SARS-CoV-2 Related Myocarditis: What We Know So Far
Abstract
:1. Introduction
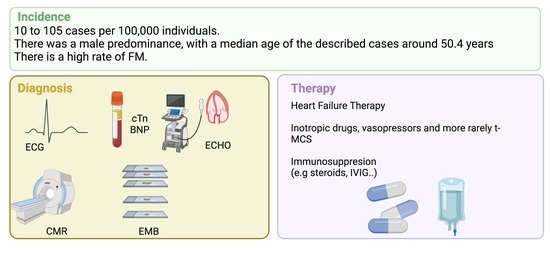
2. Myocardial Injury during COVID-19 and COVID-19-Related Myocarditis: Epidemiological Aspects
3. Diagnostic Evaluation in COVID-19-Related Myocarditis
4. Pathophysiology of COVID-19 Related Myocarditis
5. Prognosis of COVID-19 Related Myocarditis
6. Treatment of COVID-19 Related Myocarditis
7. Update of Myocarditis after SARS-CoV-2 Vaccination
8. Conclusions
Funding
Conflicts of Interest
References
- Chang, D.; Chang, X.; He, Y.; Tan, K.J.K. The Determinants of COVID-19 Morbidity and Mortality across Countries. Sci. Rep. 2022, 12, 5888. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.; Whittaker, C.; Semenova, E.; Rashid, T.; Parks, R.M.; Blenkinsop, A.; Unwin, H.J.T.; Mishra, S.; Bhatt, S.; Gurdasani, D.; et al. Assessment of COVID-19 as the Underlying Cause of Death Among Children and Young People Aged 0 to 19 Years in the US. JAMA Netw. Open 2023, 6, e2253590. [Google Scholar] [CrossRef] [PubMed]
- Vihta, K.D.; Pouwels, K.B.; Peto, T.E.A.; Pritchard, E.; Eyre, D.W.; House, T.; Gethings, O.; Studley, R.; Rourke, E.; Cook, D.; et al. Symptoms and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Positivity in the General Population in the United Kingdom. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, e329–e337. [Google Scholar] [CrossRef]
- Baj, J.; Karakuła-Juchnowicz, H.; Teresiński, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, R.; Forma, A.; Karakuła, K.; Flieger, W.; Portincasa, P.; et al. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef]
- Brosnahan, S.B.; Jonkman, A.H.; Kugler, M.C.; Munger, J.S.; Kaufman, D.A. COVID-19 and Respiratory System Disorders. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2586–2597. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Tsioufis, C.; Oikonomou, E.; Antoniades, C.; Crea, F.; Kaski, J.C.; Tousoulis, D. Inflammatory Mechanisms in COVID-19 and Atherosclerosis: Current Pharmaceutical Perspectives. Int. J. Mol. Sci. 2021, 22, 6607. [Google Scholar] [CrossRef]
- Annie, F.H.; Alkhaimy, H.; Nanjundappa, A.; Elashery, A. Association Between Myocarditis and Mortality in COVID-19 Patients in a Large Registry. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 114–119. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. ESC Scientific Document Group Fourth Universal Definition of Myocardial Infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Cheng, M.P.; Cau, A.; Lee, T.C.; Brodie, D.; Slutsky, A.; Marshall, J.; Murthy, S.; Lee, T.; Singer, J.; Demir, K.K.; et al. Acute Cardiac Injury in Coronavirus Disease 2019 and Other Viral Infections-A Systematic Review and Meta-Analysis. Crit. Care Med. 2021, 49, 1558–1566. [Google Scholar] [CrossRef]
- Abate, S.M.; Mantefardo, B.; Nega, S.; Chekole, Y.A.; Basu, B.; Ali, S.A.; Taddesse, M. Global Burden of Acute Myocardial Injury Associated with COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression. Ann. Med. Surg. 2021, 68, 102594. [Google Scholar] [CrossRef] [PubMed]
- Italia, L.; Tomasoni, D.; Bisegna, S.; Pancaldi, E.; Stretti, L.; Adamo, M.; Metra, M. COVID-19 and Heart Failure: From Epidemiology During the Pandemic to Myocardial Injury, Myocarditis, and Heart Failure Sequelae. Front. Cardiovasc. Med. 2021, 8, 713560. [Google Scholar] [CrossRef] [PubMed]
