-
PDF
- Split View
-
Views
-
Cite
Cite
Anh D Ngo, Richard Taylor, Christine L Roberts, Tuan V Nguyen, Association between Agent Orange and birth defects: systematic review and meta-analysis, International Journal of Epidemiology, Volume 35, Issue 5, October 2006, Pages 1220–1230, https://doi.org/10.1093/ije/dyl038
Close - Share Icon Share
Abstract
Background The association between parental exposure to Agent Orange or dioxin and birth defects is controversial, due to inconsistent findings in the literature. The principal aim of this study was to conduct a meta-analysis of relevant epidemiological studies that examined this association and to assess the heterogeneity among studies.
Methods Relevant studies were identified through a computerized literature search of Medline and Embase from 1966 to 2002; reviewing the reference list of retrieved articles and conference proceedings; and contacting researchers for unpublished studies. A specified protocol was followed to extract data on study details and outcomes. Both fixed-effects and random-effects models were used to synthesize the results of individual studies. The Cochrane Q test and index of heterogeneity (I2) were used to evaluate heterogeneity, and a funnel plot and Egger's test were used to evaluate publication bias.
Results In total, 22 studies including 13 Vietnamese and nine non-Vietnamese studies were identified. The summary relative risk (RR) of birth defects associated with exposure to Agent Orange was 1.95 [95% confidence interval (95% CI) 1.59–2.39], with substantial heterogeneity across studies. Vietnamese studies showed a higher summary RR (RR = 3.00; 95% CI 2.19–4.12) than non-Vietnamese studies (RR = 1.29; 95% CI 1.04–1.59). Sub-group analyses found that the magnitude of association tended to increase with greater degrees of exposure to Agent Orange, rated on intensity and duration of exposure and dioxin concentrations measured in affected populations.
Conclusion Parental exposure to Agent Orange appears to be associated with an increased risk of birth defects.
During the Vietnam War, in an attempt to deprive the vegetation cover used by North Vietnamese forces for concealment, the US Air Force initiated a military campaign named the ‘Operation Ranch Hand’, by which herbicides were repeatedly sprayed in South Vietnam. It is estimated that between 1961 and 1971, ∼77 million litres of herbicides, including 49.3 million litres of Agent Orange containing more than 360 kg of dioxin-contaminated defoliants was sprayed multiple times over 2.6 million acres.1,2 In Vietnam, the number of individuals exposed or potentially exposed to Agent Orange was estimated to be 4.8 million.2 Many military personnel from the United States, Australia, New Zealand, South Korea, North Vietnam, and South Vietnam were also exposed. Furthermore, exposure of civilian populations in Vietnam has continued because of persistence of dioxin in the environment.3
Since the first report of an increase in congenital anomalies in stillbirths and in the offspring of mice following administration of high doses of 2,4,5-T, one component of Agent Orange in 1969,4 concern has been raised that exposure to Agent Orange/dioxin may cause human birth defects. A number of studies have been conducted to determine whether exposure to Agent Orange/dioxin in Vietnam may have increased the risk of having children with birth defects, and the results have often been inconsistent.5–7 The Air Force Health Study compared health outcomes among US veterans of the Operation Ranch Hand (who conducted aerial herbicide spray operations during the Vietnam War) and other Air Force veterans who were not exposed to Agent Orange and found a modest albeit statistically insignificant association between paternal dioxin levels and birth defects.8 However, an Australian study found that ‘the evidence from all available studies supports a causal contribution to defects in veterans' children from a paternally mediated genetic effect’.9
In addition, a number of qualitative reviews have also been performed, attempting to address the issue of Agent Orange/dioxin and birth defects, but no definite conclusion has been made.10–13 A qualitative review by The Institute of Medicine concluded that there was ‘inadequate/insufficient evidence’ to determine whether an association between Agent Orange exposure and birth defects (except for spina bifida) exists.5
A major limitation of these reviews is that they primarily relied on published studies in English and, thus, missed a number of unpublished studies from Vietnam. Given the availability of unpublished data from Vietnam, there is a need to combine results from all previous studies in a systematic and quantitative way. The present study was designed to fill that gap in knowledge by performing a systematic review and meta-analysis of all data, either published or unpublished, relating to Agent Orange/dioxin exposure and birth defects. The advantage of meta-analysis is that it increases statistical power by pooling the results from small individual studies and also permits examination of the variability between studies.14
Methods
Data sources
We performed a systematic electronic search of Medline (from 1966 to June 2002) and Embase (1974 to June 2002) databases to identify published studies, using the following exploded terms: ‘Agent Orange’ and ‘Vietnam’ in conjunction with one of following terms: ‘birth defects’, ‘congenital malformations’, ‘congenital anomalies’, ‘adverse reproductive effects’, and ‘adverse developmental effects’. We extended our search to reviewing the reference list of retrieved articles and performing a manual search of all unpublished studies and papers in three international conference proceedings on Agent Orange/dioxin (1983, 1994, and 2001). Furthermore, we contacted researchers in the field and consulted The National Committee for Investigation of the Consequences of the Chemical Used in the Vietnam War (the 10-80 Committee) to obtain unpublished data from Vietnam.
The criteria for admitting studies to this meta-analysis were: (i) All published and unpublished studies providing relative risk (RR) or odds ratio (OR) on the association between exposure to Agent Orange/dioxin and birth defects or providing data that permit the calculation of these; (ii) All meta-analyses of relevant studies in the literature; (iii) International veteran studies that compared the incidence of birth defects among children of ex-servicemen involved in the Vietnam War with other ex-servicemen or general populations. Although not all international veterans involved in the Vietnam War were exposed to Agent Orange, the inclusion of studies of these veterans can serve as a comparison group when assessing the risk of having children with birth defects in other exposed populations.
Case reports, letters, and review articles were excluded from the analysis. When a study had duplicate publications, only the most inclusive publication was considered. For studies with multiple outcomes, only data concerning birth defects were included in the analysis.
The electronic search yielded 313 publications. After screening these studies based on inclusion and exclusion criteria, nine publications were considered eligible for the meta-analysis. The manual search yielded an additional 17 studies; however, four studies were excluded because of duplication. Ultimately, 22 studies, including 13 Vietnamese studies (two published and 11 unpublished), seven US studies, and two Australian studies were included in this meta-analysis.
All studies were reviewed by a single reviewer (A.D.N.), and data extraction was double checked by another reviewer (R.T.). For the classification of study types, a group of five reviewers deliberated together to reach a consensus. Each study was classified as a cohort study, case–control study, or cross-sectional study. Studies were further allocated into individual or aggregate studies according to the assessment of exposure assigned to groups or measured in individuals. In addition, all studies that met eligibility criteria were assessed for the following characteristics: location of studies (Vietnam vs other countries), the time when studies were carried out, levels of exposure, sample size and sampling methods, the source of exposure and outcome data, the types of parental exposure (mother, father or both), measurement and strength of association, and potential confounding and bias.
Statistical methods
In each study, the data were extracted and summarized into a 2 × 2 table format, by parental exposure to Agent Orange/dioxin (yes, no) and birth defects in children (yes, no). The rates of birth defects among exposed and unexposed groups were computed. If studies did not provide point and interval estimates of OR or RR, using the data of a 2 × 2 table, a spreadsheet package was used for the calculation of these. If available, the estimate of OR or RR and 95% CI adjusted for confounder was obtained from each study, otherwise the crude OR or RR and 95% CI was used.
The synthesis of data was performed using both fixed-effects and random-effects models weighting each study by a measure of its precision, the inverse of the estimate variance.15 The fixed-effects model16 assumes that there is a common effect of the exposure to Agent Orange across different studies and results differ only by chance, whereas the random-effects model (Der Simonian & Laird method)17 incorporates the between-study heterogeneity into the within-study heterogeneity and does not assume that the effect of Agent Orange exposure is common across different studies. Generally, when heterogeneity is present, the random-effects model is considered to be more appropriate because both the random variation within studies and variation among studies can be taken into account. In the presence of between-study variation, random-effects estimates have wider confidence intervals than fixed-effects estimates. When there is no detectable heterogeneity, the two estimates coincide. In the Results section, while random-effects estimates are reported as the primary analysis, fixed-effects estimates are also provided for comparison.
Heterogeneity of effects across studies was assessed by the Cochran's Q statistic15 and was deemed significant when P < 0.05. In addition, the coefficient of inconsistency (I2) as described by Higgins and Thompson18 was also computed to assess the heterogeneity. I2 is an estimate of the proportion of total variation in study estimates that is due to heterogeneity.
