- 1IRCCS Istituti Clinici Scientifici Maugeri, Milan, Italy
- 2BIOMETRA Department, University of Milan, Milan, Italy
- 3Exercise Medicine Unit, IRCCS, Istituto Auxologico Italiano, Milan, Italy
- 4Department of Physical Therapy, Federal University of São Carlos, São Carlos, Brazil
- 5Department of Biomedical Sciences for Health, University of Milan, Milan, Italy
- 6Department of Cardiothoracic, Vascular Anesthesia and Intensive Care, Policlinico San Donato, San Donato Milanese, Milan, Italy
QT interval (QT) variability analysis provides pathophysiological and prognostic information utilized in cardiac and non-cardiac diseases, complementary to those obtained from the analysis of heart period (HP) variability. An increased QT variability has been associated to a higher risk for cardiac events and poorest prognosis. Autonomic cardiovascular adaptation to internal and external challenges, such those occurring in athletes exposed to high levels of physical stress and in ageing could also be deepen by analyzing QT variability, searching for early prognostic signatures. The aim of the study was to analyze the QT variability and cardiac control complexity in a group of middle-aged half-marathon runners at baseline (B) and at a 10-year follow-up (FU). We found that the overall QT variability decreased at FU, despite the inescapable increase in age (52.3 ± 8.0 years at FU). This change was accompanied by an increase of the HP variability complexity without changes of the QT variability complexity. Of notice, over the years, the group of athletes maintained their regular physical activity by switching to a moderate intensity rather than strenuous. In conclusion, regular and moderate exercise over the years was beneficial for this group of athletes, as reflected by the decreased overall QT variability that is known to be associated to lower cardiovascular risk. The concomitant enhanced cardiac control complexity also suggests a trend opposite to what usually occurs with ageing, resulting in a more flexible cardiac control, typical of younger people.
Introduction
In the last years, more and more attention has been paid to the evaluation of the cardiovascular risk in athletes exposed to very high levels of physical stress, focusing on the medium-long term effects (Albert et al., 2000; Kim et al., 2012; Dalla Vecchia et al., 2014; Lear et al., 2017; Eijsvogels et al., 2018; Dalla Vecchia et al., 2019; De Maria et al., 2021).
A recent study (De Maria et al., 2021) demonstrated that in a group of middle-aged amateur half-marathon runners, considering a 10-year follow-up, the shift from a strenuous exercise training to a still regular but lower level of exercise, namely a moderate one, was associated to a rebalancing of the sympatho-vagal modulation directed to the heart. Such changes were accompanied by a very low occurrence of cardiovascular disease (De Maria et al., 2021). This would represent a key finding, as an enhanced vagal and decreased sympathetic cardiac modulation to the heart is known to be associated with a reduced risk of cardiovascular events (La Rovere et al., 1998; La Rovere et al., 2001; Thayer et al., 2010; Pinna et al., 2017), confirming physical exercise among the effective cardiovascular prevention measures (Thompson et al., 2003). The aforementioned study was based on the analysis of the heart period (HP) variability, without considering the analysis of the spontaneous fluctuations of the QT interval (QT), that has been successfully applied to study the cardiac neural control to provide, together with HP variability analysis, complementary and non-redundant information (De Maria et al., 2019). Indeed, QT variability analysis provides important pathophysiological insights and is useful for risk stratification in many diseases (Baumert et al., 2016). An increased QT variability has been observed in several cardiac, non-cardiac and metabolic diseases, such as coronary artery disease, hypertension, mental disorders and diabetes mellitus (Baumert et al., 2016). An increased QT variability has also been associated to sudden cardiac death (Maison-Blanche and Coumel, 1997; Singh et al., 1997) and adverse prognosis in patients after myocardial infarction (El-Hamad et al., 2020).
The application of QT variability analysis for the study of cardiac control in athletes is still limited (Yanık et al., 2021; Cavarretta et al., 2022). The aim of the present study is to evaluate the complementary information derived by QT variability assessment, including its complexity, with respect to HP variability, in a group of middle-aged half-marathon runners studied over a 10-year follow-up period. The results of the QT variability and complexity analyses will be compared and discussed in relation to previous data derived from the HP variability analysis (De Maria et al., 2021).
Together with the more traditional and widely applied power spectral analysis, the analysis in the information domain, i.e. complexity analysis, provides information about the regularity of the HP and QT variability series. Complexity analyses are based on the estimation of the complexity of HP and QT series via the assessment of the information carried by a new sample of the series that cannot be explained by a combination of past samples (Porta et al., 1998a; Porta et al., 2013).