- Santoso, A.; Pranata, R.; Wibowo, A.; Al-Farabi, M.J.; Huang, I.; Antariksa, B. Cardiac Injury Is Associated with Mortality and Critically Ill Pneumonia in COVID-19: A Meta-Analysis. Am. J. Emerg. Med. 2021, 44, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Lupi, L.; Palazzini, M.; Hendren, N.S.; Grodin, J.L.; Cannistraci, C.V.; Schmidt, M.; Hekimian, G.; Peretto, G.; Bochaton, T.; et al. Prevalence, Characteristics, and Outcomes of COVID-19-Associated Acute Myocarditis. Circulation 2022, 145, 1123–1139. [Google Scholar] [CrossRef]
- Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 301 Acute and Chronic Diseases and Injuries in 188 Countries, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [CrossRef] [Green Version]
- Golpour, A.; Patriki, D.; Hanson, P.J.; McManus, B.; Heidecker, B. Epidemiological Impact of Myocarditis. J. Clin. Med. 2021, 10, 603. [Google Scholar] [CrossRef]
- Singer, M.E.; Taub, I.B.; Kaelber, D.C. Risk of Myocarditis from COVID-19 Infection in People Under Age 20: A Population-Based Analysis. medRxiv 2022. [Google Scholar] [CrossRef]
- Moulson, N.; Petek, B.J.; Drezner, J.A.; Harmon, K.G.; Kliethermes, S.A.; Patel, M.R.; Baggish, A.L.; Outcomes Registry for Cardiac Conditions in Athletes Investigators. SARS-CoV-2 Cardiac Involvement in Young Competitive Athletes. Circulation 2021, 144, 256–266. [Google Scholar] [CrossRef]
- Theocharis, P.; Wong, J.; Pushparajah, K.; Mathur, S.K.; Simpson, J.M.; Pascall, E.; Cleary, A.; Stewart, K.; Adhvaryu, K.; Savis, A.; et al. Multimodality Cardiac Evaluation in Children and Young Adults with Multisystem Inflammation Associated with COVID-19. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 896–903. [Google Scholar] [CrossRef]
- Urban, S.; Fułek, M.; Błaziak, M.; Iwanek, G.; Jura, M.; Fułek, K.; Guzik, M.; Garus, M.; Gajewski, P.; Lewandowski, Ł.; et al. COVID-19 Related Myocarditis in Adults: A Systematic Review of Case Reports. J. Clin. Med. 2022, 11, 5519. [Google Scholar] [CrossRef]
- Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K.; Harmon, K.G.; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 79, 1717–1756. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, S.; Li, Z.; Zhou, P.; Huang, W.; Wang, H.; Shi, J.; Ni, Y.; Lin, L.; Lei, Y. Role of Electrocardiograms in Assessment of Severity and Analysis of the Characteristics of ST Elevation in Acute Myocarditis: A Two-Centre Study. Exp. Ther. Med. 2020, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Buttà, C.; Zappia, L.; Laterra, G.; Roberto, M. Diagnostic and Prognostic Role of Electrocardiogram in Acute Myocarditis: A Comprehensive Review. Ann. Noninvasive Electrocardiol. 2020, 25, e12726. [Google Scholar] [CrossRef] [Green Version]
- Carrizales-Sepúlveda, E.F.; Vera-Pineda, R.; Flores-Ramírez, R.; Hernández-Guajardo, D.A.; Pérez-Contreras, E.; Lozano-Ibarra, M.M.; Ordaz-Farías, A. Echocardiographic Manifestations in COVID-19: A Review. Heart Lung Circ. 2021, 30, 1117–1129. [Google Scholar] [CrossRef]
- Fayol, A.; Livrozet, M.; Boutouyrie, P.; Khettab, H.; Betton, M.; Tea, V.; Blanchard, A.; Bruno, R.-M.; Hulot, J.-S.; French COVID Cohort Study Group. Cardiac Performance in Patients Hospitalized with COVID-19: A 6 Month Follow-up Study. ESC Heart Fail. 2021, 8, 2232–2239. [Google Scholar] [CrossRef] [PubMed]