In a meta-analysis of observational studies, heterogeneity across studies could be present owing to several factors including, but not limited to, the wide range of study designs, populations under investigation, levels of exposure, methods of ascertainment of the outcome, and study quality deficiencies. In order to explore potential sources of the variability between studies and compare the strength of association among different study groups, we performed sub-group analyses. Studies were stratified according to study designs (case–control, cohort, or cross-sectional), study populations (Vietnamese vs non-Vietnamese), degrees of exposure, and types of parental exposure (maternal vs paternal or both).
To examine the possibility that publication bias may have affected the results, a funnel plot of the natural logarithm of OR or RR vs the inverse of variance of the studies was constructed, and the regression test for small study effects19 was used for quantitative assessment of publication bias and funnel plot asymmetry. The data on ORs or RRs and 95% CIs were entered into the STATA statistical package to perform these calculations. We used the META command to calculate a summary RR and 95% CI, and heterogeneity statistics, and the META-BIAS command to conduct the Egger's test.20
Results
This analysis was based on 22 studies with 205 102 individuals (range per study, 119–127 985).8,9,21–40 The heterogeneity of these studies with respect to time, study design, the population under investigation, and sources of exposure and outcome data are summarized in chronological order in Table 1 (a: non-Vietnamese cohort studies; b: Vietnamese cohort and cross-sectional studies) and Table 2 (Vietnamese and non-Vietnamese case–control studies). Among the 22 studies identified, 16 were retrospective cohort studies, five were case–control studies, and one was a cross-sectional study. Almost 60% (n = 13) of the studies were performed in Vietnam,28–40 and the rest were predominantly conducted in the US (n = 7).8,21,22,24–27 Two US studies22,26 and 11 Vietnamese studies28,29,31–33,35–40 have not been published in any peer-reviewed journal.
Summary of non-Vietnamese cohort studies, and Vietnamese cohort and cross-sectional studies on the association between Agent Orange and birth defects
| First author, year (Country)
. |
Study period
. |
Exposed
. |
Unexposed
. |
Exposure definition/data source and measurement
. |
Case ascertainment
. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (a) Non-Vietnamese cohort studies | ||||||||||
| Lathrop, 1984a (US)22 | 1965–82 | 3293 conceptions among 1174 Ranch Hand veterans | 4106 conceptions among 1531 non-Ranch Hand air force personnel | Aerial spraying /handling of Agent Orange/group exposure based on military records | All congenital anomalies by self-reports | |||||
| CDC, 1988 (Sub-study) (US)24 | 1965–87 | 1791 offspring of American-Vietnam veterans | 1575 offspring of US non-Vietnam veterans | Vietnam military service/group exposure based military records | All congenital anomalies by self-reports and verified by hospital records | |||||
| Field, 1988 (Australia)9 | 1965–87 | 357 Australian-Vietnam veterans in Tasmania | 281 Australian non-veterans in Tasmania | Vietnam military service/group exposure based on military records | Self-reports and verified by physician's examination | |||||
| Wolfe, 1992a (US)26 | 1965–87 | 2533 conceptions among 791 Ranch Hand veterans | 2074 conceptions among 768 non-Ranch Hand air-force veterans | Aerial spraying/handling of Agent Orange/individual exposure measured based on military records, serum dioxin levels | All types of birth defects/self-reports verified by hospital medical records | |||||
| Wolfe, 1995 (US)8 | 1965–92 | 1006 conceptions among 454 Ranch Hand veterans | 1235 conceptions among 570 non-Ranch Hand veterans | Spraying and handling of Agent Orange/individual exposure measured based on military records, serum dioxin levels | All congenital anomalies noted in hospital and medical records | |||||
| Kang, 2000 (US)27 | 1965–99 | 4140 US-Vietnam women veterans | 4140 US non-Vietnam women veterans | Vietnam military service/group exposure based on military records | All congenital malformations by self-reports | |||||
| (b) Vietnamese cohort and cross-sectional studies | ||||||||||
| Khoa, 198328a | 1965–82 | 1163 births of 313 families in a heavily sprayed village between 1965–82 | 587 births of 134 families in an unsprayed village during the same period of time | Direct exposure to the spraying/group exposure based on spraying history | All types of birth defects by self-reports and medical records | |||||
| Can, 198329a | 1965–82 | 11 023 wives of North Vietnam veterans exposed to Agent Orange in the South | 29 041 wives of North Vietnam veterans living in the North only | Military service in sprayed areas/exposure measured based on self-reports and military records | Living children with birth defects by self-reports, hospital records, and physician verification | |||||
| Phuong, 198330 | 1952–81 | 7327 pregnancies of 1219 exposed families in a sprayed commune | 6690 pregnancies of 1126 unexposed families in an unsprayed district in South Vietnam. | Direct exposure to spraying/group exposure based on spraying history | All types of birth defects by self-reports and blindly verified by medical examination | |||||
| Lang, 198332a | 1965–82 | 1142 veterans having been to the South during the war | 613 veterans only living in the North during the war | Military service in sprayed areas/exposure measured based on self-reports, military records | Living children with birth defects by self-reports, hospital records, and blind physician verification | |||||
| Tanh, 198733a | 1965–85 | 1023 families in a sprayed village | 1087 families in unsprayed village in the South | Direct exposure to spraying. group exposure based on spraying history | All types of birth defects by self-reports | |||||
| Phong, 199435a | 1982–92 | 1122 veterans having been to the South during the war | 354 veterans living in the North only | Military service in the South/group exposure based on military records, self-reports | All types of birth defects by self-reports/medical examination | |||||
| Phuong, 199436a | 1965–92 | 394 children of 416 women in a sprayed village born during the spraying time (1964–70) | 2272 children of 159 women of the same village born before the spraying time (1938–1963) | Intra-uterine or breast milk exposure/group exposure based on spraying history | All types of birth defects by self-reports | |||||
| Dai LC, 199437a | 1965–1992 | 1576 North veterans have been to the sprayed areas during spraying time | 153 North veterans only served in the North during the war | Military service in spraying areas/exposure measured based on military records, self-reports | All types of birth defects by self-reports and blind medical examination | |||||
| Dai B, 199438a | 1965–92 | 533 veteran fathers having been to the sprayed areas in South during spraying time | 477 Veteran fathers living in the North during the war | Military service in sprayed areas/exposure measured based on military records, self reports | Self-reported | |||||
| 10-80 Committee, 200039a | 1965–99 | 4310 live births in a period from 1965 to 1999 of a heavily contam-inated commune due to spraying and storing of Agent Orange | 1423 births in the same period of time in three other less contaminated communes. | Direct exposure to spraying/group exposure based on spraying records, dioxin levels in food, soil | All types of birth defects self-reported | |||||
| Hung, 200040a | 1999 | 213 children of 108 veteran fathers having been to the sprayed areas in South during spraying time | 210 children of 141 veteran fathers living in the North during the war | Military service in the sprayed areas/exposure measured based on self-reports | X-ray test to detect spina bifida | |||||
| First author, year (Country)
. |
Study period
. |
Exposed
. |
Unexposed
. |
Exposure definition/data source and measurement
. |
Case ascertainment
. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (a) Non-Vietnamese cohort studies | ||||||||||
| Lathrop, 1984a (US)22 | 1965–82 | 3293 conceptions among 1174 Ranch Hand veterans | 4106 conceptions among 1531 non-Ranch Hand air force personnel | Aerial spraying /handling of Agent Orange/group exposure based on military records | All congenital anomalies by self-reports | |||||
| CDC, 1988 (Sub-study) (US)24 | 1965–87 | 1791 offspring of American-Vietnam veterans | 1575 offspring of US non-Vietnam veterans | Vietnam military service/group exposure based military records | All congenital anomalies by self-reports and verified by hospital records | |||||
| Field, 1988 (Australia)9 | 1965–87 | 357 Australian-Vietnam veterans in Tasmania | 281 Australian non-veterans in Tasmania | Vietnam military service/group exposure based on military records | Self-reports and verified by physician's examination | |||||
| Wolfe, 1992a (US)26 | 1965–87 | 2533 conceptions among 791 Ranch Hand veterans | 2074 conceptions among 768 non-Ranch Hand air-force veterans | Aerial spraying/handling of Agent Orange/individual exposure measured based on military records, serum dioxin levels | All types of birth defects/self-reports verified by hospital medical records | |||||
| Wolfe, 1995 (US)8 | 1965–92 | 1006 conceptions among 454 Ranch Hand veterans | 1235 conceptions among 570 non-Ranch Hand veterans | Spraying and handling of Agent Orange/individual exposure measured based on military records, serum dioxin levels | All congenital anomalies noted in hospital and medical records | |||||