Materials and Methods
Population and Experimental Protocol
The experimental protocol has fully been described previously (Dalla Vecchia et al., 2014; De Maria et al., 2021). The study population consisted of a group of 18 half-marathon runners (17 males) first studied at the fifth Corripavia race and then at a 10-year follow-up. For convenience, the data collected firstly will hereinafter be referred to as baseline (B) (Dalla Vecchia et al., 2014), while those collected at follow-up as FU (De Maria et al., 2021). The demographic and clinical features of the enrolled population at FU are shown in Table 1.
Briefly, the athletes were studied during supine resting (REST) and active standing (STAND), both at B and FU. The electrocardiogram (ECG) from a modified lead II (AT-MIO 16E2, National Instrument at B and LAB3, Marazza, Monza Italy at FU) was acquired for 5 min at REST and for 4 min during STAND. Sampling rate was fixed at 300 Hz at B and at 1000 Hz at FU. Participants were asked to avoid caffeinated and alcoholic beverages in the 24 h preceding the test. They were also asked to avoid physical exercise in the 72 h preceding the experimental session (Furlan et al., 1993).
Written informed consent was obtained from all subjects involved in the study. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of IRCCS Istituti Clinici Scientifici Maugeri (protocol code 2277CE, date of approval 12/03/2019).
Heart Period and QT Interval Time Series Extraction
HP and QT beat-to-beat time series were derived from the ECG acquired during the protocol. The HP was approximated as the time distance between two consecutive R wave apexes detected on the ECG by an automatic algorithm, while the QT interval was approximated as the temporal distance between the second R peak of the correspondent HP and the end of the following T wave (Porta et al., 2010; De Maria et al., 2019). The end of the T wave was fixed where the absolute value of the first derivative calculated on the T-wave downslope was smaller than 30% of the value of the steepest slope of the T-wave (Porta et al., 2010; De Maria et al., 2019). None of the analyzed signals exhibited biphasic T waves (Porta et al., 1998b).
R wave peak detections were visually checked to avoid misidentification. Correction of the HP and QT time series was implemented in case of ectopic beats, which never exceeded 5% of the series length.
For each subject, stationary segments of 300 consecutive beats were selected for further analysis in each experimental condition, i.e. REST and STAND.
After linear detrending of the selected segments, mean and variance of the HP and QT series were calculated and named as μHP and σ2HP, μQT and σ2QT, respectively.
Power Spectral Analysis
Parametric power spectral analysis was performed over the QT and HP series. The series were modelized as an autoregressive model whose order was chosen according to Akaike information criterion in the range from 10 to 16 and the coefficients were estimated via least squares method solved via the Levinson-Durbin recursion. The series were then decomposed into power spectral components. The sum of the absolute power of the spectral components of QT series whose central frequency dropped in the low frequency band (LF band, 0.04–0.15 Hz) was labelled as LFQT and taken as an index of the sympathetic modulation directed to the ventricles (Baumert et al., 2011; Porta et al., 2011; De Maria et al., 2019). The sum of the absolute power of the spectral components of the HP series whose central frequency dropped in the high frequency band (HF band, 0.15–0.4 Hz) was labelled as HFHP and taken as an index of the vagal modulation directed to the sinus node (Malliani et al., 1991). HFHP was expressed in absolute and normalized units (Malliani et al., 1991).
Heart Period and QT Interval Complexity Analyses
The complexity of the HP and QT series was calculated by the corrected conditional entropy (CCE) method, originally described in (Porta et al., 1998a). Briefly, the conditional entropy is based on the estimation of the complexity of a series via the assessment of the information carried by a new sample that cannot be explained by a combination of past samples.
The dynamic of the QT and HP series was quantized into six levels and conditional entropy was assessed over the quantized series. The strategy followed to correct the estimate of conditional entropy and to make robust its estimate was described in (Porta et al., 1998a). The application of a correction allowed us to find a minimum of CCE with respect to the number of past samples utilized to condition the evolution of the current one. This minimum represents the best compromise between the ability of past sample to condition the temporal evolution of the series by favoring the reduction of future uncertainty and the natural tendency of conditional probability to decrease towards 0 when the length of the conditioning patterns increases, thus leading to the unavoidable decline of the conditional entropy to 0. The minimum of the CCE was taken as complexity index (CI). CI was computed over HP and QT series (CIHP and CIQT), respectively. CI ranges from 0 (the current value does not carry information given past samples indicating maximum predictability and null complexity) to the Shannon entropy, representing the maximum information carried by the current value. Normalized CI of HP (NCIHP) and QT (NCIQT) series were calculated by dividing CIHP and CIQT by the Shannon entropy of HP and QT series, respectively. Both NCIHP and NCIQT range from 0 (null predictability) to 1 (perfect predictability). The higher the CIHP, CIQT, NCIHP and NCIQT, the higher the complexity of the HP and QT series and the lower their predictability (Porta et al., 2013).
Statistical Analysis
Two-way repeated measure analysis of variance (ANOVA, one factor repetition, Holm-Sidak test for multiple comparison) was applied to test the differences between the two experimental conditions (i.e. REST and STAND) at B and FU.