- Castiello, T.; Georgiopoulos, G.; Finocchiaro, G.; Claudia, M.; Gianatti, A.; Delialis, D.; Aimo, A.; Prasad, S. COVID-19 and Myocarditis: A Systematic Review and Overview of Current Challenges. Heart Fail. Rev. 2022, 27, 251–261. [Google Scholar] [CrossRef]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Caforio, A.L.P. Acute Myocardial Injury, MINOCA, or Myocarditis? Improving Characterization of Coronavirus-Associated Myocardial Involvement. Eur. Heart J. 2020, 41, 2124–2125. [Google Scholar] [CrossRef]
- Cooper, L.T.; Keren, A.; Sliwa, K.; Matsumori, A.; Mensah, G.A. The Global Burden of Myocarditis: Part 1: A Systematic Literature Review for the Global Burden of Diseases, Injuries, and Risk Factors 2010 Study. Glob. Heart 2014, 9, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- From, A.M.; Maleszewski, J.J.; Rihal, C.S. Current Status of Endomyocardial Biopsy. Mayo Clin. Proc. 2011, 86, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease: A Scientific Statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J. Am. Coll. Cardiol. 2007, 50, 1914–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T.; Chahal, C.A.A. Recognizing COVID-19-Related Myocarditis: The Possible Pathophysiology and Proposed Guideline for Diagnosis and Management. Heart Rhythm 2020, 17, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Sarfraz, Z.; Sarfraz, A.; Mukherjee, D.; Batra, N.; Hitawala, G.; Yaqoob, S.; Patel, A.; Agarwala, P.; Ruchika; et al. COVID-19 Infection and Myocarditis: A State-of-the-Art Systematic Review. J. Prim. Care Community Health 2021, 12, 21501327211056800. [Google Scholar] [CrossRef]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheiss, M.; et al. Detection of Viral SARS-CoV-2 Genomes and Histopathological Changes in Endomyocardial Biopsies. ESC Heart Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Leone, O.; Rizzo, S.; De Gaspari, M.; van der Wal, A.C.; Aubry, M.-C.; Bois, M.C.; Lin, P.T.; Maleszewski, J.J.; Stone, J.R. Pathological Features of COVID-19-Associated Myocardial Injury: A Multicentre Cardiovascular Pathology Study. Eur. Heart J. 2020, 41, 3827–3835. [Google Scholar] [CrossRef]
- Greulich, S.; Klingel, K. COVID-19 and Myocarditis: Findings from Cardiac Magnetic Resonance Imaging and Endomyocardial Biopsies. Hamostaseologie 2021, 41, 366–370. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, D.; Fu, S.; Zhang, J.; Yang, X.; Xu, L.; Xu, J.; Wu, Y.; Huang, C.; Ouyang, Y.; et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: A Cross-Sectional Study. Crit. Care 2020, 24, 219. [Google Scholar] [CrossRef]
- Bajaj, R.; Sinclair, H.C.; Patel, K.; Low, B.; Pericao, A.; Manisty, C.; Guttmann, O.; Zemrak, F.; Miller, O.; Longhi, P.; et al. Delayed-Onset Myocarditis Following COVID-19. Lancet Respir. Med. 2021, 9, e32–e34. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-Term Cardiovascular Outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Ammirati, E.; Cipriani, M.; Moro, C.; Raineri, C.; Pini, D.; Sormani, P.; Mantovani, R.; Varrenti, M.; Pedrotti, P.; Conca, C.; et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients With Acute Myocarditis: Multicenter Lombardy Registry. Circulation 2018, 138, 1088–1099. [Google Scholar] [CrossRef]
