Abstract
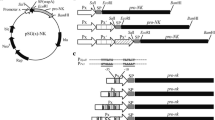
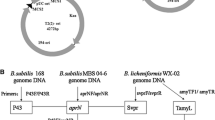
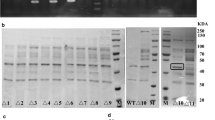
Nattokinase (NK) is a health product for the prevention and potential control of thrombosis diseases. To explore the possibility of enhancing NK production in Bacillus subtilis by altering the promoter of NK gene (PaprN), we tested several methods. We substituted the wild-type −10 box (TACAAT) of PaprN with the consensus sequence (TATAAT) of σA-dependent promoters, mutated the original −35 box (TACTAA) to a partial consensus sequence (TACACA), and expressed aprN from two tandem promoters, respectively. The efficacies of these changes were monitored by fibrinolytic activity, SDS-PAGE, and northern blotting analyses. Fibrinolytic activity analysis showed that altering the −10 region of PaprN could increase NK production by 136%. This production is significantly higher than those reported in the literatures. Similar results were obtained in SDS-PAGE and northern blotting analyses. This engineered promoter was also able to enhance the expression of β-glucuronidase (GUS) by 249%. Partial alteration of the −35 element could slightly improve the production of NK by 13%, while two tandem promoters just had marginal effects on the production of NK. Our study showed that alteration of −10 or −35 elements in PaprN, especially −10 element, is an effective way to enhance the production of heterologous proteins in B. subtilis.





Similar content being viewed by others
References
Akamatsu T, Taguchi H, Okada H (2000) A simple and rapid extraction of high molecular weight chromosomal DNA from Bacillus subtilis protoplasts for cosmid cloning and interspecific transformation. Biosci Biotechnol Biochem 64:1082–1083
Astrup T, Mullertz S (1952) The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys 40:346–351
Chen P, Chao Y (2006) Enhanced production of recombinant nattokinase in Bacillus subtilis by the elimination of limiting factors. Biotechnol Lett 28:1595–1600
Chen P, Chiang C, Chao Y (2007a) Strategy to approach stable production of recombinant nattokinase in Bacillus subtilis. Biotechnol Prog 23(4):808–813
Chen P, Chiang C, Chao Y (2007b) Medium optimization for the production of recombinant nattokinase by Bacillus subtilis using response surface methodology. Biotechnol Prog 23(6):1327–1332
de Ruyter PG, Kuipers OP, de Vos WM (1996) Controlled gene expression system for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol 62:3662–3667
Fujita M, Hong K, Nishimuro S (1995) Characterization of nattokinase-degraded products from human fibrinogen or cross-linked fibrin. Fibrinolysis 9:157–164
Helmann JD (1995) Compilation and analysis of Bacillus subtilis σA-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res 23:2351–2360
Horton R, Hunt H, Ho S, Pullen J, Pease L (1989) Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68
Ishiwa H, Shibahara H (1985) New shuttle vectors for Escherichia coli and Bacillus subtilis. II. Plasmid pHY300PLK, a multipurpose cloning vector with a polylinker, derived from pHY460. Jpn J Genet 60:235–243
Jan J, Valle F, Bolivar F, Merino E (2001) Construction of protein overproducer strains in Bacillus subtilis by an integrative approach. Appl Microbiol Biotechnol 55:69–75
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kawamura F, Doi RH (1984) Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J Bacteriol 160:442–444
Liu B, Song H (2002) Molecular cloning and expression of nattokinase gene in Bacillus subtilis. Acta Biochim Biophys Sin 34:338–340
Sumi H, Hamada N, Tsushima H (1987) A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto: a typical and popular soybean food in the Japanese diet. Experientia 43:1110–1111
Tai M (2006) Nattokinase for prevention of thrombosis. Am J Health Syst Pharm 63:1121–1123
Wei W, Xiang H, Tan H (2002) Two tandem promoters to increase gene expression in Lactococcus lactis. Biotechnol Lett 24:1669–1672
Widner B, Thomas M, Sternberg D, Lammon D, Behr R, Sloma A (2000) Development of marker-free stains of Bacillus subtilis capable of secreting high levels of industrial enzymes. J Ind Microbiol Biotechnol 25:201–212
Wu S, Feng C, Zhong J, Huan L (2007) Roles of S3 site residues of nattokinase on its activity and substrate specificity. J Biochem 142:357–364
Xue GP, Johason JS, Dalrymple BP (1999) High osmolarity improves the electro-transformation efficiency of gram-positive bacteria Bacillus subtilis and Bacillus licheniformis. J Microbiol Methods 34:183–191
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119
Acknowledgments
This work was supported by grants from National Programs for High Technology Research and Development of China: 2006AA10Z319, the Key Project of the Chinese Academy of Sciences (KSCXZ-YW-G-016) and the Top Field Program in Institute of Microbiology, CAS (KSCXZ-SW-113). The authors are grateful to Dr. Juanmei Ye for kindly providing B. subtilis DB104 and to Dr. Zhang Liqun for pHY300PLK. We are also grateful to Xiaobo Liang, Shifang Jia, and Meiling Chen for helping us in fibrinolytic activity analysis, and to Dr. Zhaoqing Luo and Jie Li for critical reading and helpful comments of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, SM., Feng, C., Zhong, J. et al. Enhanced production of recombinant nattokinase in Bacillus subtilis by promoter optimization. World J Microbiol Biotechnol 27, 99–106 (2011). https://doi.org/10.1007/s11274-010-0432-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0432-5