Dosing & Uses
Dosage Forms & Strengths
capsule
- 72mcg
- 145mcg
- 290mcg
Irritable Bowel Syndrome
Indicated for irritable bowel syndrome with constipation (IBS-C)
290 mcg PO qDay
Chronic Idiopathic Constipation
Indicated for chronic idiopathic constipation (CIC)
145 mcg PO qDay
72 mcg PO qDay may be used based on individual presentation
Dosage Modifications
Renal or hepatic impairment
- Not expected to affect clearance of linaclotide or its active metabolite because linaclotide metabolism occurs within gastrointestinal tract and plasma concentrations are not measurable following administration of recommended dosage
Dosage Forms & Strengths
capsule
- 72mcg
Functional Constipation
Indicated for treatment of functional constipation (FC) in pediatric patients aged 6-17 years
72 mg PO qDay
Dosage Modifications
Renal or hepatic impairment
- Not expected to affect clearance of linaclotide or its active metabolite because linaclotide metabolism occurs within gastrointestinal tract and plasma concentrations are not measurable following administration of recommended dosage
Adverse Effects
>10%
IBS-C
- Diarrhea (20%)
CIC
- Diarrhea (16-22%)
1-10%
IBS-C
- Abdominal pain (7%)
- Flatulence (4%)
- Headache (4%)
- Viral gastroenteritis (3%)
- Abdominal distension (2%)
- Severe diarrhea (2%)
- Defecation urgency (<2%)
- Fecal incontinence (<2%)
- Vomiting (<2%)
- Gastroesophageal reflux disease (<2%)
CIC
- Abdominal pain (7%)
- Flatulence (6%)
- Upper respiratory tract infection (5%)
- Sinusitis (3%)
- Abdominal distension (1-3%)
- Defecation urgency (<2%)
- Fecal incontinence (<2%)
- Dyspepsia (<2%)
- Viral gastroenteritis (<2%)
FC
- Diarrhea (4%)
Frequency Not Defined
FC
- Nausea
- Abdominal discomfort
- Dehydration
Postmarketing Reports
Hypersensitivity reactions: Anaphylaxis, angioedema, rash (including hives or urticaria)
Gastrointestinal reactions: Hematochezia, nausea, rectal hemorrhage
Warnings
Black Box Warnings
Risk of serious dehydration in pediatric patients <2 years
- Contraindicated in pediatric patients aged <2 years
- In nonclinical studies in neonatal mice, oral administration of a single, clinically relevant adult dose of linaclotide caused deaths due to dehydration
Contraindications
Patients aged <2 years due to risk of serious dehydration
Known or suspected mechanical gastrointestinal obstruction
Cautions
Pediatric risk
- Contraindicated in patients <2 years of age
- In neonatal mice, fluid secretion increased because of GC-C agonism resulting in mortality within the first 24 hr due to dehydration
- There was no age-dependent trend in GC-C intestinal expression in a clinical study of children 2 to <18 years of age; there are insufficient data available on GC-C intestinal expression in children aged <2 years to assess the risk of developing diarrhea and its potentially serious consequences in these patients
Diarrhea
- Diarrhea was the most common adverse reaction in clinical trials
- In postmarketing reports, severe diarrhea associated with dizziness, syncope, hypotension and electrolyte abnormalities (hypokalemia and hyponatremia) requiring hospitalization or IV fluid administration have been reported
- Diarrhea reported in pediatric patients 6-17 years with functional constipation receiving therapy
- If severe diarrhea occurs, suspend dosing and rehydrate
Pregnancy & Lactation
Pregnancy
Linaclotide and its active metabolite are negligibly absorbed systemically following oral administration, and maternal use is not expected to result in fetal exposure
Insufficient data available data on use in pregnant women to inform any drug-associated risk for major birth defects and miscarriage
Animal data
- No effects on embryofetal development were observed with oral administration of linaclotide in rats and rabbits during organogenesis at doses much higher than the maximum recommended human dosage
- Severe maternal toxicity associated with effects on fetal morphology were observed in mice
Lactation
Linaclotide and its active metabolite were not detected in the milk of lactating women; in adults, concentrations of linaclotide and its active metabolite were below the limit of quantitation in plasma following multiple doses of the drug
Maternal use of the drug is not expected to result in exposure to the drug or its active metabolite in breastfed infants; there is no information on effects on milk production; consider developmental and health benefits of breastfeeding along with the mother’s clinical need for therapy and any potential adverse effects on breastfed infant from the drug or from the underlying maternal condition
No lactation studies in animals have been conducted
Unknown whether negligible systemic absorption of linaclotide by adults will result in a clinically relevant exposure to breastfed infants
Pregnancy Categories
A: Generally acceptable. Controlled studies in pregnant women show no evidence of fetal risk.