| Kang, 2000 (US)27 | 1965–99 | 4140 US-Vietnam women veterans | 4140 US non-Vietnam women veterans | Vietnam military service/group exposure based on military records | All congenital malformations by self-reports | |||||
| (b) Vietnamese cohort and cross-sectional studies | ||||||||||
| Khoa, 198328a | 1965–82 | 1163 births of 313 families in a heavily sprayed village between 1965–82 | 587 births of 134 families in an unsprayed village during the same period of time | Direct exposure to the spraying/group exposure based on spraying history | All types of birth defects by self-reports and medical records | |||||
| Can, 198329a | 1965–82 | 11 023 wives of North Vietnam veterans exposed to Agent Orange in the South | 29 041 wives of North Vietnam veterans living in the North only | Military service in sprayed areas/exposure measured based on self-reports and military records | Living children with birth defects by self-reports, hospital records, and physician verification | |||||
| Phuong, 198330 | 1952–81 | 7327 pregnancies of 1219 exposed families in a sprayed commune | 6690 pregnancies of 1126 unexposed families in an unsprayed district in South Vietnam. | Direct exposure to spraying/group exposure based on spraying history | All types of birth defects by self-reports and blindly verified by medical examination | |||||
| Lang, 198332a | 1965–82 | 1142 veterans having been to the South during the war | 613 veterans only living in the North during the war | Military service in sprayed areas/exposure measured based on self-reports, military records | Living children with birth defects by self-reports, hospital records, and blind physician verification | |||||
| Tanh, 198733a | 1965–85 | 1023 families in a sprayed village | 1087 families in unsprayed village in the South | Direct exposure to spraying. group exposure based on spraying history | All types of birth defects by self-reports | |||||
| Phong, 199435a | 1982–92 | 1122 veterans having been to the South during the war | 354 veterans living in the North only | Military service in the South/group exposure based on military records, self-reports | All types of birth defects by self-reports/medical examination | |||||
| Phuong, 199436a | 1965–92 | 394 children of 416 women in a sprayed village born during the spraying time (1964–70) | 2272 children of 159 women of the same village born before the spraying time (1938–1963) | Intra-uterine or breast milk exposure/group exposure based on spraying history | All types of birth defects by self-reports | |||||
| Dai LC, 199437a | 1965–1992 | 1576 North veterans have been to the sprayed areas during spraying time | 153 North veterans only served in the North during the war | Military service in spraying areas/exposure measured based on military records, self-reports | All types of birth defects by self-reports and blind medical examination | |||||
| Dai B, 199438a | 1965–92 | 533 veteran fathers having been to the sprayed areas in South during spraying time | 477 Veteran fathers living in the North during the war | Military service in sprayed areas/exposure measured based on military records, self reports | Self-reported | |||||
| 10-80 Committee, 200039a | 1965–99 | 4310 live births in a period from 1965 to 1999 of a heavily contam-inated commune due to spraying and storing of Agent Orange | 1423 births in the same period of time in three other less contaminated communes. | Direct exposure to spraying/group exposure based on spraying records, dioxin levels in food, soil | All types of birth defects self-reported | |||||
| Hung, 200040a | 1999 | 213 children of 108 veteran fathers having been to the sprayed areas in South during spraying time | 210 children of 141 veteran fathers living in the North during the war | Military service in the sprayed areas/exposure measured based on self-reports | X-ray test to detect spina bifida | |||||
aUnpublished study.
Summary of non-Vietnamese cohort studies, and Vietnamese cohort and cross-sectional studies on the association between Agent Orange and birth defects
| First author, year (Country)
. |
Study period
. |
Exposed
. |
Unexposed
. |
Exposure definition/data source and measurement
. |
Case ascertainment
. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (a) Non-Vietnamese cohort studies | ||||||||||
| Lathrop, 1984a (US)22 | 1965–82 | 3293 conceptions among 1174 Ranch Hand veterans | 4106 conceptions among 1531 non-Ranch Hand air force personnel | Aerial spraying /handling of Agent Orange/group exposure based on military records | All congenital anomalies by self-reports | |||||
| CDC, 1988 (Sub-study) (US)24 | 1965–87 | 1791 offspring of American-Vietnam veterans | 1575 offspring of US non-Vietnam veterans | Vietnam military service/group exposure based military records | All congenital anomalies by self-reports and verified by hospital records | |||||
| Field, 1988 (Australia)9 | 1965–87 | 357 Australian-Vietnam veterans in Tasmania | 281 Australian non-veterans in Tasmania | Vietnam military service/group exposure based on military records | Self-reports and verified by physician's examination | |||||
| Wolfe, 1992a (US)26 | 1965–87 | 2533 conceptions among 791 Ranch Hand veterans | 2074 conceptions among 768 non-Ranch Hand air-force veterans | Aerial spraying/handling of Agent Orange/individual exposure measured based on military records, serum dioxin levels | All types of birth defects/self-reports verified by hospital medical records | |||||
| Wolfe, 1995 (US)8 | 1965–92 | 1006 conceptions among 454 Ranch Hand veterans | 1235 conceptions among 570 non-Ranch Hand veterans | Spraying and handling of Agent Orange/individual exposure measured based on military records, serum dioxin levels | All congenital anomalies noted in hospital and medical records | |||||
| Kang, 2000 (US)27 | 1965–99 | 4140 US-Vietnam women veterans | 4140 US non-Vietnam women veterans | Vietnam military service/group exposure based on military records | All congenital malformations by self-reports | |||||
| (b) Vietnamese cohort and cross-sectional studies | ||||||||||
| Khoa, 198328a | 1965–82 | 1163 births of 313 families in a heavily sprayed village between 1965–82 | 587 births of 134 families in an unsprayed village during the same period of time | Direct exposure to the spraying/group exposure based on spraying history | All types of birth defects by self-reports and medical records | |||||
| Can, 198329a | 1965–82 | 11 023 wives of North Vietnam veterans exposed to Agent Orange in the South | 29 041 wives of North Vietnam veterans living in the North only | Military service in sprayed areas/exposure measured based on self-reports and military records | Living children with birth defects by self-reports, hospital records, and physician verification | |||||
| Phuong, 198330 | 1952–81 | 7327 pregnancies of 1219 exposed families in a sprayed commune | 6690 pregnancies of 1126 unexposed families in an unsprayed district in South Vietnam. | Direct exposure to spraying/group exposure based on spraying history | All types of birth defects by self-reports and blindly verified by medical examination | |||||
| Lang, 198332a | 1965–82 | 1142 veterans having been to the South during the war | 613 veterans only living in the North during the war | Military service in sprayed areas/exposure measured based on self-reports, military records | Living children with birth defects by self-reports, hospital records, and blind physician verification | |||||
| Tanh, 198733a | 1965–85 | 1023 families in a sprayed village | 1087 families in unsprayed village in the South | Direct exposure to spraying. group exposure based on spraying history | All types of birth defects by self-reports | |||||
| Phong, 199435a | 1982–92 | 1122 veterans having been to the South during the war | 354 veterans living in the North only | Military service in the South/group exposure based on military records, self-reports | All types of birth defects by self-reports/medical examination | |||||
| Phuong, 199436a | 1965–92 | 394 children of 416 women in a sprayed village born during the spraying time (1964–70) | 2272 children of 159 women of the same village born before the spraying time (1938–1963) | Intra-uterine or breast milk exposure/group exposure based on spraying history | All types of birth defects by self-reports | |||||
| Dai LC, 199437a | 1965–1992 | 1576 North veterans have been to the sprayed areas during spraying time | 153 North veterans only served in the North during the war | Military service in spraying areas/exposure measured based on military records, self-reports | All types of birth defects by self-reports and blind medical examination | |||||
| Dai B, 199438a | 1965–92 | 533 veteran fathers having been to the sprayed areas in South during spraying time | 477 Veteran fathers living in the North during the war | Military service in sprayed areas/exposure measured based on military records, self reports | Self-reported | |||||
| 10-80 Committee, 200039a | 1965–99 | 4310 live births in a period from 1965 to 1999 of a heavily contam-inated commune due to spraying and storing of Agent Orange | 1423 births in the same period of time in three other less contaminated communes. | Direct exposure to spraying/group exposure based on spraying records, dioxin levels in food, soil | All types of birth defects self-reported | |||||
| Hung, 200040a | 1999 | 213 children of 108 veteran fathers having been to the sprayed areas in South during spraying time | 210 children of 141 veteran fathers living in the North during the war | Military service in the sprayed areas/exposure measured based on self-reports | X-ray test to detect spina bifida | |||||
| First author, year (Country)