In both figures and tables, continuous data are presented as mean ± standard deviation, while categorical variables as absolute number (percentage).
All the statistical analyses were performed using the commercial software Sigmaplot (version 11.0, Systat Software, Inc., Chicago, IL, United States). A p-value <0.05 was always considered as significant.
Results
Table 1 summarizes the demographic and clinical features of the studied population. Compared to B, the athletes’ weekly training was significantly lower at FU, from 14.6 ± 2.9 to 6.5 ± 3.1 h per week, as already described in (De Maria et al., 2021).
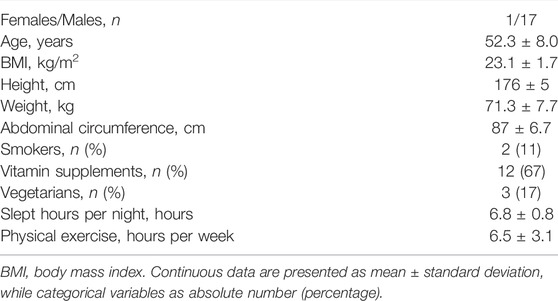
Figure 1 summarizes the results of the HP variability analysis at B and FU in the group of half-marathon runners, as already presented in (De Maria et al., 2021).
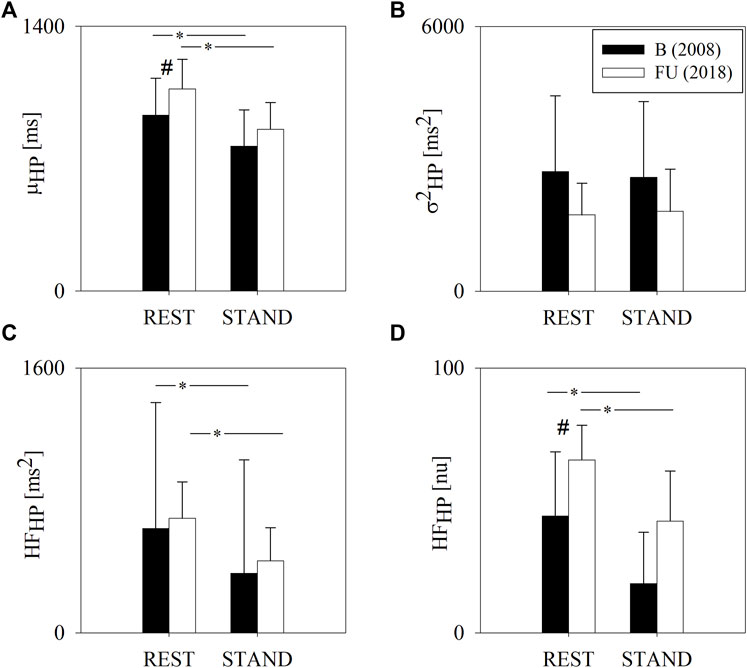
FIGURE 1. Results of the HP variability analysis in a group of amateur half-marathon runners at baseline (B, black bars) and at a 10-year follow-up (FU, white bars) in resting condition (REST) and during active standing (STAND). The bar graphs show the HP interval mean (μHP, panel (A)), HP variance (σ2HP, panel (B)) and the power of HP variability in the high frequency band (HFHP expressed in absolute and normalized units, panels (C,D), respectively). Data are presented as mean ± standard deviation. # indicates p < 0.05 FU vs B, while *p < 0.05 REST vs STAND.
Figure 2 shows the results of the QT variability analysis at B and FU in the two experimental conditions, i.e. REST and STAND. At REST, μQT was higher at FU compared to B. STAND induced a decrease of μQT both at B and FU. σ2QT was lower at FU compared to B both at REST and during STAND. At FU, from REST to STAND there were no σ2QT changes, while at B, STAND induced an increase of σ2QT. Analogously, LFQT was lower at FU compared to B, both at REST and during STAND, where the difference was significant solely during STAND. LFQT increased significantly from REST to STAND only at B and not at FU.