- Ammirati, E.; Veronese, G.; Bottiroli, M.; Wang, D.W.; Cipriani, M.; Garascia, A.; Pedrotti, P.; Adler, E.D.; Frigerio, M. Update on acute myocarditis. Trends Cardiovasc. Med. 2020, 31, 370–379. [Google Scholar] [CrossRef]
- Radovanovic, M.; Petrovic, M.; Barsoum, M.K.; Nordstrom, C.W.; Calvin, A.D.; Dumic, I.; Jevtic, D.; Hanna, R.D. Influenza Myopericarditis and Pericarditis: A Literature Review. J. Clin. Med. 2022, 11, 4123. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.W.; Tucker, A.M.; Bloom, O.J.; Green, G.; DiFiori, J.P.; Solomon, G.; Phelan, D.; Kim, J.H.; Meeuwisse, W.; Sills, A.K.; et al. Prevalence of Inflammatory Heart Disease Among Professional Athletes With Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol. 2021, 6, 745–752. [Google Scholar] [CrossRef]
- Abou Hassan, O.K.; Sheng, C.C.; Wang, T.K.M.; Cremer, P.C. SARS-CoV-2 Myocarditis: Insights Into Incidence, Prognosis, and Therapeutic Implications. Curr. Cardiol. Rep. 2021, 23, 129. [Google Scholar] [CrossRef]
- The Writing Committee for the REMAP-CAP Investigators. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA 2020, 324, 1317–1329. [Google Scholar] [CrossRef]
- Chau, V.Q.; Giustino, G.; Mahmood, K.; Oliveros, E.; Neibart, E.; Oloomi, M.; Moss, N.; Mitter, S.S.; Contreras, J.P.; Croft, L.; et al. Cardiogenic Shock and Hyperinflammatory Syndrome in Young Males With COVID-19. Circ. Heart Fail. 2020, 13, e007485. [Google Scholar] [CrossRef] [PubMed]
- Dequin, P.-F.; Heming, N.; Meziani, F.; Plantefève, G.; Voiriot, G.; Badié, J.; François, B.; Aubron, C.; Ricard, J.-D.; Ehrmann, S.; et al. Effect of Hydrocortisone on 21-Day Mortality or Respiratory Support Among Critically Ill Patients With COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1298–1306. [Google Scholar] [CrossRef]
- Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [CrossRef] [PubMed]
- Cavalli, G.; De Luca, G.; Campochiaro, C.; Della-Torre, E.; Ripa, M.; Canetti, D.; Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Boffini, N.; et al. Interleukin-1 Blockade with High-Dose Anakinra in Patients with COVID-19, Acute Respiratory Distress Syndrome, and Hyperinflammation: A Retrospective Cohort Study. Lancet Rheumatol. 2020, 2, e325–e331. [Google Scholar] [CrossRef]
- Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; Van Bentum-Puijk, W.; Berry, L.R.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Ucciferri, C.; Auricchio, A.; Di Nicola, M.; Potere, N.; Abbate, A.; Cipollone, F.; Vecchiet, J.; Falasca, K. Canakinumab in a Subgroup of Patients with COVID-19. Lancet Rheumatol. 2020, 2, e457–e458. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Wang, W.; Hayek, S.S.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19. JAMA Intern. Med. 2021, 181, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Heidecker, B.; Dagan, N.; Balicer, R.; Eriksson, U.; Rosano, G.; Coats, A.; Tschöpe, C.; Kelle, S.; Poland, G.A.; Frustaci, A.; et al. Myocarditis Following COVID-19 Vaccine: Incidence, Presentation, Diagnosis, Pathophysiology, Therapy, and Outcomes Put into Perspective. A Clinical Consensus Document Supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2022, 24, 2000–2018. [Google Scholar] [CrossRef]
- VAERS—Guide to Interpreting VAERS Data. Available online: https://vaers.hhs.gov/data/dataguide.html (accessed on 21 May 2023).
