Approach Considerations
The diagnosis of Barrett esophagus does not lead to specific therapy. Little evidence supports the assumption that antisecretory agents or antireflux surgery prevents the occurrence of adenocarcinoma or leads to regression of Barrett esophagus. [20]
In the early to mid-1980s, histamine 2 (H2)-receptor antagonists were the most commonly prescribed agents for treatment of GERD. However, a number of studies were conducted with either cimetidine or ranitidine, and none documented regression of Barrett esophagus. As of April 1, 2020, ranitidine was withdrawn from the market due to an increasing number of products containing the contaminant known as N-nitrosodimethylamine (NDMA), a possible carcinogen.
In the late 1980s, proton pump inhibitors (PPIs) were introduced and proved to be much more efficacious at reducing gastric acid secretion. Even so, the supposition that better acid suppression could induce Barrett esophagus regression was met with optimism, and studies on this to date have been inconclusive. Only 2 of 7 investigators demonstrated some regression. Most were unable to detect any regression, despite documentation of complete normalization of esophageal pH by pH testing.
Currently, the indications for medical therapy in Barrett esophagus—control of symptoms and healing of esophageal mucosa—are the same as those for GERD. An important, as yet unanswered, question is whether abolishing acid completely with high-dose PPIs decreases the risk for adenocarcinoma of the esophagus and warrants the cost and possible adverse effects of this therapy.
Surgery
In addition to acid, the reflux of pancreatic and biliary secretions into the esophagus has been implicated in the pathogenesis of Barrett esophagus. Because medications are effective at reducing only the acid component in reflux, surgical therapy may have an advantage.
However, while studies have shown surgery to be efficacious in the control of GERD symptoms, the results regarding Barrett esophagus regression are inconclusive. No good evidence indicates that surgical therapy provides regression in Barrett esophagus. Thus, antireflux surgery, such as Nissen fundoplication, is not indicated for eradication of Barrett esophagus, but it certainly is reasonable for appropriate patients who desire surgery for control of GERD symptoms.
With relation to reduction of cancer risk in Barrett esophagus, evidence remains insufficient to recommend surgery over medical therapy, although regression of features associated with cancer risk appears to be more common following surgical intervention than medical therapy. [21]
When high-grade dysplasia is discovered and confirmed by a second pathologist, endoscopic ablation is the standard of care.
A systematic review found relatively high pooled incidence rates of recurrence of intestinal metaplasia after achieving complete remission through radiofrequency ablation (9.5% per patient year) and endoscopic therapy (7.1% per patient year) of Barrett esophagus. [22] Predictors of recurrence were increasing age and length of Barrett esophagus. Other pooled incidence rates of recurrence (per patient year) included the following [22] :
-
Dysplastic Barrett esophagus: 2.0% for radiofrequency ablation; 1.3% for endoscopic therapy
-
High-grade dysplasia/esophageal adenocarcinoma: 1.2%for radiofrequency ablation; 0.8% for endoscopic therapy
Diet
Data remain inconclusive regarding the relationship between Barrett esophagus and dietary fruit, fat, and red/processed meat intake, although dietary vegetable intake may lower the risk. [23] Thus, the diet for patients with Barrett esophagus is the same as that recommended for patients with GERD. Patients should avoid the following:
-
Fried or fatty foods
-
Chocolate
-
Peppermint
-
Alcohol
-
Coffee
-
Carbonated beverages
-
Citrus fruits or juices
-
Tomato sauce
-
Ketchup
-
Mustard
-
Vinegar
-
Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs)
Patients also should decrease the size of portions at mealtime, avoid eating 3 hours prior to bedtime, elevate the head of the bed 6 inches, lose weight (if overweight), and stop smoking.