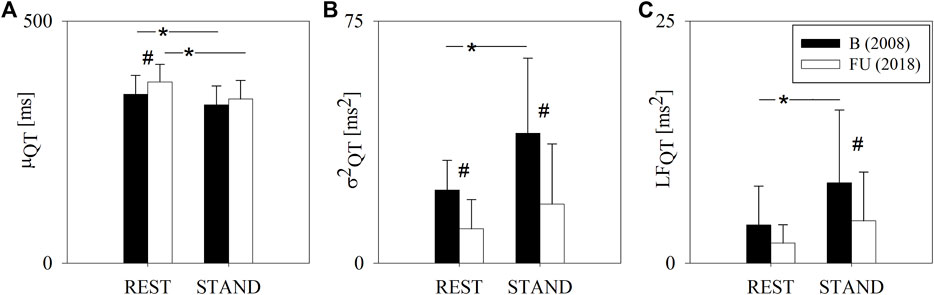
FIGURE 2. Results of the QT variability analysis in a group of amateur half-marathon runners at baseline (B, black bars) and at a 10-year follow-up (FU, white bars) in resting condition (REST) and during active standing (STAND). The bar graphs show the QT interval mean (μQT, panel (A)), QT interval variance (σ2QT, panel (B)) and the absolute power of QT variability in the low frequency band (LFQT, panel (C)). Data are presented as mean ± standard deviation. # indicates p < 0.05 FU vs B, while *p < 0.05 REST vs STAND.
Figure 3 shows the results of the complexity analysis over the HP series. Both the CIHP and NCIHP were higher at FU compared to B in both the experimental conditions (i.e. REST and STAND). NCIHP decreased from REST to STAND only at B, while CIHP remained unchanged both at B and FU.
FIGURE 3. Results of the complexity analysis of the HP series in a group of amateur half-marathon runners at baseline (B, black bars) and at a 10-year follow-up (FU, white bars) in resting condition (REST) and during active standing (STAND). The bar graphs show the complexity index (CIHP, panel (A)) and the normalized complexity index (NCIHP, panel (B)). Data are presented as mean ± standard deviation. # indicates p < 0.05 FU vs B, while *p < 0.05 REST vs STAND.
Figure 4 shows the results of the complexity analysis over the QT series. No variation was observed for any of the considered indices between FU and B and between REST and STAND.
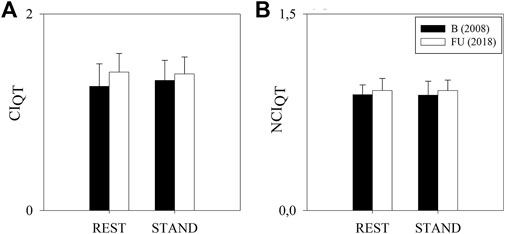
FIGURE 4. Results of the complexity analysis of the QT series in a group of amateur half-marathon runners at baseline (B, white bars) and at a 10-year follow-up (FU, black bars) in resting condition (REST) and during active standing (STAND). The bar graphs show the complexity index (CIQT, panel (A)) and the normalized complexity index (NCIQT, panel (B)). Data are presented as mean ± standard deviation.
When the analyses were repeated excluding the only female subject of the study, the above results were confirmed.
Discussion
The present study was carried out in a group of middle-aged non-elite half-marathon runners within the Corripavia protocol (Dalla Vecchia et al., 2014; De Maria et al., 2021). They were evaluated by means of the QT variability and QT complexity analyses at baseline (fifth Corripavia race) and at a 10-year follow-up.
At FU, the following findings were identified: 1) the QT variability decreased; 2) the complexity of the HP variability increased; 3) the QT variability complexity did not vary.
Effects of Regular Physical Exercise on QT Variability
The analysis of the HP variability, utilized in the previous study (De Maria et al., 2021), demonstrated that this same group of athletes exhibited an increased cardiac vagal modulation and a decreased cardiac sympathetic modulation at FU compared to B (Dalla Vecchia et al., 2014; De Maria et al., 2021), the opposite of what usually occurs with ageing (Lakatta and Levy, 2003). These paradoxical findings have been interpreted as the result of the regular physical activity, that in our group of middle-aged athletes was changed over the years in terms of intensity, i.e. from a strenuous to a moderate level of exercise, but has remained regular. The consequent favorable changes in autonomic balance would imply a decreased risk for cardiovascular events, as lower cardiac sympathetic modulation and higher vagal are known to be associated with reduced risk and better outcomes in several pathological conditions, such as heart failure, myocardial infarction, hypertension and diabetes (Liao et al., 1996; Rovere et al., 1998; La Rovere et al., 2001; Thayer et al., 2010; Pinna et al., 2017).
It is recognized that QT variability analysis may furnish additional prognostic indices (Baumert et al., 2016), in particular augmented QT variability has been associated to sudden cardiac death (Maison-Blanche and Coumel, 1997; Singh et al., 1997; Atiga et al., 1998; Haigney et al., 2004), which represents an open issue in both elite and non-elite athletes (Marijon et al., 2015). In the present study, we focused on the analysis of QT variability and cardiac neural control complexity to test the hypothesis that the more balanced HP variability demonstrated in (De Maria et al., 2021) would also be accompanied by a reduction of the overall QT variability. Indeed, the relation between HP and QT variabilities is complex, where only a portion of the QT variability is related to the HP variability (Porta et al., 1998c; El-Hamad et al., 2019), while some changes in the QT variability are independent. The proportion of the QT variability unrelated to HP variability is not negligible, has been proven to be clinically significant (El-Hamad et al., 2019) and is the only portion responsible of the overall increase of QT variability caused by sympathetic enhancement during head-up tilt test (Porta et al., 2010). In addition, decoupling between HP and QT variability has been observed with the ageing process (Baumert et al., 2013).
