Abstract
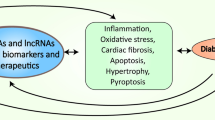
MicroRNAs (miRNAs) are a novel class of noncoding, conserved, tiny (19–24 nt) RNAs that regulate gene expression either by RNA interference (RNAi), where they target 3′-UTR and degrade mRNA or repress translation, or by RNA activation (RNAa), where they target promoter elements at 5′-UTR and induce gene transcription. They have emerged as a therapeutic target for diabetes and cardiovascular diseases because each miRNA has several targets that allows it to make a layer of regulatory network. Diabetes is recognized as a multifactorial metabolic disease that increases the chances of heart failure and exacerbates mortality. MiRNAs regulates insulin production, beta-cell differentiation, cardiac hypertrophy, fibrosis, and rhythm, and thereby plays a crucial role in cardiac remodeling in diabetes. Differential expression of circulatory miRNAs has potential as a biomarker for heart failure in diabetes. It is documented that miRNAs regulate inflammation, epigenetic modifications, and autophagy and are altered by matrix metalloproteinase 9, homocysteine, and exercise, which are associated with diabetic cardiomyopathy. This chapter embodies the differentially expressed miRNAs in diabetic hearts, their plausible causes of deregulation, and the therapeutic potential of miRNAs in ameliorating diabetic cardiomyopathy.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Tyagi AC, Sen U, Mishra PK (2011) Synergy of microRNA and stem cell: a novel therapeutic approach for diabetes mellitus and cardiovascular diseases. Curr Diabetes Rev 7:367–376
Huang V, Li LC (2012) miRNA goes nuclear. RNA Biol 9:269–273
Liao JY, Ma LM, Guo YH, Zhang YC et al (2010) Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3′ trailers. PLoS One 5:e10563
Huang V, Place RF, Portnoy V, Wang J et al (2012) Upregulation of cyclin B1 by miRNA and its implications in cancer. Nucleic Acids Res 40:1695–1707
Place RF, Li LC, Pookot D, Noonan EJ et al (2008) MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci USA 105:1608–1613
Younger ST, Corey DR (2011) Transcriptional gene silencing in mammalian cells by miRNA mimics that target gene promoters. Nucleic Acids Res 39:5682–5691
Lytle JR, Yario TA, Steitz JA (2007) Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc Natl Acad Sci USA 104:9667–9672
Younger ST, Corey DR (2011) Transcriptional regulation by miRNA mimics that target sequences downstream of gene termini. Mol Biosyst 7:2383–2388
Berezikov E, Chung WJ, Willis J, Cuppen E et al (2007) Mammalian mirtron genes. Mol Cell 28:328–336
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20
Asrih M, Steffens S (2013) Emerging role of epigenetics and miRNA in diabetic cardiomyopathy. Cardiovasc Pathol 22:117–125
Chavali V, Tyagi SC, Mishra PK (2013) Predictors and prevention of diabetic cardiomyopathy. Diabetes Metab Syndr Obes 6:151–160
Tijsen AJ, Pinto YM, Creemers EE (2012) Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases. Am J Physiol Heart Circ Physiol 303:H1085–H1095
Latronico MV, Condorelli G (2011) MicroRNAs in hypertrophy and heart failure. Exp Biol Med (Maywood) 236:125–131
Port JD, Sucharov C (2010) Role of microRNAs in cardiovascular disease: therapeutic challenges and potentials. J Cardiovasc Pharmacol 56:444–453
Topkara VK, Mann DL (2011) Role of microRNAs in cardiac remodeling and heart failure. Cardiovasc Drugs Ther 25:171–182
Mishra PK, Tyagi N, Kumar M, Tyagi SC (2009) MicroRNAs as a therapeutic target for cardiovascular diseases. J Cell Mol Med 13:778–789
Montgomery RL, Hullinger TG, Semus HM, Dickinson BA et al (2011) Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 124:1537–1547
Sun LL, Jiang BG, Li WT, Zou JJ et al (2011) MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract 91:94–100
Tang X, Muniappan L, Tang G, Ozcan S (2009) Identification of glucose-regulated miRNAs from pancreatic beta cells reveals a role for miR-30d in insulin transcription. RNA 15:287–293
Chavali V, Tyagi SC, Mishra PK (2013) Differential expression of dicer, miRNAs, and inflammatory markers in diabetic Ins2+/- Akita hearts. Cell Biochem Biophys. June 15, Epub ahead of print
He A, Zhu L, Gupta N, Chang Y et al (2007) Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol 21:2785–2794
Caporali A, Meloni M, Vollenkle C, Bonci D et al (2011) Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation 123:282–291
Li Y, Song YH, Li F, Yang T et al (2009) MicroRNA-221 regulates high glucose-induced endothelial dysfunction. Biochem Biophys Res Commun 381:81–83
Lu H, Buchan RJ, Cook SA (2010) MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc Res 86:410–420
Shan ZX, Lin QX, Deng CY, Zhu JN et al (2010) miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett 584:3592–3600
Wang XH, Qian RZ, Zhang W, Chen SF (2009) MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol 36:181–188