Barrett Esophagus Screening and Surveillance
The Practice Parameters Committee of the American College of Gastroenterology recommends that patients with long-standing GERD symptoms (>5y), particularly those aged 50 years or older, have an upper endoscopy to detect or screen for Barrett esophagus. Once identified, patients with Barrett esophagus should undergo periodic surveillance endoscopy to identify histologic markers for increased cancer risk (dysplasia) or cancer that is at an earlier stage and is amenable to therapy. Preliminary data suggest that surveillance endoscopy does just that. Still, esophageal cancer is an uncommon cause of death.
In a cohort study of patients with Barrett esophagus not undergoing surveillance, only 2.5% of 155 patients died as a result of esophageal cancer, with a mean of 9 years follow-up. Patients with Barrett esophagus should be considered candidates for surveillance only if a potential to prolong life expectancy exists and only if they are eligible for therapy when dysplasia or early cancer is detected. Age and comorbidity are important factors to consider.
The goal of surveillance is the detection of dysplasia or early cancer. Currently, dysplasia is the best histologic marker for cancer risk. The appropriate surveillance interval is based on published data on the natural history of dysplasia and primarily is a function of the grade of dysplasia. Surveillance involves repeated upper endoscopy with systematic 4-quadrant biopsies at 2cm intervals along the entire length of the segment of Barrett esophagus, with additional biopsy of any mucosal abnormalities.
Patients with Barrett esophagus in whom dysplasia is lacking for two consecutive yearly endoscopies may be extended to follow-up at 3-year intervals. Patients with persistent low-grade dysplasia on repeat endoscopy should undergo surveillance every 6 months for 2 cycles; if no progression of disease is noted, surveillance may be extended to yearly follow-up. Management of high-grade dysplasia is more controversial.
Managing high-grade dysplasia
Observer variation is a problem in the grading of dysplasia, and the first step in management of a patient with high-grade dysplasia always is confirmation of the diagnosis by a pathologist who is an expert at reading esophageal biopsies. The surgical literature suggests that as many as 40% of patients who undergo esophagectomy for high-grade dysplasia have concomitant cancer in the resected specimen.
Currently, three management options for high-grade dysplasia exist. One is surveillance endoscopy, with intensive biopsy at 3-month intervals until cancer is detected. The second is endoscopic ablation, and the third is surgical resection.
Because dysplasia and cancer are patchy and cannot be visualized endoscopically, the diagnosis is difficult with even the most intensive surveillance. Current research is focused on developing endoscopic techniques that will highlight dysplastic tissue to allow directed biopsy and also on finding surrogate cellular markers and the like that may help in predicting which patients will develop cancer in the absence of biopsy-proven dysplasia.
Ablative Therapy for Barrett Esophagus
The goal of ablative therapy is to destroy the Barrett epithelium to a sufficient depth to eliminate the intestinal metaplasia and allow regrowth of squamous epithelium. A number of modalities have been tried, usually in combination with medical or surgical therapy because successful ablation appears to require an antacid environment.
Human studies have been performed with radiofrequency ablation (RFA), photodynamic therapy (PDT), argon plasma coagulation (APC), multipolar electrocoagulation (MPEC), heater probes, various forms of lasers, endoscopic mucosal resection (EMR), and cryotherapy.
Ablative therapy is emerging as a viable alternative to surgical resection or esophagectomy for patients with high-grade dysplasia in Barrett esophagus. In fact, in most major medical centers ablation is first-line therapy. A study by Prasad found that the 5-year survival rate for patients with high-grade dysplasia in Barrett esophagus who were treated with PDT and EMR was comparable to that of patients treated with esophagectomy. [24]
A retrospective cohort study of 166 patients with dysplastic Barrett esophagus showed that endoluminal therapy combining endoscopic mucosal resection and ablation is a safe and effective treatment for Barrett esophagus. [25, 26]
Radiofrequency ablation
RFA is FDA approved for eradication of high-grade dysplasia in Barrett esophagus. It is also a treatment option for low-grade dysplasia in Barrett esophagus, provided the risks and benefits are thoroughly discussed with the patient. [27] RFA is a balloon-based, bipolar radiofrequency ablation system. This technique requires the use of sizing balloons to determine the inner diameter of the targeted portion of the esophagus. This is followed by placement of a balloon-based electrode with a 3-cm long treatment area that incorporates tightly spaced, bipolar electrodes that alternate in polarity. The electrode is then attached to a radiofrequency generator and a preselected amount of energy is delivered in less than 1 second at 350 W.