Our results showed a decreased overall QT variability at FU, both while supine and during active standing, together with a tendency to a diminished LFQT index, a marker of the sympathetic modulation directed to the ventricles (De Maria et al., 2019). These results corroborate those derived from the HP variability analysis (De Maria et al., 2021) and reinforce the concept that a regular and moderate dose of physical exercise is effective in reducing the cardiovascular risk (Shiroma and Lee, 2010; Wen et al., 2011; Dalla Vecchia et al., 2014; Tesema et al., 2019; De Maria et al., 2021).
These findings are further confirmed by those derived from the complexity analysis of the HP variability, as an overall increase of the HP complexity has been observed, independently from the experimental condition. In this group of middle-aged athletes, the augmented HP complexity found at FU is in keeping with a reduced overall sympathetic cardiac modulation and enhanced vagal one (Catai et al., 2014; Milan-Mattos et al., 2018).
The female participant showed similar results as the male ones. This finding is not obviously sufficient to draw any conclusion about sex influences in the response of the cardiovascular neural control to medium and long-term physical training.
Effect of Regular Physical Exercise on Ageing
Interestingly, the observed increase of HP complexity after at least 10 years of regular exercise might sound paradoxical, as cardiac control complexity is usually reduced by ageing (Kaplan et al., 1991; Catai et al., 2014), as well as by a number of pathological conditions (Goldberger et al., 2002). Therefore, a regular and moderate physical exercise in this group of athletes seems to counteract some effects of ageing on heart control.
The interpretation of QT variability results with respect to ageing is less straightforward. In fact, the effects of ageing processes on the QT variability are still debated (Baumert et al., 2016; Piccirillo et al., 2020), because of the paucity of studies and some contrasting results. However, most studies reported an increased QT and HP variability ratio with ageing, probably dragged by the HP variability decrease more than the QT variability increase (Baumert et al., 2016).
In the athletes of the current study, QT variability decreased at the 10-year follow-up, thus suggesting a rejuvenation of the cardiac neural control. Indeed, in an aged group of athletes regularly exercising, we observed a trend of QT variability opposite to that expected with ageing only. Consistently, they were characterized by a very low cardiovascular disease occurrence (De Maria et al., 2021). An additional confounding factor is the QT-HP relationship that induces a transfer of information from HP to QT and might influence the amount of complexity genuinely attributable to QT dynamics. Future studies should check the impact of this issue via ad hoc multivariate information domain approaches (Porta et al., 2017).
Limitations
The main limitation of the present study is the small sample size and the lack of a control group of sedentary subjects. However, the assessment of the long-term effects of physical training in a group of athletes followed from their forties to fifties, an age particularly at risk for cardiac events, represents a strength of the study worth sharing. It must also be emphasized that the athletes of this study have changed the intensity of training over the years, a limitation inherent in the observational design of the study, which does not allow for considerations on the differences in the effects of strenuous or moderate exercise. Ad hoc studies are needed to elucidate the open questions.
Conclusion
In the present study, we analyzed the QT variability and the HP and QT cardiac control complexity in a small group of middle-aged non-elite half-marathon runners evaluated at baseline and after 10 years.
Regular and moderate exercise over the years was beneficial for this group of athletes, as reflected by the decreased overall QT variability and the concomitant enhanced cardiac control complexity.