. |
Study period
. |
Exposed
. |
Unexposed
. |
Exposure definition/data source and measurement
. |
Case ascertainment
. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (a) Non-Vietnamese cohort studies | ||||||||||
| Lathrop, 1984a (US)22 | 1965–82 | 3293 conceptions among 1174 Ranch Hand veterans | 4106 conceptions among 1531 non-Ranch Hand air force personnel | Aerial spraying /handling of Agent Orange/group exposure based on military records | All congenital anomalies by self-reports | |||||
| CDC, 1988 (Sub-study) (US)24 | 1965–87 | 1791 offspring of American-Vietnam veterans | 1575 offspring of US non-Vietnam veterans | Vietnam military service/group exposure based military records | All congenital anomalies by self-reports and verified by hospital records | |||||
| Field, 1988 (Australia)9 | 1965–87 | 357 Australian-Vietnam veterans in Tasmania | 281 Australian non-veterans in Tasmania | Vietnam military service/group exposure based on military records | Self-reports and verified by physician's examination | |||||
| Wolfe, 1992a (US)26 | 1965–87 | 2533 conceptions among 791 Ranch Hand veterans | 2074 conceptions among 768 non-Ranch Hand air-force veterans | Aerial spraying/handling of Agent Orange/individual exposure measured based on military records, serum dioxin levels | All types of birth defects/self-reports verified by hospital medical records | |||||
| Wolfe, 1995 (US)8 | 1965–92 | 1006 conceptions among 454 Ranch Hand veterans | 1235 conceptions among 570 non-Ranch Hand veterans | Spraying and handling of Agent Orange/individual exposure measured based on military records, serum dioxin levels | All congenital anomalies noted in hospital and medical records | |||||
| Kang, 2000 (US)27 | 1965–99 | 4140 US-Vietnam women veterans | 4140 US non-Vietnam women veterans | Vietnam military service/group exposure based on military records | All congenital malformations by self-reports | |||||
| (b) Vietnamese cohort and cross-sectional studies | ||||||||||
| Khoa, 198328a | 1965–82 | 1163 births of 313 families in a heavily sprayed village between 1965–82 | 587 births of 134 families in an unsprayed village during the same period of time | Direct exposure to the spraying/group exposure based on spraying history | All types of birth defects by self-reports and medical records | |||||
| Can, 198329a | 1965–82 | 11 023 wives of North Vietnam veterans exposed to Agent Orange in the South | 29 041 wives of North Vietnam veterans living in the North only | Military service in sprayed areas/exposure measured based on self-reports and military records | Living children with birth defects by self-reports, hospital records, and physician verification | |||||
| Phuong, 198330 | 1952–81 | 7327 pregnancies of 1219 exposed families in a sprayed commune | 6690 pregnancies of 1126 unexposed families in an unsprayed district in South Vietnam. | Direct exposure to spraying/group exposure based on spraying history | All types of birth defects by self-reports and blindly verified by medical examination | |||||
| Lang, 198332a | 1965–82 | 1142 veterans having been to the South during the war | 613 veterans only living in the North during the war | Military service in sprayed areas/exposure measured based on self-reports, military records | Living children with birth defects by self-reports, hospital records, and blind physician verification | |||||
| Tanh, 198733a | 1965–85 | 1023 families in a sprayed village | 1087 families in unsprayed village in the South | Direct exposure to spraying. group exposure based on spraying history | All types of birth defects by self-reports | |||||
| Phong, 199435a | 1982–92 | 1122 veterans having been to the South during the war | 354 veterans living in the North only | Military service in the South/group exposure based on military records, self-reports | All types of birth defects by self-reports/medical examination | |||||
| Phuong, 199436a | 1965–92 | 394 children of 416 women in a sprayed village born during the spraying time (1964–70) | 2272 children of 159 women of the same village born before the spraying time (1938–1963) | Intra-uterine or breast milk exposure/group exposure based on spraying history | All types of birth defects by self-reports | |||||
| Dai LC, 199437a | 1965–1992 | 1576 North veterans have been to the sprayed areas during spraying time | 153 North veterans only served in the North during the war | Military service in spraying areas/exposure measured based on military records, self-reports | All types of birth defects by self-reports and blind medical examination | |||||
| Dai B, 199438a | 1965–92 | 533 veteran fathers having been to the sprayed areas in South during spraying time | 477 Veteran fathers living in the North during the war | Military service in sprayed areas/exposure measured based on military records, self reports | Self-reported | |||||
| 10-80 Committee, 200039a | 1965–99 | 4310 live births in a period from 1965 to 1999 of a heavily contam-inated commune due to spraying and storing of Agent Orange | 1423 births in the same period of time in three other less contaminated communes. | Direct exposure to spraying/group exposure based on spraying records, dioxin levels in food, soil | All types of birth defects self-reported | |||||
| Hung, 200040a | 1999 | 213 children of 108 veteran fathers having been to the sprayed areas in South during spraying time | 210 children of 141 veteran fathers living in the North during the war | Military service in the sprayed areas/exposure measured based on self-reports | X-ray test to detect spina bifida | |||||
aUnpublished study.
Summary of case–control studies on the association between Agent Orange and birth defects
| First author, year, (Country)
. |
Study period
. |
Cases
. |
Controls
. |
Exposure definition/data source and measurement
. |
Case ascertainment
. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Vietnamese studies | ||||||||||
| Erickson, 1984 (US)21 | 1968–80 | 4815 babies with major structural congenital malformations | 2967 normal babies in the same population born at the same period of time (1968–80) | Vietnam military service/Individual exposure measured based on self-report validated by an Exposure Opportunity Index (EOI) | All serious congenital malformations at birth provided by birth defects registry and vital statistics | |||||
| Donovan, 1984 (Australia)23 | 1968–80 | 329 infants with anomalies of all types | 328 normal babies in the same population born at the same period of time | Vietnam military service/Group exposure based on military records | All congenital anomalies diagnosed at, or shortly after births in hospital records | |||||
| Aschengrau, 1990 (US)25 | 1977–80 | 857 live-born or still born infants with one or more congenital anomalies at a Hospital for Women | 998 normal live-born infants at the same hospital | Vietnam military service/Group exposure based on military records | All types of birth defects diagnosed within a few days of births | |||||
| Vietnamese studies | ||||||||||
| Can, 1983a (Vietnam)31 | 1975–82 | 61 living children with observable anomalies in one district in North of Vietnam | 183 living children without anomalies at the same agein the district | Paternal military service in the South/Group exposure based on military records | All type of anomalies detected by a medical examination | |||||
| Phuong, 1989 (Vietnam)34 | 1982 | 15 mothers giving birth to deformed babies a Gyn-Ob hospital in the South | 104 mothers giving birth to normal babies at the same hospital | Direct exposure to spraying/Group exposure based on self-report, spraying history | Birth defects diagnosed at birth | |||||
| First author, year, (Country)
. |
Study period
. |
Cases
. |
Controls
. |
Exposure definition/data source and measurement
. |
Case ascertainment
. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Vietnamese studies | ||||||||||
| Erickson, 1984 (US)21 | 1968–80 | 4815 babies with major structural congenital malformations | 2967 normal babies in the same population born at the same period of time (1968–80) | Vietnam military service/Individual exposure measured based on self-report validated by an Exposure Opportunity Index (EOI) | All serious congenital malformations at birth provided by birth defects registry and vital statistics | |||||
| Donovan, 1984 (Australia)23 | 1968–80 | 329 infants with anomalies of all types | 328 normal babies in the same population born at the same period of time | Vietnam military service/Group exposure based on military records | All congenital anomalies diagnosed at, or shortly after births in hospital records | |||||
| Aschengrau, 1990 (US)25 | 1977–80 | 857 live-born or still born infants with one or more congenital anomalies at a Hospital for Women | 998 normal live-born infants at the same hospital | Vietnam military service/Group exposure based on military records | All types of birth defects diagnosed within a few days of births | |||||
| Vietnamese studies | ||||||||||
| Can, 1983a (Vietnam)31 | 1975–82 | 61 living children with observable anomalies in one district in North of Vietnam | 183 living children without anomalies at the same agein the district | Paternal military service in the South/Group exposure based on military records | All type of anomalies detected by a medical examination | |||||
| Phuong, 1989 (Vietnam)34 | 1982 | 15 mothers giving birth to deformed babies a Gyn-Ob hospital in the South | 104 mothers giving birth to normal babies at the same hospital | Direct exposure to spraying/Group exposure based on self-report, spraying history | Birth defects diagnosed at birth | |||||
aUnpublished study in Vietnam.