Shaheen et al demonstrated that RFA was associated with a high rate of complete eradication of dysplasia and intestinal metaplasia and a reduced risk of disease progression in patients with dysplastic Barrett esophagus. [28]
In the study, complete eradication occurred in 90.5% of patients with low-grade dysplasia who received radiofrequency ablation, compared with 22.7% of patients in the control group, who underwent a sham procedure. Among patients with high-grade dysplasia, complete eradication occurred in 81% of those in the ablation group, whereas complete eradication occurred in only 19% of patients in the control group.
Patients in the ablation group had less disease progression than did those in the control group (3.6% vs 16.3%, respectively) and fewer cancers than did patients in the control group (1.2% vs 9.3%, respectively).
In a randomized study of 136 patients with Barrett esophagus and low-grade dysplasia, Phoa et al found that in comparison with endoscopic surveillance, endoscopic radiofrequency ablation (RFA) significantly reduced the rate of neoplastic progression to high-grade dysplasia or adenocarcinoma. [29, 30, 31]
Over 3 years of follow-up, RFA reduced the risk of progression to high-grade dysplasia or adenocarcinoma from 26.5% to 1.5% (P < 0.001) and lowered the risk of progression to adenocarcinoma from 8.8% to 1.5% (P = 0.03). [30] Among patients in the ablation group, the rate of complete eradication was 92.6% for dysplasia and 88.2% for intestinal metaplasia, compared with 27.9% for dysplasia and 0% for intestinal metaplasia among patients in the surveillance group.
Use of endoscopic mucosal resection before RFA appears to significantly reduce the risk of treatment failure for Barrett esophagus–associated dysplasia and Barrett esophagus–associated intramucosal adenocarcinoma, whereas a significant predictor of treatment failure appears to be the presence of intramucosal adenocarcinoma involving 50% or more of the columnar metaplastic area on index examination. [32, 33]
Photodynamic therapy
Photodynamic therapy (PDT) involves the use of a photosensitizing agent that accumulates in tissue and induces local necrosis through the production of intracellular free radicals following exposure to light at a certain wavelength. Typically, a hematoporphyrin is used as the photosensitizing agent because it has a greater affinity for neoplastic tissue.
Another agent, 5-aminolevulinic acid (ALA), which induces endogenous protoporphyrin IX and has selectivity for the mucosa over deeper submucosal layers, has also been used. The results have been promising for the regression of Barrett esophagus, as well as for the treatment of dysplasia and superficial carcinoma.
Using PTD to treat 100 patients—including 73 with high-grade dysplasia and 13 with superficial adenocarcinoma—Overholt et al found that Barrett mucosa was completely eliminated in 43 patients and dysplasia was eliminated in 78 patients. [34]
Other studies have shown similar response rates, but Barrett epithelium beneath the superficial squamous layer has been observed, indicating that deeper-placed pluripotent cells may be preserved. Additionally, PDT is an expensive and time-consuming endeavor, and early use was complicated by esophageal stricture requiring dilation in 58% of patients. PDT has been largely replaced by RFA in most medical centers performing ablation.
Argon plasma coagulation (APC) ablation
APC is a method of contact-free high-frequency current coagulation in which the burning of tissue stops as soon as the area is ablated. One study using high-power APC was reported to result in complete restoration of squamous mucosa in 33 out of 33 patients after a mean 1.96 sessions. The major complications were chest pain and odynophagia, which occurred in 57.5% of patients and lasted 3-10 days. Only three patients experienced stricture, which was treated easily with dilation. Only one endoscopic, as well as histologic, recurrence was observed at 10.6-month mean follow-up, but this was in a patient with an ineffective Nissen fundoplication.
