evolution by selection. Journal of Theoretical Biology, 110, 155–172. doi:10.1016/S0022-5193(84)80050-8.
Cheverud, J. M. (1988). A comparison of genetic and phenotypic correlations. Evolution; International Journal of Organic Evolution, 42, 958–968. doi:10.2307/2408911.
Cheverud, J. M. (1989). A comparative analysis of morphological variation patterns in the papionins. Evolution; International Journal of Organic Evolution, 43(8), 1737–1747. doi:10.2307/2409389.
Cheverud, J. M. (1995). Morphological integration in the saddle-back tamarin (Saguinus fuscicollis) cranium. American Naturalist, 145, 63–89. doi:10.1086/285728.
Cheverud, J. M. (1996). Developmental integration and the evolution of pleiotropy. American Zoologist, 36, 44–50.
Cheverud, J. M., Ehrich, T. H., Vaughn, T. T., Koreishi, S. F., Linsey, R. B., & Pletscher, L. S. (2004). Pleiotropic effects on mandibular morphology II: Differential epistasis and genetic variation in morphological integration. Journal of Experimental Zoology Part B, 302(5), 424–435. doi:10.1002/jez.b.21008.
Cheverud, J. M., & Marroig, G. (2007). Comparing covariance matrices: Random skewers method compared to the common principal components model. Genetics and Molecular Biology, 30(2), 461–469. doi:10.1590/S1415-47572007000300027.
Cheverud, J. M., Wagner, G., & Dow, M. M. (1989). Methods for the comparative analysis of variation patterns. Systematic Zoology, 38(3), 201–213. doi:10.2307/2992282.
De Conto, V. (2007). Genética quantitativa e evolução morfológica em Akodon cursor. PhD thesis, Universidade Federal do Rio de Janeiro, RJ.
Eble, G. (2004). The macroevolution of phenotypic integration. In M. Pigliucci & K. Preston (Eds.), Phenotypic integration: Studying the ecology and evolution of complex phenotypes (pp. 253–273). Oxford: Oxford University Press.
Ehrich, T., Vaughn, T. T., Koreishi, S. F., Linsey, R. B., Pletscher, L. S., & Cheverud, J. M. (2003). Pleiotropic effects on mandibular morphology I. Developmental morphological integration and differential dominance. Journal of Experimental Zoology (Molecular and Developmental Evolution), 296B, 58–79.
Falconer, D. S., & Mackay, T. F. C. (1996). Introduction to quantitative genetics (ed. 4). Harlow, Essex, UK: Longmans Green.
Gonzalez-Jose, R., van der Molen, S., Gonzalez-Perez, E., & Hernandez, M. (2004). Patterns of phenotypic covariation and correlation in modern humans as viewed from morphological integration. American Journal of Physical Anthropology, 123(1), 69–77. doi:10.1002/ajpa.10302.
Hansen, T. F., & Houle, D. (2008). Measuring and comparing evolvability and constraint in multivariate characters. Journal of Evolutionary Biology, 21(5), 1201–1219. doi:10.1111/j.1420-9101.2008.01573.x.
Helms, J. A., Cordero, D., & Tapadia, M. D. (2005). New insights into craniofacial morphogenesis. Development, 132, 851–861. doi:10.1242/dev.01705.
Kenney-Hunt, J. P., Wang, B., Norgard, E. A., Fawcett, G., Falk, D., Pletscher, L. S., et al. (2008). Pleiotropic patterns of quantitative trait loci for seventy murine skeletal traits. Genetics, 178, 2275–2288. doi:10.1534/genetics.107.084434.
Kitazoe, Y., Kishino, H., Waddell, P. J., Nakajima, N., Okabayashi, T., Watabe, T., et al. (2007). Robust time estimation reconciles views of the antiquity of placental mammals. PLoS ONE, 2, e384. doi:10.1371/journal.pone.0000384.
Klingenberg, C. P. (2004). Integration, modules and development: Molecules to morphology to evolution. In M. Pigliucci & K. Preston (Eds.), Phenotypic integration: Studying the ecology and evolution of complex phenotypes (pp. 213–230). Oxford: Oxford University Press.
Lande, R. (1979). Quantitative genetic analysis of multivariate evolution applied to brain: Body size allometry. Evolution; International Journal of Organic Evolution, 33, 402–416. doi:10.2307/2407630.
Marroig, G., & Cheverud, J. M. (2001). A comparison of phenotypic variation and covariation patterns and the role of phylogeny ecology and ontogeny during cranial evolution of New World monkeys. Evolution; International Journal of Organic Evolution, 55(12), 2576–2600.