B: May be acceptable. Either animal studies show no risk but human studies not available or animal studies showed minor risks and human studies done and showed no risk. C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done. D: Use in LIFE-THREATENING emergencies when no safer drug available. Positive evidence of human fetal risk. X: Do not use in pregnancy. Risks involved outweigh potential benefits. Safer alternatives exist. NA: Information not available.Pharmacology
Mechanism of Action
Guanylate cyclase C (GC-C) agonist; activation of GC-C located on the luminal surface of intestinal epithelial cells leads to increased cyclic guanosine monophosphate (cGMP), anion secretion, fluid secretion, and intestinal transit
Appears to work topically rather than systemically; elevation in intracellular cGMP stimulates secretion of chloride and bicarbonate into the intestinal lumen, mainly through activation of the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel, resulting in increased intestinal fluid and accelerated transit
Absorption
Minimal systemic absorption
Concentrations of linaclotide and its active metabolite in plasma are below the limit of quantitation after oral doses of 145 mcg or 290 mcg
Metabolism
Metabolized within the GI tract to its principal, active metabolite by loss of the terminal tyrosine moiety
Both linaclotide and the metabolite are proteolytically degraded within the intestinal lumen to smaller peptides and naturally occurring amino acids
Elimination
Excretion: 3-5% feces (parent compound) and virtually 100% of active metabolite
Administration
Oral Administration
Take on an empty stomach at least 30 minutes before first meal of the day, at approximately the same time each day
Swallow capsule whole; do not chew or crush
Missed dose
- Skip missed dose and take next dose at regular time; do not take 2 doses at the same time
Difficulty swallowing tablets
- May sprinkle capsule contents on applesauce or in water; not tested in other soft foods or liquids
Administration in applesauce
- Place 1 teaspoonful of applesauce at room temperature into a clean container
- Open capsule
- Sprinkle entire contents (beads) on applesauce
- Consume entire contents immediately; do not chew beads; do not store applesauce-beads mixture for later use
Administration in water
- Pour ~1 ounce (30 mL) of bottled water at room-temperature into clean cup
- Open capsule
- Sprinkle entire contents (beads) into water
- Gently swirl beads and water for at least 20 sec
- Swallow entire mixture of beads and water immediately
- Add another 30 mL of water to any beads remaining in cup, swirl for 20 seconds, and swallow immediately
- Do not store the bead-water mixture for later use
- Note: Drug is coated on surface of the beads and will dissolve off the beads into water; beads will remain visible and will not dissolve; therefore, it is not necessary to consume all beads to deliver complete dose
Nasogastric or gastric feeding tube administration in water
- Open capsule and empty the beads into clean container with 1 ounce (30 mL) of room-temperature bottled water
- Mix by gently swirling beads for at least 20 sec
- Draw-up beads and water mixture to an appropriately sized catheter-tipped syringe and apply rapid and steady pressure (10 mL/10 sec) to dispense syringe contents into the tube
- After administering the bead-water mixture, flush nasogastric/gastric tube with a minimum of 10 mL of water
- Note: It is not necessary to flush all the beads through to deliver complete dose
Storage
Store at 25ºC (77ºF); excursions permitted between 15-30ºC (59-86ºF)
Keep in the original container; do not subdivide or repackage
Protect from moisture
Do not remove desiccant from the container
Images
| BRAND | FORM. | UNIT PRICE | PILL IMAGE |
|---|---|---|---|
| Linzess oral
-
|
145 mcg capsule |  |
|
| Linzess oral
-
|
72 mcg capsule | 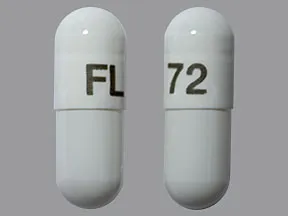 |
|
| Linzess oral
-
|
290 mcg capsule |  |
Copyright © 2010 First DataBank, Inc.
Patient Handout
Formulary
Adding plans allows you to compare formulary status to other drugs in the same class.
To view formulary information first create a list of plans. Your list will be saved and can be edited at any time.
Adding plans allows you to:
- View the formulary and any restrictions for each plan.
- Manage and view all your plans together – even plans in different states.
- Compare formulary status to other drugs in the same class.
- Access your plan list on any device – mobile or desktop.