Summary of case–control studies on the association between Agent Orange and birth defects
| First author, year, (Country)
. |
Study period
. |
Cases
. |
Controls
. |
Exposure definition/data source and measurement
. |
Case ascertainment
. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Vietnamese studies | ||||||||||
| Erickson, 1984 (US)21 | 1968–80 | 4815 babies with major structural congenital malformations | 2967 normal babies in the same population born at the same period of time (1968–80) | Vietnam military service/Individual exposure measured based on self-report validated by an Exposure Opportunity Index (EOI) | All serious congenital malformations at birth provided by birth defects registry and vital statistics | |||||
| Donovan, 1984 (Australia)23 | 1968–80 | 329 infants with anomalies of all types | 328 normal babies in the same population born at the same period of time | Vietnam military service/Group exposure based on military records | All congenital anomalies diagnosed at, or shortly after births in hospital records | |||||
| Aschengrau, 1990 (US)25 | 1977–80 | 857 live-born or still born infants with one or more congenital anomalies at a Hospital for Women | 998 normal live-born infants at the same hospital | Vietnam military service/Group exposure based on military records | All types of birth defects diagnosed within a few days of births | |||||
| Vietnamese studies | ||||||||||
| Can, 1983a (Vietnam)31 | 1975–82 | 61 living children with observable anomalies in one district in North of Vietnam | 183 living children without anomalies at the same agein the district | Paternal military service in the South/Group exposure based on military records | All type of anomalies detected by a medical examination | |||||
| Phuong, 1989 (Vietnam)34 | 1982 | 15 mothers giving birth to deformed babies a Gyn-Ob hospital in the South | 104 mothers giving birth to normal babies at the same hospital | Direct exposure to spraying/Group exposure based on self-report, spraying history | Birth defects diagnosed at birth | |||||
| First author, year, (Country)
. |
Study period
. |
Cases
. |
Controls
. |
Exposure definition/data source and measurement
. |
Case ascertainment
. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Vietnamese studies | ||||||||||
| Erickson, 1984 (US)21 | 1968–80 | 4815 babies with major structural congenital malformations | 2967 normal babies in the same population born at the same period of time (1968–80) | Vietnam military service/Individual exposure measured based on self-report validated by an Exposure Opportunity Index (EOI) | All serious congenital malformations at birth provided by birth defects registry and vital statistics | |||||
| Donovan, 1984 (Australia)23 | 1968–80 | 329 infants with anomalies of all types | 328 normal babies in the same population born at the same period of time | Vietnam military service/Group exposure based on military records | All congenital anomalies diagnosed at, or shortly after births in hospital records | |||||
| Aschengrau, 1990 (US)25 | 1977–80 | 857 live-born or still born infants with one or more congenital anomalies at a Hospital for Women | 998 normal live-born infants at the same hospital | Vietnam military service/Group exposure based on military records | All types of birth defects diagnosed within a few days of births | |||||
| Vietnamese studies | ||||||||||
| Can, 1983a (Vietnam)31 | 1975–82 | 61 living children with observable anomalies in one district in North of Vietnam | 183 living children without anomalies at the same agein the district | Paternal military service in the South/Group exposure based on military records | All type of anomalies detected by a medical examination | |||||
| Phuong, 1989 (Vietnam)34 | 1982 | 15 mothers giving birth to deformed babies a Gyn-Ob hospital in the South | 104 mothers giving birth to normal babies at the same hospital | Direct exposure to spraying/Group exposure based on self-report, spraying history | Birth defects diagnosed at birth | |||||
aUnpublished study in Vietnam.
Overall association
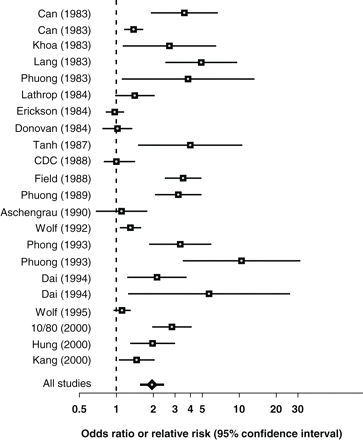
Table 3 lists the data from the 2 × 2 tables, ORs or RRs and the incidence of birth defects in the unexposed for each study, and the summary estimate from meta-analysis of the 22 studies. The overall estimate of the RR of birth defects in the Agent Orange exposed group as compared with the non-exposed group was 1.95 (95% CI 1.59–2.39) by the random-effects model. There was a significant variability across studies, with the heterogeneity Q statistic being 163 (P < 0.001). The coefficient of inconsistency was estimated at 0.87, suggesting that 87% of total variability in effect sizes was due to heterogeneity among studies. Figure 1 (forest plot) shows in chronological order the studies that contribute to an assessment of the overall RR of birth defects associated with Agent Orange exposure with their estimated ORs or RRs and 95% confidence limits.
Pooling analysis for association between Agent Orange exposure and all malformations
| . | . | Birth defects
. |
. | Exposed
. |
. | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author (Reference)
|
Study design
|
Yes
|
No
|
Yes
|
No
|
OR or RR
|
95% confidence interval
|
% Malformed in the unexposed
|
||||||||
| Non-Vietnamese studies | ||||||||||||||||
| Erickson (21) | CC | 428 | 4387 | 268 | 2699 | 0.97a | 0.83–1.14 | NA | ||||||||
| Lathrop (22) | Cohort | 76 | 44 | 757 | 638 | 1.41 | 0.99–2.02 | 6.5 | ||||||||
| Donovan (23) | CC | 127 | 202 | 123 | 205 | 1.02a | 0.78–1.33 | NA | ||||||||
| CDC (24) | Cohort | 130 | 112 | 1661 | 1463 | 1.00a | 0.80–1.40 | 7.1 | ||||||||
| Field (9) | Cohort | 137 | 41 | 437 | 558 | 3.49 | 2.51–4.85 | 5.6 | ||||||||
| Aschengrau (25) | CC | 55 | 146 | 52 | 166 | 1.10a | 0.69–1.76 | NA | ||||||||
| Wolf (26) | Cohort | 229 | 289 | 816 | 1313 | 1.30a | 1.08–1.57 | 18 | ||||||||
| Wolf (8) | Cohort | 177 | 204 | 615 | 777 | 1.11a | 0.96–1.29 | 22.3 | ||||||||
| Kang (27) | Cohort | 95 | 85 | 1570 | 1827 | 1.46a | 1.06–2.02 | 5.8 | ||||||||
| Vietnamese studies | ||||||||||||||||
| Khoa (28) | Cohort | 33 | 6 | 1163 | 581 | 2.70 | 1.14–6.40 | 1.02 | ||||||||
| Can (29) | CC | 30 | 31 | 39 | 144 | 3.57 | 1.93–6.60 | NA | ||||||||
| Phuong (30) | Cohort | 81 | 29 | 5543 | 6389 | 3.19 | 2.09–4.86 | 0.45 | ||||||||
| Can (31) | Cohort | 189 | 521 | 26 501 | 100 774 | 1.38 | 1.17–1.63 | 0.51 | ||||||||
| Lang (32) | Cohort | 71 | 10 | 3076 | 2162 | 4.90 | 2.53–9.48 | 0.46 | ||||||||
| Tanh (33) | Cohort | 24 | 5 | 5395 | 4495 | 3.99 | 1.52–10.44 | 0.10 | ||||||||
| Phuong (34) | CC | 5 | 10 | 12 | 92 | 3.83 | 1.12–13.12 | NA | ||||||||
| Phong (35) | Cohort | 118 | 13 | 3403 | 1269 | 3.31 | 1.87–5.84 | 1.01 | ||||||||
| Phuong (36) | Cohort | 9 | 5 | 385 | 2276 | 10.42 | 3.51–30.93 | 0.22 | ||||||||
| DaiLC (37) | Cohort | 151 | 14 | 6356 | 1275 | 2.14 | 1.24–3.68 | 1.09 | ||||||||
| DaiB (38) | Cohort | 11 | 2 | 1639 | 1700 | 5.67 | 1.26–25.56 | 0.12 | ||||||||
| 10-80 Committee (39) | Cohort | 283 | 33 | 4027 | 1390 | 2.83 | 1.98–4.04 | 2.30 | ||||||||
| Hung (40) | CS | 131 | 94 | 82 | 116 | 1.97 | 1.31–2.96 | 44.00 | ||||||||