Marroig, G., & Cheverud, J. M. (2005). Size as a line of least evolutionary resistance: Diet and adaptive morphological radiation in New World monkeys. Evolution; International Journal of Organic Evolution, 59, 1128–1142.
Marroig, G., et al. (2008). Companion paper.
Mitteroecker, P., & Bookstein, F. (2008). The evolutionary role of modularity and integration in the hominoid cranium. Evolution; International Journal of Organic Evolution, 62, 943–958. doi:10.1111/j.1558-5646.2008.00321.x.
Moore, W. J. (1981). The mammalian skull. Cambridge: Cambridge University Press.
Morriss-Kay, G. M. (2001). Derivation of the mammalian skull vault. Journal of Anatomy, 199, 143–151. doi:10.1017/S0021878201008093.
Murphy, W. J., Eizirik, E., Johnson, W. E., Zhang, Y. P., Ryder, O. A., & O’Brien, S. J. (2001). Molecular phylogenetics and the origins of placental mammals. Nature, 409, 614–618. doi:10.1038/35054550.
Oliveira, F. B., Porto, A., & Marroig, G. Similarity of phenotypic covariance and correlation patterns in the skull along the evolution of Old World Monkeys: A case for the G evolutionary stasis. (Submitted to the Journal of Human Evolution).
Olson, E. C., & Miller, R. L. (1958). Morphological integration. Chicago: University of Chicago Press.
Pavlicev, M., Kenney-Hunt, J. P., Norgard, E. A., Roseman, C. C., Wolf, J. J., & Cheverud, J. M. (2008). Genetic variation in pleiotropy: Differential epistasis as a source of variation in the allometric relationship between long bone lengths and body weight. Evolution; International Journal of Organic Evolution, 62(1), 199–213.
Preston, K. A., & Ackerly, D. D. (2004). Allometry and evolution in modular organisms. In M. Pigliucci & K. Preston (Eds.), Phenotypic integration: Studying the ecology and evolution of complex phenotypes (pp. 80–106). Oxford: Oxford University Press.
Revell, L., Harmon, L. J., Langerhans, R. B., & Kolbe, J. J. (2007). A phylogenetic approach to determining the importance of constraint on phenotypic evolution in the neotropical lizard, Anolis cristatellus. Evolutionary Ecology Research, 9, 261–282.
Roff, D. A. (1997). Evolutionary quantitative genetics. New York: Chapman and Hall.
Roff, D. A., & Mousseau, T. (2005). The evolution of the phenotypic covariance matrix: Evidence for selection and drift in Melanoplus. Journal of Evolutionary Biology, 18(4), 1104–1114. doi:10.1111/j.1420-9101.2005.00862.x.
Shirai, L. T., & Marroig, G. Evolutionary constraint and freedom: A comparison between New World marsupials and monkeys skull (submitted).
Smith, K. K. (1996). Integration of craniofacial structures during development in mammals. American Zoologist, 36, 70–79.
Smith, K. K. (1997). Comparative patterns of craniofacial development in Eutherian and Metatherian mammals. Evolution; International Journal of Organic Evolution, 51(5), 1663–1678. doi:10.2307/2411218.
Smith, K. K. (2001). The evolution of mammalian development. Bulletin of the Museum of Comparative Zoology, 156, 119–135.
Sneath, P. H., & Sokal, R. R. (1973). Numerical taxonomy. San Francisco: WH Freeman and Company.
Springer, M. S., Stanhope, M. J., Madsen, O., & de Jong, W. W. (2004). Molecules consolidate the placental mammal tree. Trends in Ecology & Evolution, 19(8), 430–438. doi:10.1016/j.tree.2004.05.006.
Steppan, S. J., Phillips, P. C., & Houle, D. (2002). Comparative quantitative genetics: Evolution of the G matrix. Trends in Ecology & Evolution, 17(7), 320–327. doi:10.1016/S0169-5347(02)02505-3.
Tapadia, M. D., Cordero, D. R., & Helms, J. A. (2005). It’s all in your head: New insights into craniofacial development and deformation. Journal of Anatomy, 207, 461–477.
Wagner, G. P., & Altenberg, L. (1996). Complex adaptations and the evolution of evolvability. Evolution; International Journal of Organic Evolution, 50(3), 967–976. doi:10.2307/2410639.
Wagner, G. P., Kenney-Hunt, J. P., Pavlicev, M., Peck, J. R., Waxman, D., & Cheverud, J. M. (2008). Pleiotropic scaling of gene effects and the “Cost of Complexity”. Nature, 452, 470–473. doi:10.1038/nature06756.