Taken together, these results point to a protective effect of physical exercise with respect to both ageing and cardiovascular risk, linked to a more flexible cardiac control with higher ability to interact and counterbalance external inputs, as it occurs in younger people.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of IRCCS Istituti Clinici Scientifici Maugeri (protocol code 2277CE, date of approval 12/03/2019). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization, LADV and BMD; methodology, AP and BMD; software, AP and BMD; investigation, MOG, AC, FP, DL and MM; resources, LADV; data curation, BMD and MOG; writing—original draft preparation, BMD and LADV; writing—review and editing, BMD, DL, MOG, AC, FP, MM, MP, AP and LADV; supervision, LADV and AP; project administration, DL and LADV. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all the volunteers who adhered to the experimental protocol. They thank Franco Corona, President of the Asd Atletica Cento Torri Pavia for the organizational support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2022.880250/full#supplementary-material
References
Albert C. M., Mittleman M. A., Chae C. U., Lee I.-M., Hennekens C. H., Manson J. E. (2000). Triggering of Sudden Death from Cardiac Causes by Vigorous Exertion. N. Engl. J. Med. 343, 1355–1361. doi:10.1056/nejm200011093431902
Atiga W. L., Calkins H., Lawrence J. H., Tomaselli G. F., Smith J. M., Berger R. D. (1998). Beat-to-Beat Repolarization Lability Identifies Patients at Risk for Sudden Cardiac Death. J. Cardiovasc. Electrophysiol. 9, 899–908. doi:10.1111/j.1540-8167.1998.tb00130.x
Baumert M., Czippelova B., Porta A., Javorka M. (2013). Decoupling of QT Interval Variability from Heart Rate Variability with Ageing. Physiol. Meas. 34, 1435–1448. doi:10.1088/0967-3334/34/11/1435
Baumert M., Porta A., Vos M. A., Malik M., Couderc J.-P., Laguna P., et al. (2016). QT Interval Variability in Body Surface ECG: Measurement, Physiological Basis, and Clinical Value: Position Statement and Consensus Guidance Endorsed by the European Heart Rhythm Association Jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace 18, 925–944. doi:10.1093/europace/euv405
Baumert M., Schlaich M. P., Nalivaiko E., Lambert E., Sari C. I., Kaye D. M., et al. (2011). Relation between QT Interval Variability and Cardiac Sympathetic Activity in Hypertension. Am. J. Physiol. Heart Circ. Physiol. 300, H1412–H1417. doi:10.1152/ajpheart.01184.2010
Catai A., Takahashi A., Perseguini N., Milan J., Minatel V., Rehder-Santos P., et al. (2014). Effect of the Postural Challenge on the Dependence of the Cardiovascular Control Complexity on Age. Entropy 16, 6686–6704. doi:10.3390/e16126686
Cavarretta E., Sciarra L., Biondi-Zoccai G., Maffessanti F., Nigro A., Sperandii F., et al. (2022). Age-Related Electrocardiographic Characteristics of Male Junior Soccer Athletes. Front. Cardiovasc. Med. 8, 784170. doi:10.3389/fcvm.2021.784170
Dalla Vecchia L. A., Barbic F., De Maria B., Cozzolino D., Gatti R., Dipaola F., et al. (2019). Can Strenuous Exercise Harm the Heart? Insights from a Study of Cardiovascular Neural Regulation in Amateur Triathletes. PLoS One 14, e0216567. doi:10.1371/journal.pone.0216567
Dalla Vecchia L., Traversi E., Porta A., Lucini D., Pagani M. (2014). On Site Assessment of Cardiac Function and Neural Regulation in Amateur Half marathon Runners. Open Heart 1, e000005. doi:10.1136/openhrt-2013-000005
De Maria B., Bari V., Sgoifo A., Carnevali L., Cairo B., Vaini E., et al. (2019). Concomitant Evaluation of Heart Period and QT Interval Variability Spectral Markers to Typify Cardiac Control in Humans and Rats. Front. Physiol. 10, 1478. doi:10.3389/fphys.2019.01478
De Maria B., de Oliveira Gois M., Catai A. M., Marra C., Lucini D., Porta A., et al. (2021). Ten-Year Follow-Up of Cardiac Function and Neural Regulation in a Group of Amateur Half-Marathon Runners. Open Heart 8, e001561. doi:10.1136/openhrt-2020-001561
Eijsvogels T. M. H., Thompson P. D., Franklin B. A. (2018). The "Extreme Exercise Hypothesis": Recent Findings and Cardiovascular Health Implications. Curr. Treat. Options. Cardio Med. 20, 84. doi:10.1007/s11936-018-0674-3
El-Hamad F., Javorka M., Czippelova B., Krohova J., Turianikova Z., Porta A., et al. (2019). Repolarization Variability Independent of Heart Rate during Sympathetic Activation Elicited by Head-Up Tilt. Med. Biol. Eng. Comput. 57, 1753–1762. doi:10.1007/s11517-019-01998-9