| Summary (all studies) | 2590 | 6283 | 63 920 | 132 309 | 1.95 | 1.59–2.39 | ||||||||||
| . | . | Birth defects
. |
. | Exposed
. |
. | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author (Reference)
|
Study design
|
Yes
|
No
|
Yes
|
No
|
OR or RR
|
95% confidence interval
|
% Malformed in the unexposed
|
||||||||
| Non-Vietnamese studies | ||||||||||||||||
| Erickson (21) | CC | 428 | 4387 | 268 | 2699 | 0.97a | 0.83–1.14 | NA | ||||||||
| Lathrop (22) | Cohort | 76 | 44 | 757 | 638 | 1.41 | 0.99–2.02 | 6.5 | ||||||||
| Donovan (23) | CC | 127 | 202 | 123 | 205 | 1.02a | 0.78–1.33 | NA | ||||||||
| CDC (24) | Cohort | 130 | 112 | 1661 | 1463 | 1.00a | 0.80–1.40 | 7.1 | ||||||||
| Field (9) | Cohort | 137 | 41 | 437 | 558 | 3.49 | 2.51–4.85 | 5.6 | ||||||||
| Aschengrau (25) | CC | 55 | 146 | 52 | 166 | 1.10a | 0.69–1.76 | NA | ||||||||
| Wolf (26) | Cohort | 229 | 289 | 816 | 1313 | 1.30a | 1.08–1.57 | 18 | ||||||||
| Wolf (8) | Cohort | 177 | 204 | 615 | 777 | 1.11a | 0.96–1.29 | 22.3 | ||||||||
| Kang (27) | Cohort | 95 | 85 | 1570 | 1827 | 1.46a | 1.06–2.02 | 5.8 | ||||||||
| Vietnamese studies | ||||||||||||||||
| Khoa (28) | Cohort | 33 | 6 | 1163 | 581 | 2.70 | 1.14–6.40 | 1.02 | ||||||||
| Can (29) | CC | 30 | 31 | 39 | 144 | 3.57 | 1.93–6.60 | NA | ||||||||
| Phuong (30) | Cohort | 81 | 29 | 5543 | 6389 | 3.19 | 2.09–4.86 | 0.45 | ||||||||
| Can (31) | Cohort | 189 | 521 | 26 501 | 100 774 | 1.38 | 1.17–1.63 | 0.51 | ||||||||
| Lang (32) | Cohort | 71 | 10 | 3076 | 2162 | 4.90 | 2.53–9.48 | 0.46 | ||||||||
| Tanh (33) | Cohort | 24 | 5 | 5395 | 4495 | 3.99 | 1.52–10.44 | 0.10 | ||||||||
| Phuong (34) | CC | 5 | 10 | 12 | 92 | 3.83 | 1.12–13.12 | NA | ||||||||
| Phong (35) | Cohort | 118 | 13 | 3403 | 1269 | 3.31 | 1.87–5.84 | 1.01 | ||||||||
| Phuong (36) | Cohort | 9 | 5 | 385 | 2276 | 10.42 | 3.51–30.93 | 0.22 | ||||||||
| DaiLC (37) | Cohort | 151 | 14 | 6356 | 1275 | 2.14 | 1.24–3.68 | 1.09 | ||||||||
| DaiB (38) | Cohort | 11 | 2 | 1639 | 1700 | 5.67 | 1.26–25.56 | 0.12 | ||||||||
| 10-80 Committee (39) | Cohort | 283 | 33 | 4027 | 1390 | 2.83 | 1.98–4.04 | 2.30 | ||||||||
| Hung (40) | CS | 131 | 94 | 82 | 116 | 1.97 | 1.31–2.96 | 44.00 | ||||||||
| Summary (all studies) | 2590 | 6283 | 63 920 | 132 309 | 1.95 | 1.59–2.39 | ||||||||||
The summary OR or RR was based on the random-effects model. Test for heterogeneity: Q = 163; P < 0.001; Index of inconsistency (I2) = 0.87. CC: case–control studies; CS: Cross-sectional studies; NA: Not applicable.
aAdjusted OR or RR.
Pooling analysis for association between Agent Orange exposure and all malformations
| . | . | Birth defects
. |
. | Exposed
. |
. | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author (Reference)
|
Study design
|
Yes
|
No
|
Yes
|
No
|
OR or RR
|
95% confidence interval
|
% Malformed in the unexposed
|
||||||||
| Non-Vietnamese studies | ||||||||||||||||
| Erickson (21) | CC | 428 | 4387 | 268 | 2699 | 0.97a | 0.83–1.14 | NA | ||||||||
| Lathrop (22) | Cohort | 76 | 44 | 757 | 638 | 1.41 | 0.99–2.02 | 6.5 | ||||||||
| Donovan (23) | CC | 127 | 202 | 123 | 205 | 1.02a | 0.78–1.33 | NA | ||||||||
| CDC (24) | Cohort | 130 | 112 | 1661 | 1463 | 1.00a | 0.80–1.40 | 7.1 | ||||||||
| Field (9) | Cohort | 137 | 41 | 437 | 558 | 3.49 | 2.51–4.85 | 5.6 | ||||||||
| Aschengrau (25) | CC | 55 | 146 | 52 | 166 | 1.10a | 0.69–1.76 | NA | ||||||||
| Wolf (26) | Cohort | 229 | 289 | 816 | 1313 | 1.30a | 1.08–1.57 | 18 | ||||||||
| Wolf (8) | Cohort | 177 | 204 | 615 | 777 | 1.11a | 0.96–1.29 | 22.3 | ||||||||
| Kang (27) | Cohort | 95 | 85 | 1570 | 1827 | 1.46a | 1.06–2.02 | 5.8 | ||||||||
| Vietnamese studies | ||||||||||||||||
| Khoa (28) | Cohort | 33 | 6 | 1163 | 581 | 2.70 | 1.14–6.40 | 1.02 | ||||||||
| Can (29) | CC | 30 | 31 | 39 | 144 | 3.57 | 1.93–6.60 | NA | ||||||||
| Phuong (30) | Cohort | 81 | 29 | 5543 | 6389 | 3.19 | 2.09–4.86 | 0.45 | ||||||||
| Can (31) | Cohort | 189 | 521 | 26 501 | 100 774 | 1.38 | 1.17–1.63 | 0.51 | ||||||||
| Lang (32) | Cohort | 71 | 10 | 3076 | 2162 | 4.90 | 2.53–9.48 | 0.46 | ||||||||
| Tanh (33) | Cohort | 24 | 5 | 5395 | 4495 | 3.99 | 1.52–10.44 | 0.10 | ||||||||
| Phuong (34) | CC | 5 | 10 | 12 | 92 | 3.83 | 1.12–13.12 | NA | ||||||||
| Phong (35) | Cohort | 118 | 13 | 3403 | 1269 | 3.31 | 1.87–5.84 | 1.01 | ||||||||
| Phuong (36) | Cohort | 9 | 5 | 385 | 2276 | 10.42 | 3.51–30.93 | 0.22 | ||||||||
| DaiLC (37) | Cohort | 151 | 14 | 6356 | 1275 | 2.14 | 1.24–3.68 | 1.09 | ||||||||
| DaiB (38) | Cohort | 11 | 2 | 1639 | 1700 | 5.67 | 1.26–25.56 | 0.12 | ||||||||
| 10-80 Committee (39) | Cohort | 283 | 33 | 4027 | 1390 | 2.83 | 1.98–4.04 | 2.30 | ||||||||
| Hung (40) | CS | 131 | 94 | 82 | 116 | 1.97 | 1.31–2.96 | 44.00 | ||||||||
| Summary (all studies) | 2590 | 6283 | 63 920 | 132 309 | 1.95 | 1.59–2.39 | ||||||||||
| . | . | Birth defects
. |
. | Exposed
. |
. | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author (Reference)
|
Study design
|
Yes
|
No
|
Yes
|
No
|
OR or RR
|
95% confidence interval
|
% Malformed in the unexposed
|
||||||||
| Non-Vietnamese studies | ||||||||||||||||
| Erickson (21) | CC | 428 | 4387 | 268 | 2699 | 0.97a | 0.83–1.14 | NA | ||||||||
| Lathrop (22) | Cohort | 76 | 44 | 757 | 638 | 1.41 | 0.99–2.02 | 6.5 | ||||||||
| Donovan (23) | CC | 127 | 202 | 123 | 205 | 1.02a | 0.78–1.33 | NA | ||||||||
| CDC (24) | Cohort | 130 | 112 | 1661 | 1463 | 1.00a | 0.80–1.40 | 7.1 | ||||||||
| Field (9) | Cohort | 137 | 41 | 437 | 558 | 3.49 | 2.51–4.85 | 5.6 | ||||||||
| Aschengrau (25) | CC | 55 | 146 | 52 | 166 | 1.10a | 0.69–1.76 | NA | ||||||||
| Wolf (26) | Cohort | 229 | 289 | 816 | 1313 | 1.30a | 1.08–1.57 | 18 | ||||||||
| Wolf (8) | Cohort | 177 | 204 | 615 | 777 | 1.11a | 0.96–1.29 | 22.3 | ||||||||
| Kang (27) | Cohort | 95 | 85 | 1570 | 1827 | 1.46a | 1.06–2.02 | 5.8 | ||||||||
| Vietnamese studies | ||||||||||||||||
| Khoa (28) | Cohort | 33 | 6 | 1163 | 581 | 2.70 | 1.14–6.40 | 1.02 | ||||||||
| Can (29) | CC | 30 | 31 | 39 | 144 | 3.57 | 1.93–6.60 | NA | ||||||||
| Phuong (30) | Cohort | 81 | 29 | 5543 | 6389 | 3.19 | 2.09–4.86 | 0.45 | ||||||||
| Can (31) | Cohort | 189 | 521 | 26 501 | 100 774 | 1.38 | 1.17–1.63 | 0.51 | ||||||||
| Lang (32) | Cohort | 71 | 10 | 3076 | 2162 | 4.90 | 2.53–9.48 | 0.46 | ||||||||
| Tanh (33) | Cohort | 24 | 5 | 5395 | 4495 | 3.99 | 1.52–10.44 | 0.10 | ||||||||
| Phuong (34) | CC | 5 | 10 | 12 | 92 | 3.83 | 1.12–13.12 | NA | ||||||||
| Phong (35) | Cohort | 118 | 13 | 3403 | 1269 | 3.31 | 1.87–5.84 | 1.01 | ||||||||
| Phuong (36) | Cohort | 9 | 5 | 385 | 2276 | 10.42 | 3.51–30.93 | 0.22 | ||||||||
| DaiLC (37) | Cohort | 151 | 14 | 6356 | 1275 | 2.14 | 1.24–3.68 | 1.09 | ||||||||
| DaiB (38) | Cohort | 11 | 2 | 1639 | 1700 | 5.67 | 1.26–25.56 | 0.12 | ||||||||
| 10-80 Committee (39) | Cohort | 283 | 33 | 4027 | 1390 | 2.83 | 1.98–4.04 | 2.30 | ||||||||
| Hung (40) | CS | 131 | 94 | 82 | 116 | 1.97 | 1.31–2.96 | 44.00 | ||||||||
| Summary (all studies) | 2590 | 6283 | 63 920 | 132 309 | 1.95 | 1.59–2.39 | ||||||||||
The summary OR or RR was based on the random-effects model. Test for heterogeneity: Q = 163; P < 0.001; Index of inconsistency (I2) = 0.87. CC: case–control studies; CS: Cross-sectional studies; NA: Not applicable.