Chavali V, Tyagi SC, Mishra PK (2012) MicroRNA-133a regulates DNA methylation in diabetic cardiomyocytes. Biochem Biophys Res Commun 425:668–672
Glass C, Singla DK (2011) MicroRNA-1 transfected embryonic stem cells enhance cardiac myocyte differentiation and inhibit apoptosis by modulating the PTEN/Akt pathway in the infarcted heart. Am J Physiol Heart Circ Physiol 301:H2038–H2049
Liu C, Teng ZQ, McQuate AL, Jobe EM et al (2013) An epigenetic feedback regulatory loop involving microRNA-195 and MBD1 governs neural stem cell differentiation. PLoS One 8:e51436
Vinas JL, Ventayol M, Brune B, Jung M et al (2013) miRNA let-7e modulates the Wnt pathway and early nephrogenic markers in mouse embryonic stem cell differentiation. PLoS One 8:e60937
Wang Y, Du L, Li X, Zhang S et al (2011) Functional homogeneity in microRNA target heterogeneity: a new sight into human microRNomics. OMICS 15:25–35
Zhang C (2008) MicroRNomics: a newly emerging approach for disease biology. Physiol Genomics 33:139–147
van Rooij E, Olson EN (2007) MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest 117:2369–2376
Krol J, Loedige I, Filipowicz W (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11:597–610
Macfarlane LA, Murphy PR (2010) MicroRNA: biogenesis, function and role in cancer. Curr Genomics 11:537–561
Denli AM, Tops BB, Plasterk RH, Ketting RF et al (2004) Processing of primary microRNAs by the microprocessor complex. Nature 432:231–235
Gregory RI, Yan KP, Amuthan G, Chendrimada T et al (2004) The microprocessor complex mediates the genesis of microRNAs. Nature 432:235–240
Han J, Lee Y, Yeom KH, Kim YK et al (2004) The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev 18:3016–3027
Han J, Lee Y, Yeom KH, Nam JW et al (2006) Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 125:887–901
Lee Y, Jeon K, Lee JT, Kim S et al (2002) MicroRNA maturation: stepwise processing and subcellular localization. EMBO J 21:4663–4670
Lee Y, Ahn C, Han J, Choi H et al (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425:415–419
Bohnsack MT, Czaplinski K, Gorlich D (2004) Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 10:185–191
Lund E, Dahlberg JE (2006) Substrate selectivity of exportin 5 and Dicer in the biogenesis of microRNAs. Cold Spring Harb Symp Quant Biol 71:59–66
Okada C, Yamashita E, Lee SJ, Shibata S (2009) A high-resolution structure of the pre-microRNA nuclear export machinery. Science 326:1275–1279
Yi R, Qin Y, Macara IG, Cullen BR (2003) Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev 17:3011–3016
Zeng Y, Cullen BR (2004) Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucleic Acids Res 32:4776–4785
Okamura K, Hagen JW, Duan H, Tyler DM (2007) The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 130:89–100
Du T, Zamore PD (2005) microPrimer: the biogenesis and function of microRNA. Development 132:4645–4652
King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 21:1414–1431
Tabak AG, Herder C, Rathmann W, Brunner EJ et al (2012) Prediabetes: a high-risk state for diabetes development. Lancet 379:2279–2290
Wild S, Roglic G, Green A, Sicree R et al (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Mathew V, Gersh BJ, Williams BA, Laskey WK et al (2004) Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 109:476–480
Pignone M, Alberts MJ, Colwell JA, Cushman M et al (2010) Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care 33:1395–1402
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T et al (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30:595–602
Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME (2008) Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 121:748–757
Maisch B, Alter P, Pankuweit S (2011) Diabetic cardiomyopathy—fact or fiction? Herz 36:102–115
Miki T, Yuda S, Kouzu H, Miura T (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18:149–166
Sharma V, McNeill JH (2006) Diabetic cardiomyopathy: where are we 40 years later? Can J Cardiol 22:305–308
Depre C, Shipley GL, Chen W, Han Q (1998) Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med 4:1269–1275
Goodwin GW, Taylor CS, Taegtmeyer H (1998) Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem 273:29530–29539
Rodrigues B, Cam MC, McNeill JH (1998) Metabolic disturbances in diabetic cardiomyopathy. Mol Cell Biochem 180:53–57
Poornima IG, Parikh P, Shannon RP (2006) Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 98:596–605
Boudina S, Abel ED (2006) Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 21:250–258
Du X, Matsumura T, Edelstein D, Rossetti L et al (2003) Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 112:1049–1057
Nishikawa T, Edelstein D, Brownlee M (2000) The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl 77:S26–S30
Nishikawa T, Edelstein D, Du XL, Yamagishi S et al (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790