Wagner, G. P., Pavlicev, M., & Cheverud, J. M. (2007). The road to modularity. Nature Reviews Genetics, 8, 921–931. doi:10.1038/nrg2267.
Wible, J. R., Rougier, G. W., Novacek, M. J., & Asher, R. J. (2007). Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature, 447, 1003–1006. doi:10.1038/nature05854.
Willis, J. H., Coyne, J. A., & Kirkpatrick, M. (1991). Can one predict the evolution of quantitative characters without genetics? Evolution; International Journal of Organic Evolution, 45(2), 441–444. doi:10.2307/2409678.
Wilson, D. E., & Reeder, D. M. (Eds.). (2005). Mammal species of the world: A taxonomic and geographic reference. Baltimore: Johns Hopkins University Press.
Young, R. L., & Badyaev, A. V. (2006). Evolutionary persistence of phenotypic integration: Influence of developmental and functional relationships on complex trait evolution. Evolution; International Journal of Organic Evolution, 60(6), 1291–1299.
Acknowledgements
We thank Campbell Rolian and Katherine Willmore for the opportunity to present this data in the 2008 AAPA meeting. Benedikt Hallgrímsson and 2 anonymous referees made constructive comments on an earlier version of this paper. We are also grateful to the people and institutions that provided generous help and access to mammal collections: E. Westwig, N. Simmons, R. Voss and R. MacPhee (AMNH); L. Tomsett, P. Jenkins and D. Hills (BMNH); B. Paterson, W. Stanley, and L. Heaney (FMNH); J. Chupasko and M. Omura (MCZ); M. Godinot, F. Renoult, C. Lefrève and J. Cuisin (MNHN); L. Salles, J. Oliveira, F. Barbosa, and S. Franco (MNRJ); S. Costa and J. de Queiroz (MPEG); Staff at the Museo de la Universidad Nacional Mayor de San Marcos; M. de Vivo and J. Gualda (MZUSP); H. van Grouw and B. Bekkum-Ansari (Naturalis); R. Thorington, R. Chapman and L. Gordon (NMNH); M. Harman (Powell-Cotton Museum); Georges Lenglet (RBINS); E. Gilissen and W. Wendelen (RMCA); R. Asher, I. Thomas and D. Willborn (ZMB); F. Smith and S. Tardif (University of Tennessee, and the Oak Ridge Associated Universities Marmoset Research Center); C. Zollikofer, M. Ponce de Léon and T. Jashashvili (Zürich Universität); R. Smith (Museu de Anatomia da UNIFESP); E. Liberti (Museu de Anatomia “Professor Alfonso Bovero”). This research was supported by grants and fellowships from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES), Conselho Nacional de Pesquisas (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação José Bonifácio (FUJB), Projeto de Conservação e Utilização Sustentável da Diversidade Biológica (PROBIO), and an American Museum of Natural History Collections Study Grant.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Landmark abbreviations and definitions
Landmarks |
||
|---|---|---|
Abbreviations |
Landmarks |
Position |
IS |
lntradentale superior |
Midline |
PM |
Premaxillary-maxillary suture at the alveolus |
Both sides |
NSL |
Nasale |
Midline |
NA |
Nasion |
Midline |
BR |
Bregma |
Both sides |
PT |
Pterion |
Both sides |
ZS |
Zygomaxillare superior |
Both sides |
ZI |
Zygomaxillare inferior |
Both sides |
MT |
Maxillary tuberosity |
Both sides |
PNS |
Posterior nasal spine |
Midline |
APET |
Anterior petrous temporal |
Both sides |
BA |
Basion |
Midline |
OPI |
Opisthion |
Midline |
EAM |
Anterior external auditory meatus |
Both sides |
PEAM |
Posterior external auditory meatus |
Both sides |
ZYGO |
Inferior zygo-temporal suture |
Both sides |
TSP |
Temporo-spheno-parietal junction |
Both sides |
TS |
Temporo-sphenoidal junction at petrous |
Both sides |
JP |
Jugular process |
Both sides |
LD |
Lambda |
Midline |
AS |
Asterion |
Both sides |
Rights and permissions
About this article
Cite this article
Porto, A., de Oliveira, F.B., Shirai, L.T. et al. The Evolution of Modularity in the Mammalian Skull I: Morphological Integration Patterns and Magnitudes. Evol Biol 36, 118–135 (2009). https://doi.org/10.1007/s11692-008-9038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-008-9038-3