aAdjusted OR or RR.
Odds ratio or relative risk estimate and 95% confidence intervals for individual studies
Sub-group analysis
Table 4 represents the results of sub-group analyses. The magnitude of association was higher in the Vietnamese population (RR: 3.0; 95% CI 2.19–4.12) than in non-Vietnamese veterans (RR: 1.29; 95% CI 1.04–1.59). In the Vietnamese studies, the magnitude of association was lower in cohort studies than in case–control studies. However, in non-Vietnamese populations, the association between Agent Orange and birth defect was only found in cohort studies, not in case–control studies.
Summary of estimates of risk for congenital anomalies associated with Agent Orange exposure in Vietnam war
| . | . | . | Measure of heterogeneity
. |
. | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup
. |
Number of studies
. |
Summary OR or RR (95% CI)
. |
Q-value
. |
P-value
. |
I2
. |
Analysis model . | ||||||
| All studies | 22 | 1.95 (1.59–2.39) | 163.0 | <0.0001 | 0.87 | RE | ||||||
| Study design | ||||||||||||
| Cohort | 16 | 2.16 (1.68–2.76) | 119.0 | <0.0001 | 0.87 | RE | ||||||
| Case–control | 5 | 1.40 (0.95–2.06) | 20.0 | <0.0001 | 0.80 | RE | ||||||
| Cross-sectional | 1 | 3.00 (2.02–4.47) | NA | NA | NA | NA | ||||||
| Study population | ||||||||||||
| Vietnamese | ||||||||||||
| Cohort | 10 | 3.11 (2.11–4.61) | 50.26 | <0.0001 | 0.82 | RE | ||||||
| Case–control | 2 | 3.55 (2.06–6.14) | 0.00 | 0.965 | . | FE | ||||||
| Cross-sectional | 1 | 1.97 (1.31–2.96) | NA | NA | NA | NA | ||||||
| Total | 13 | 3.00 (2.19–4.12) | 54.6 | <0.0001 | 0.78 | RE | ||||||
| International veterans | ||||||||||||
| Cohort | 6 | 1.45 (1.07–1.96) | 45.63 | <0.0001 | 0.89 | RE | ||||||
| Case–control | 3 | 0.99 (0.87–1.13) | 0.3 | 0.8607 | 0.00 | FE | ||||||
| Total | 9 | 1.29 (1.04–1.59) | 55.0 | <0.0001 | 0.85 | RE | ||||||
| Total (Lathrop22 removed) | 8 | 1.28 (1.02–1.61) | 53.5 | <0.0001 | 0.87 | RE | ||||||
| Exposure | ||||||||||||
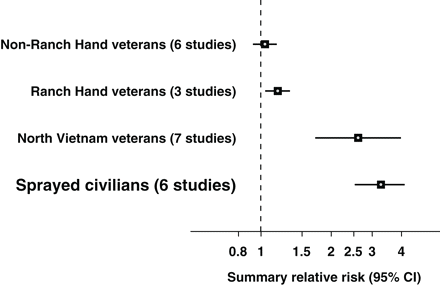
| International non-Ranch Hand veteransa | 6 | 1.04 (0.93–1.16) | 5.14 | 0.273 | 0.03 | FE | ||||||
| Ranch Hand veteransb | 3 | 1.20 (1.08–1.34) | 2.559 | 0.278 | 0.46 | FE | ||||||
| Ranch Hand veteransb (Lathrop22 removed) | 2 | 1.18 (1.05–1.32) | 1.688 | 0.194 | 0.41 | FE | ||||||
| North Vietnam veteransb | 7 | 2.61 (1.72–3.95) | 29.82 | <0.0001 | 0.80 | RE | ||||||
| North Vietnam veteransb (Can et al.31 removed) | 6 | 2.75 (2.17–3.49) | 8.32 | 0.1395 | 0.40 | FE | ||||||
| Sprayed civiliansc | 6 | 3.27 (2.54–4.10) | 5.41 | 0.3679 | 0.08 | FE | ||||||
| . | . | . | Measure of heterogeneity
. |
. | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup
. |
Number of studies
. |
Summary OR or RR (95% CI)
. |
Q-value
. |
P-value
. |
I2
. |
Analysis model . | ||||||
| All studies | 22 | 1.95 (1.59–2.39) | 163.0 | <0.0001 | 0.87 | RE | ||||||
| Study design | ||||||||||||
| Cohort | 16 | 2.16 (1.68–2.76) | 119.0 | <0.0001 | 0.87 | RE | ||||||
| Case–control | 5 | 1.40 (0.95–2.06) | 20.0 | <0.0001 | 0.80 | RE | ||||||
| Cross-sectional | 1 | 3.00 (2.02–4.47) | NA | NA | NA | NA | ||||||
| Study population | ||||||||||||
| Vietnamese | ||||||||||||
| Cohort | 10 | 3.11 (2.11–4.61) | 50.26 | <0.0001 | 0.82 | RE | ||||||
| Case–control | 2 | 3.55 (2.06–6.14) | 0.00 | 0.965 | . | FE | ||||||
| Cross-sectional | 1 | 1.97 (1.31–2.96) | NA | NA | NA | NA | ||||||
| Total | 13 | 3.00 (2.19–4.12) | 54.6 | <0.0001 | 0.78 | RE | ||||||
| International veterans | ||||||||||||
| Cohort | 6 | 1.45 (1.07–1.96) | 45.63 | <0.0001 | 0.89 | RE | ||||||
| Case–control | 3 | 0.99 (0.87–1.13) | 0.3 | 0.8607 | 0.00 | FE | ||||||
| Total | 9 | 1.29 (1.04–1.59) | 55.0 | <0.0001 | 0.85 | RE | ||||||
| Total (Lathrop22 removed) | 8 | 1.28 (1.02–1.61) | 53.5 | <0.0001 | 0.87 | RE | ||||||
| Exposure | ||||||||||||
| International non-Ranch Hand veteransa | 6 | 1.04 (0.93–1.16) | 5.14 | 0.273 | 0.03 | FE | ||||||
| Ranch Hand veteransb | 3 | 1.20 (1.08–1.34) | 2.559 | 0.278 | 0.46 | FE | ||||||
| Ranch Hand veteransb (Lathrop22 removed) | 2 | 1.18 (1.05–1.32) | 1.688 | 0.194 | 0.41 | FE | ||||||
| North Vietnam veteransb | 7 | 2.61 (1.72–3.95) | 29.82 | <0.0001 | 0.80 | RE | ||||||
| North Vietnam veteransb (Can et al.31 removed) | 6 | 2.75 (2.17–3.49) | 8.32 | 0.1395 | 0.40 | FE | ||||||
| Sprayed civiliansc | 6 | 3.27 (2.54–4.10) | 5.41 | 0.3679 | 0.08 | FE | ||||||
RE: Random-effects model; FE: fixed-effects model; I 2: Index of inconsistency.
aNo or minimal exposure.
bFather exposed.
cBoth parents exposed.