Mishra PK, Tyagi N, Sen U, Joshua IG et al (2010) Synergism in hyperhomocysteinemia and diabetes: role of PPAR-gamma and tempol. Cardiovasc Diabetol 9:49
Mishra PK, Chavali V, Metreveli N, Tyagi SC (2012) Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix. Can J Physiol Pharmacol 90:353–360
Mishra PK, Metreveli N, Tyagi SC (2010) MMP-9 gene ablation and TIMP-4 mitigate PAR-1-mediated cardiomyocyte dysfunction: a plausible role of dicer and miRNA. Cell Biochem Biophys 57:67–76
Wakasaki H, Koya D, Schoen FJ, Jirousek MR et al (1997) Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci USA 94:9320–9325
Mishra PK, Givvimani S, Metreveli N, Tyagi SC (2010) Attenuation of beta2-adrenergic receptors and homocysteine metabolic enzymes cause diabetic cardiomyopathy. Biochem Biophys Res Commun 401:175–181
Mishra PK, Awe O, Metreveli N, Qipshidze N et al (2011) Exercise mitigates homocysteine—beta2-adrenergic receptor interactions to ameliorate contractile dysfunction in diabetes. Int J Physiol Pathophysiol Pharmacol 3:97–106
Wang G, Zhu X, Xie W, Han P et al (2010) Rad as a novel regulator of excitation-contraction coupling and beta-adrenergic signaling in heart. Circ Res 106:317–327
Tarquini R, Lazzeri C, Pala L, Rotella CM, Gensini GF (2011) The diabetic cardiomyopathy. Acta Diabetol 48:173–181
Guay C, Roggli E, Nesca V, Jacovetti C et al (2011) Diabetes mellitus, a microRNA-related disease? Transl Res 157:253–264
Hennessy E, O’Driscoll L (2008) Molecular medicine of microRNAs: structure, function and implications for diabetes. Expert Rev Mol Med 10:e24
Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S et al (2004) A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432:226–230
Zampetaki A, Kiechl S, Drozdov I, Willeit P et al (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107:810–817
Feng B, Chen S, George B, Feng Q et al (2010) miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes Metab Res Rev 26:40–49
Care A, Catalucci D, Felicetti F, Bonci D et al (2007) MicroRNA-133 controls cardiac hypertrophy. Nat Med 13:613–618
Matkovich SJ, Wang W, Tu Y, Eschenbacher WH et al (2010) MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res 106:166–175
Duisters RF, Tijsen AJ, Schroen B, Leenders JJ et al (2009) miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res 104:170–178, 6p
Shan H, Zhang Y, Lu Y, Zhang Y et al (2009) Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res 83:465–472
Chen JF, Murchison EP, Tang R, Callis TE et al (2008) Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci USA 105:2111–2116
Latronico MV, Elia L, Condorelli G, Catalucci D (2008) Heart failure: targeting transcriptional and post-transcriptional control mechanisms of hypertrophy for treatment. Int J Biochem Cell Biol 40:1643–1648
van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM et al (2008) Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA 105:13027–13032
Thum T, Gross C, Fiedler J, Fischer T et al (2008) MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456:980–984
Brase JC, Wuttig D, Kuner R, Sultmann H (2010) Serum microRNAs as non-invasive biomarkers for cancer. Mol Cancer 9:306
Brase JC, Johannes M, Schlomm T, Falth M et al (2011) Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer 128:608–616
Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G et al (2011) MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol 8:467–477
Kosaka N, Iguchi H, Ochiya T (2010) Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 101:2087–2092
Tie Y, Liu B, Fu H, Zheng X (2009) Circulating miRNA and cancer diagnosis. Sci China C Life Sci 52:1117–1122
Ajit SK (2012) Circulating microRNAs as biomarkers, therapeutic targets, and signaling molecules. Sensors (Basel) 12:3359–3369
Dimmeler S, Zeiher AM (2010) Circulating microRNAs: novel biomarkers for cardiovascular diseases? Eur Heart J 31:2705–2707
Fichtlscherer S, Zeiher AM, Dimmeler S (2011) Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol 31:2383–2390
Li C, Pei F, Zhu X, Duan DD et al (2012) Circulating microRNAs as novel and sensitive biomarkers of acute myocardial infarction. Clin Biochem 45:727–732
Scholer N, Langer C, Kuchenbauer F (2011) Circulating microRNAs as biomarkers—True Blood? Genome Med 3:72
Acknowledgments
This work is supported in part with by National Institute of Heart HL-113281 and HL-116205 to Paras K. Mishra and HL-108621 and HL-74185 to Suresh C Tyagi.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Mishra, P.K., Tyagi, S.C. (2014). MicroRNomics of Diabetic Cardiomyopathy. In: Turan, B., Dhalla, N. (eds) Diabetic Cardiomyopathy. Advances in Biochemistry in Health and Disease, vol 9. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9317-4_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9317-4_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9316-7
Online ISBN: 978-1-4614-9317-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)