- Gargano, J.W. Use of MRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Sinagra, G.; Porcari, A.; Merlo, M.; Barillà, F.; Basso, C.; Ciccone, M.M.; Curcio, A.; Mancone, M.; Mercuro, G.; Muscoli, S.; et al. Myocarditis and pericarditis following mRNA COVID-19 vaccination. Expert opinion of the Italian Society of Cardiology. G. Ital. Cardiol. (2006) 2021, 22, 894–899. [Google Scholar] [CrossRef]
- Sinagra, G.; Porcari, A.; Merlo, M.; Fabris, E.; Imazio, M.; Barillà, F.; Basso, C.; Ciccone, M.M.; Curcio, A.; Mancone, M.; et al. 2022 Update on myocarditis and pericarditis following COVID-19 vaccination. Expert Opinion of the Italian Society of Cardiology. G. Ital. Cardiol. (2006) 2022, 23, 408–413. [Google Scholar] [CrossRef]
- Simone, A.; Herald, J.; Chen, A.; Gulati, N.; Shen, A.Y.-J.; Lewin, B.; Lee, M.-S. Acute Myocarditis Following COVID-19 MRNA Vaccination in Adults Aged 18 Years or Older. JAMA Intern. Med. 2021, 181, 1668–1670. [Google Scholar] [CrossRef]
- Ammirati, E.; Bizzi, E.; Veronese, G.; Groh, M.; Van de Heyning, C.M.; Lehtonen, J.; Pineton de Chambrun, M.; Cereda, A.; Picchi, C.; Trotta, L.; et al. Immunomodulating Therapies in Acute Myocarditis and Recurrent/Acute Pericarditis. Front. Med. 2022, 9, 838564. [Google Scholar] [CrossRef]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 MRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Abbate, A.; Gavin, J.; Madanchi, N.; Kim, C.; Shah, P.R.; Klein, K.; Boatman, J.; Roberts, C.; Patel, S.; Danielides, S. Fulminant Myocarditis and Systemic Hyperinflammation Temporally Associated with BNT162b2 MRNA COVID-19 Vaccination in Two Patients. Int. J. Cardiol. 2021, 340, 119–121. [Google Scholar] [CrossRef]
- Larson, K.F.; Ammirati, E.; Adler, E.D.; Cooper, L.T.; Hong, K.N.; Saponara, G.; Couri, D.; Cereda, A.; Procopio, A.; Cavalotti, C.; et al. Myocarditis After BNT162b2 and MRNA-1273 Vaccination. Circulation 2021, 144, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Rosner, C.M.; Genovese, L.; Tehrani, B.N.; Atkins, M.; Bakhshi, H.; Chaudhri, S.; Damluji, A.A.; de Lemos, J.A.; Desai, S.S.; Emaminia, A.; et al. Myocarditis Temporally Associated With COVID-19 Vaccination. Circulation 2021, 144, 502–505. [Google Scholar] [CrossRef]
- Corradetti, S.; Sclafani, M.; Mistrulli, R.; Gallo, G.; Pagannone, E.; Di Girolamo, M.; Autore, C.; Battistoni, A.; Volpe, M. Features and Follow-up of Patients Affected by Noninflammatory Myocarditis after Coronavirus Disease 2019 Vaccination. J. Cardiovasc. Med. 2023, 24, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.; Dionne, A.; Muniz, J.C.; McHugh, K.E.; Portman, M.A.; Lambert, L.M.; Thacker, D.; Elias, M.D.; Li, J.S.; Toro-Salazar, O.H.; et al. Clinically Suspected Myocarditis Temporally Related to COVID-19 Vaccination in Adolescents and Young Adults: Suspected Myocarditis After COVID-19 Vaccination. Circulation 2022, 145, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 MRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]


| Acute Myocarditis (41) | SARS-CoV-2 Related Myocarditis (20) | |
|---|---|---|
| Age at admission, median (years) | 34 | 44 |
| Male sex (%) | 81% | 73% |
| ECG alterations at admission (% of patients) | 85% | 95% |
Echocardiography alterations at admission
|
|
|
| Abnormal troponin findings (% of patients) | 99.3% | 95% |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
|
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mistrulli, R.; Ferrera, A.; Muthukkattil, M.L.; Volpe, M.; Barbato, E.; Battistoni, A. SARS-CoV-2 Related Myocarditis: What We Know So Far. J. Clin. Med. 2023, 12, 4700. https://doi.org/10.3390/jcm12144700
Mistrulli R, Ferrera A, Muthukkattil ML, Volpe M, Barbato E, Battistoni A. SARS-CoV-2 Related Myocarditis: What We Know So Far. Journal of Clinical Medicine. 2023; 12(14):4700. https://doi.org/10.3390/jcm12144700
Chicago/Turabian StyleMistrulli, Raffaella, Armando Ferrera, Melwyn Luis Muthukkattil, Massimo Volpe, Emanuele Barbato, and Allegra Battistoni. 2023. "SARS-CoV-2 Related Myocarditis: What We Know So Far" Journal of Clinical Medicine 12, no. 14: 4700. https://doi.org/10.3390/jcm12144700