El-Hamad F. J., Bonabi S. Y., Müller A., Steger A., Schmidt G., Baumert M. (2020). Augmented Oscillations in QT Interval Duration Predict Mortality Post Myocardial Infarction Independent of Heart Rate. Front. Physiol. 11, 578173. doi:10.3389/fphys.2020.578173
Furlan R., Piazza S., Dell'Orto S., Gentile E., Cerutti S., Pagani M., et al. (1993). Early and Late Effects of Exercise and Athletic Training on Neural Mechanisms Controlling Heart Rate. Cardiovasc. Res. 27, 482–488. doi:10.1093/cvr/27.3.482
Goldberger A. L., Peng C.-K., Lipsitz L. A. (2002). What Is Physiologic Complexity and How Does it Change with Aging and Disease? Neurobiol. Aging 23, 23–26. doi:10.1016/s0197-4580(01)00266-4
Haigney M. C., Zareba W., Gentlesk P. J., Goldstein R. E., Illovsky M., McNitt S., et al. (2004). QT Interval Variability and Spontaneous Ventricular Tachycardia or Fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II Patients. J. Am. Coll. Cardiol. 44, 1481–1487. doi:10.1016/j.jacc.2004.06.063
Kaplan D. T., Furman M. I., Pincus S. M., Ryan S. M., Lipsitz L. A., Goldberger A. L. (1991). Aging and the Complexity of Cardiovascular Dynamics. Biophysical J. 59, 945–949. doi:10.1016/s0006-3495(91)82309-8
Kim J. H., Malhotra R., Chiampas G., d'Hemecourt P., Troyanos C., Cianca J., et al. (2012). Cardiac Arrest during Long-Distance Running Races. N. Engl. J. Med. 366, 130–140. doi:10.1056/NEJMoa1106468
La Rovere M. T., Pinna G. D., Hohnloser S. H., Marcus F. I., Mortara A., Nohara R., et al. (2001). Baroreflex Sensitivity and Heart Rate Variability in the Identification of Patients at Risk for Life-Threatening Arrhythmias. Circulation 103, 2072–2077. doi:10.1161/01.cir.103.16.2072
Lakatta E. G., Levy D. (2003). Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part I: Aging Arteries: A "set up" for Vascular Disease. Circulation 107, 139–146. doi:10.1161/01.cir.0000048892.83521.58
Lear S. A., Hu W., Rangarajan S., Gasevic D., Leong D., Iqbal R., et al. (2017). The Effect of Physical Activity on Mortality and Cardiovascular Disease in 130 000 People from 17 High-Income, Middle-Income, and Low-Income Countries: The PURE Study. Lancet 390, 2643–2654. doi:10.1016/S0140-6736(17)31634-3
Liao D., Cai J., Barnes R., Tyroler H., Rautaharju P., Holme I., et al. (1996). Association of Cardiac Autonomic Function and the Development of Hypertension: the ARIC Study. Am. J. Hypertens. 9, 1147–1156. doi:10.1016/s0895-7061(96)00249-x
Maison-Blanche P., Coumel P. (1997). Changes in Repolarization Dynamicity and the Assessment of the Arrhythmic Risk. Pacing Clin. Electro 20, 2614–2624. doi:10.1111/j.1540-8159.1997.tb06111.x
Malliani A., Pagani M., Lombardi F., Cerutti S. (1991). Cardiovascular Neural Regulation Explored in the Frequency Domain. Circulation 84, 482–492. doi:10.1161/01.cir.84.2.482
Marijon E., Uy-Evanado A., Reinier K., Teodorescu C., Narayanan K., Jouven X., et al. (2015). Sudden Cardiac Arrest during Sports Activity in Middle Age. Circulation 131, 1384–1391. doi:10.1161/CIRCULATIONAHA.114.011988
Milan-Mattos J. C., Porta A., Perseguini N. M., Minatel V., Rehder-Santos P., Takahashi A. C. M., et al. (2018). Influence of Age and Gender on the Phase and Strength of the Relation between Heart Period and Systolic Blood Pressure Spontaneous Fluctuations. J. Appl. Physiol. 124, 791–804. doi:10.1152/japplphysiol.00903.2017
Piccirillo G., Moscucci F., Fabietti M., Di Iorio C., Mastropietri F., Sabatino T., et al. (2020). Age, Gender and Drug Therapy Influences on Tpeak-Tend Interval and on Electrical Risk Score. J. Electrocardiol. 59, 88–92. doi:10.1016/j.jelectrocard.2020.01.009
Pinna G. D., Porta A., Maestri R., De Maria B., Dalla Vecchia L. A., La Rovere M. T. (2017). Different Estimation Methods of Spontaneous Baroreflex Sensitivity Have Different Predictive Value in Heart Failure Patients. J. Hypertens. 35, 1666–1675. doi:10.1097/HJH.0000000000001377
Porta A., Bari V., Badilini F., Tobaldini E., Gnecchi-Ruscone T., Montano N. (2011). Frequency Domain Assessment of the Coupling Strength between Ventricular Repolarization Duration and Heart Period during Graded Head-Up Tilt. J. Electrocardiol. 44, 662–668. doi:10.1016/j.jelectrocard.2011.08.002
Porta A., Bari V., De Maria B., Baumert M. (2017). A Network Physiology Approach to the Assessment of the Link between Sinoatrial and Ventricular Cardiac Controls. Physiol. Meas. 38, 1472–1489. doi:10.1088/1361-6579/aa6e95