Other studies have been less encouraging, with persistence of residual foci of Barrett epithelium under the neosquamous lining in 22-29%; deep esophageal ulceration with massive bleeding, perforation, and even death have been reported.
Multipolar electrocoagulation (MPEC) ablation
MPEC is a method in which the mucosa is ablated by direct contact with an electrocautery probe. Sampliner et al used this technique to treat 10 patients with long-segment Barrett esophagus (LSBE), using half of the patient's own esophagus as an internal control, and found that all 10 patients had the treated segment eliminated by visual and biopsy criteria at 6 months; the untreated segment remained unchanged in the patients, despite acid suppression. Treatment took an average of 2.5 sessions, and 5 patients had complications in 75 total sessions (2 transient odynophagia, 1 transient dysphagia, 1 chest pain, and 1 upper gastrointestinal bleed). [35]
In a 10-year follow-up study of 139 patients with Barrett esophagus, MPEC ablative therapy was associated with complications (all minor) in less than 5% of patients. [36] Recurrent Barrett esophagus occurred in less than 5% of patients. No adenocarcinoma or high-grade dysplasia of the esophagus developed in any of the patients. These results indicated the long-term efficacy and safety of mucosal ablation in Barrett esophagus.
Laser ablation
Lasers have been used in numerous small studies for eradication of Barrett esophagus. Results were less consistent with this modality than with those listed above. Studies that demonstrated full or partial regression endoscopically were confounded by the persistence of glandular elements beneath the neosquamous epithelium in as many as one third of cases.
Cryoablation
One of the newer ablative techniques is low-pressure cryospray ablation using liquid nitrogen, pioneered at the author's institution. The components of the low-pressure spray cryoablation device are as follows:
-
Liquid nitrogen tank
-
Electronic console - For monitoring and controlling cryogen release
-
Dual foot pedal - For controlling cryogen release and heating of the catheter
-
Catheter - A multilayered, 7-9F, open-tipped catheter for spray of supercold nitrogen gas through an upper endoscope
The mechanism of injury is unique relative to other ablative techniques. Cryoablation induces apoptosis, causes cryonecrosis at supercold temperatures (-76°C to -196°C), results in transient ischemia, and can cause immune stimulation. The Barrett epithelium is resistant to apoptosis and, therefore, may be uniquely suited for treatment by cryoablation.
A pilot study at the author's institution using cryoablation in Barrett esophagus with degrees of dysplasia ranging from no dysplasia to multifocal, high-grade dysplasia achieved complete endoscopic reversal of Barrett esophagus in 78% of cases, with no subsquamous specialized intestinal metaplasia (SIM) or dysplasia at 6-month follow-up. These results will need confirmation at other institutions. (See the images below.)
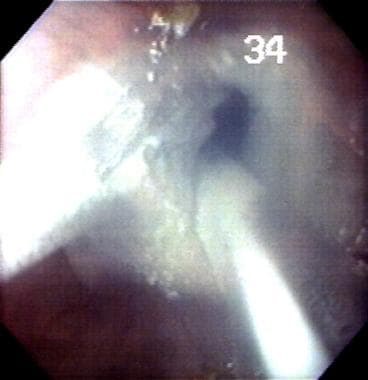 Cryoablation of esophageal lining in Barrett esophagus (BE). This is one of the newest experimental ablative therapies for the esophagus performed at the author's laboratory.
Cryoablation of esophageal lining in Barrett esophagus (BE). This is one of the newest experimental ablative therapies for the esophagus performed at the author's laboratory.
-
Barrett esophagus (BE). The salmon-pink area has specialized intestinal metaplasia. The white area is squamous epithelium.
-
Cryoablation of esophageal lining in Barrett esophagus (BE). This is one of the newest experimental ablative therapies for the esophagus performed at the author's laboratory.
-
Blistering of the esophageal mucosal layer after cryoablation in Barrett esophagus (BE).