Porta A., Baselli G., Caiani E., Malliani A., Lombardi F., Cerutti S. (1998). Quantifying Electrocardiogram RT-RR Variability Interactions. Med. Biol. Eng. Comput. 36, 27–34. doi:10.1007/BF02522854
Porta A., Baselli G., Lambardi F., Cerutti S., Antolini R., Del Greco M., et al. (1998). Performance Assessment of Standard Algorithms for Dynamic R-T Interval Measurement: Comparison between R-Tapex and R-T(end) Approach. Med. Biol. Eng. Comput. 36, 35–42. doi:10.1007/bf02522855
Porta A., Baselli G., Liberati D., Montano N., Cogliati C., Gnecchi-Ruscone T., et al. (1998). Measuring Regularity by Means of a Corrected Conditional Entropy in Sympathetic Outflow. Biol. Cybernetics 78, 71–78. doi:10.1007/s004220050414
Porta A., Castiglioni P., Bari V., Bassani T., Marchi A., Cividjian A., et al. (2013). K-Nearest-Neighbor Conditional Entropy Approach for the Assessment of the Short-Term Complexity of Cardiovascular Control. Physiol. Meas. 34, 17–33. doi:10.1088/0967-3334/34/1/17
Porta A., Tobaldini E., Gnecchi-Ruscone T., Montano N. (2010). RT Variability Unrelated to Heart Period and Respiration Progressively Increases during Graded Head-Up Tilt. Am. J. Physiology-Heart Circulatory Physiol. 298, H1406–H1414. doi:10.1152/ajpheart.01206.2009
La Rovere M. T., Bigger J. T., Marcus F. I., Mortara A., Schwartz P. J. (1998). Baroreflex Sensitivity and Heart-Rate Variability in Prediction of Total Cardiac Mortality after Myocardial Infarction. ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Lancet 351, 478–484. doi:10.1016/s0140-6736(97)11144-8
Shiroma E. J., Lee I.-M. (2010). Physical Activity and Cardiovascular Health: Lessons Learned from Epidemiological Studies across Age, Gender, and Race/Ethnicity. Circulation 122, 743–752. doi:10.1161/CIRCULATIONAHA.109.914721
Singh J. P., Sleight P., Kardos A., Hart G. (1997). QT Interval Dynamics and Heart Rate Variability Preceding a Case of Cardiac Arrest. Heart 77, 375–377. doi:10.1136/hrt.77.4.375
Tesema G., George M., Hadgu A., Haregot E., Mondal S., Mathivana D. (2019). Does Chronic High-Intensity Endurance Training Have an Effect on Cardiovascular Markers of Active Populations and Athletes? Systematic Review and Meta-Analysis. BMJ Open 9, e032832–2019. doi:10.1136/bmjopen-2019-032832
Thayer J. F., Yamamoto S. S., Brosschot J. F. (2010). The Relationship of Autonomic Imbalance, Heart Rate Variability and Cardiovascular Disease Risk Factors. Int. J. Cardiol. 141, 122–131. doi:10.1016/j.ijcard.2009.09.543
Thompson P. D., Buchner D., Piña I. L., Balady G. J., Williams M. A., Marcus B. H., et al. (2003). Exercise and Physical Activity in the Prevention and Treatment of Atherosclerotic Cardiovascular Disease: A Statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 107, 3109–3116. doi:10.1161/01.CIR.0000075572.40158.77
Wen C. P., Wai J. P. M., Tsai M. K., Yang Y. C., Cheng T. Y. D., Lee M.-C., et al. (2011). Minimum Amount of Physical Activity for Reduced Mortality and Extended Life Expectancy: A Prospective Cohort Study. The Lancet 378, 1244–1253. doi:10.1016/S0140-6736(11)60749-6
Keywords: physical exercise, heart rate variability, QT interval variability, complexity, autonomic nervous system, athletes, half-marathon, ageing
Citation: De Maria B, Lucini D, Gois MO, Catai AM, Perego F, Malacarne M, Pagani M, Porta A and Dalla Vecchia LA (2022) Improvement of Sympathovagal Balance by Regular Exercise May Counteract the Ageing Process. A Study by the Analysis of QT Variability. Front. Physiol. 13:880250. doi: 10.3389/fphys.2022.880250
Received: 21 February 2022; Accepted: 04 April 2022;
Published: 20 April 2022.
Edited by:
Tijana Bojić, University of Belgrade, SerbiaReviewed by:
Federica Moscucci, Sapienza University of Rome, ItalyTelmo Pereira, Fisiologia Clínica, Portugal
Copyright © 2022 De Maria, Lucini, Gois, Catai, Perego, Malacarne, Pagani, Porta and Dalla Vecchia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Porta, alberto.porta@unimi.it
 Beatrice De Maria
Beatrice De Maria Daniela Lucini
Daniela Lucini Mariana de Oliveira Gois
Mariana de Oliveira Gois Aparecida Maria Catai
Aparecida Maria Catai Francesca Perego
Francesca Perego Mara Malacarne
Mara Malacarne Massimo Pagani
Massimo Pagani Alberto Porta
Alberto Porta Laura Adelaide Dalla Vecchia
Laura Adelaide Dalla Vecchia