Summary of estimates of risk for congenital anomalies associated with Agent Orange exposure in Vietnam war
| . | . | . | Measure of heterogeneity
. |
. | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup
. |
Number of studies
. |
Summary OR or RR (95% CI)
. |
Q-value
. |
P-value
. |
I2
. |
Analysis model . | ||||||
| All studies | 22 | 1.95 (1.59–2.39) | 163.0 | <0.0001 | 0.87 | RE | ||||||
| Study design | ||||||||||||
| Cohort | 16 | 2.16 (1.68–2.76) | 119.0 | <0.0001 | 0.87 | RE | ||||||
| Case–control | 5 | 1.40 (0.95–2.06) | 20.0 | <0.0001 | 0.80 | RE | ||||||
| Cross-sectional | 1 | 3.00 (2.02–4.47) | NA | NA | NA | NA | ||||||
| Study population | ||||||||||||
| Vietnamese | ||||||||||||
| Cohort | 10 | 3.11 (2.11–4.61) | 50.26 | <0.0001 | 0.82 | RE | ||||||
| Case–control | 2 | 3.55 (2.06–6.14) | 0.00 | 0.965 | . | FE | ||||||
| Cross-sectional | 1 | 1.97 (1.31–2.96) | NA | NA | NA | NA | ||||||
| Total | 13 | 3.00 (2.19–4.12) | 54.6 | <0.0001 | 0.78 | RE | ||||||
| International veterans | ||||||||||||
| Cohort | 6 | 1.45 (1.07–1.96) | 45.63 | <0.0001 | 0.89 | RE | ||||||
| Case–control | 3 | 0.99 (0.87–1.13) | 0.3 | 0.8607 | 0.00 | FE | ||||||
| Total | 9 | 1.29 (1.04–1.59) | 55.0 | <0.0001 | 0.85 | RE | ||||||
| Total (Lathrop22 removed) | 8 | 1.28 (1.02–1.61) | 53.5 | <0.0001 | 0.87 | RE | ||||||
| Exposure | ||||||||||||
| International non-Ranch Hand veteransa | 6 | 1.04 (0.93–1.16) | 5.14 | 0.273 | 0.03 | FE | ||||||
| Ranch Hand veteransb | 3 | 1.20 (1.08–1.34) | 2.559 | 0.278 | 0.46 | FE | ||||||
| Ranch Hand veteransb (Lathrop22 removed) | 2 | 1.18 (1.05–1.32) | 1.688 | 0.194 | 0.41 | FE | ||||||
| North Vietnam veteransb | 7 | 2.61 (1.72–3.95) | 29.82 | <0.0001 | 0.80 | RE | ||||||
| North Vietnam veteransb (Can et al.31 removed) | 6 | 2.75 (2.17–3.49) | 8.32 | 0.1395 | 0.40 | FE | ||||||
| Sprayed civiliansc | 6 | 3.27 (2.54–4.10) | 5.41 | 0.3679 | 0.08 | FE | ||||||
| . | . | . | Measure of heterogeneity
. |
. | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup
. |
Number of studies
. |
Summary OR or RR (95% CI)
. |
Q-value
. |
P-value
. |
I2
. |
Analysis model . | ||||||
| All studies | 22 | 1.95 (1.59–2.39) | 163.0 | <0.0001 | 0.87 | RE | ||||||
| Study design | ||||||||||||
| Cohort | 16 | 2.16 (1.68–2.76) | 119.0 | <0.0001 | 0.87 | RE | ||||||
| Case–control | 5 | 1.40 (0.95–2.06) | 20.0 | <0.0001 | 0.80 | RE | ||||||
| Cross-sectional | 1 | 3.00 (2.02–4.47) | NA | NA | NA | NA | ||||||
| Study population | ||||||||||||
| Vietnamese | ||||||||||||
| Cohort | 10 | 3.11 (2.11–4.61) | 50.26 | <0.0001 | 0.82 | RE | ||||||
| Case–control | 2 | 3.55 (2.06–6.14) | 0.00 | 0.965 | . | FE | ||||||
| Cross-sectional | 1 | 1.97 (1.31–2.96) | NA | NA | NA | NA | ||||||
| Total | 13 | 3.00 (2.19–4.12) | 54.6 | <0.0001 | 0.78 | RE | ||||||
| International veterans | ||||||||||||
| Cohort | 6 | 1.45 (1.07–1.96) | 45.63 | <0.0001 | 0.89 | RE | ||||||
| Case–control | 3 | 0.99 (0.87–1.13) | 0.3 | 0.8607 | 0.00 | FE | ||||||
| Total | 9 | 1.29 (1.04–1.59) | 55.0 | <0.0001 | 0.85 | RE | ||||||
| Total (Lathrop22 removed) | 8 | 1.28 (1.02–1.61) | 53.5 | <0.0001 | 0.87 | RE | ||||||
| Exposure | ||||||||||||
| International non-Ranch Hand veteransa | 6 | 1.04 (0.93–1.16) | 5.14 | 0.273 | 0.03 | FE | ||||||
| Ranch Hand veteransb | 3 | 1.20 (1.08–1.34) | 2.559 | 0.278 | 0.46 | FE | ||||||
| Ranch Hand veteransb (Lathrop22 removed) | 2 | 1.18 (1.05–1.32) | 1.688 | 0.194 | 0.41 | FE | ||||||
| North Vietnam veteransb | 7 | 2.61 (1.72–3.95) | 29.82 | <0.0001 | 0.80 | RE | ||||||
| North Vietnam veteransb (Can et al.31 removed) | 6 | 2.75 (2.17–3.49) | 8.32 | 0.1395 | 0.40 | FE | ||||||
| Sprayed civiliansc | 6 | 3.27 (2.54–4.10) | 5.41 | 0.3679 | 0.08 | FE | ||||||
RE: Random-effects model; FE: fixed-effects model; I 2: Index of inconsistency.
aNo or minimal exposure.
bFather exposed.
cBoth parents exposed.
In either cohort or case–control studies, significant heterogeneity of risk estimates was observed. The index of inconsistency for all Vietnamese studies (I2) was 0.78 (P < 0.0001). Similarly, there was a significant heterogeneity in the international veterans study with I2 being 0.85 (P < 0.0001).
Evidence based on intensity, duration of exposure, and dioxin levels measured in human tissues41–50 indicates that sprayed civilians were the most heavily exposed, followed by North Vietnam veterans, and then US Ranch Hand veterans. International non-Ranch Hand veterans had minimal or no exposure. Sub-group meta-analyses by intensity and duration of exposure show that summary relative risks were homogeneous, except for North Vietnam veteran studies for which the heterogeneity test was significant (P < 0.0001; Table 4). The magnitude of association varied in connection with the pattern of exposure to Agent Orange/dioxin: no significant increased risk of birth defects following no or low exposure (non-Ranch Hand veterans: RR = 1.04; 95% CI 0.93–1.16), higher significant risks following higher exposure (Ranch Hand veterans: RR = 1.20; 95% CI 1.08–1.34, North Vietnam veterans: RR = 2.61, 95% CI 1.72–3.95, and Vietnamese sprayed civilians: RR = 3.27, 95% CI 2.54–4.10) (Figure 2).
Agent Orange exposure and birth defects in four differentially exposed groups
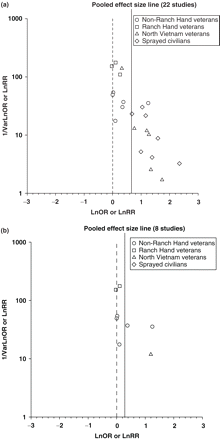
Funnel plots of all studies revealed a severe asymmetrical distribution of ORs or RRs (Figure 3a), suggesting the presence of publication bias with the absence of small studies producing no statistically significant effects (Egger's test: intercept = 3.75; P << 0.001). When studies were stratified by location of studies, the funnel plots and Egger's test indicate the possibility of publication bias among Vietnamese studies (intercept = 3.06; P << 0.001) but not among non-Vietnamese studies (intercept = 3.13; P = 0.225). Moreover, the funnel plot and Egger's test suggest some evidence of publication bias among all published studies (intercept = 3.80; P = 0.096) (Figure 3b).
Funnel plots of ln OR or ln RR and precision for (a) all studies, and (b) published studies. Precision was defined as inverse variance of ln (OR/RR)
Discussion
Principal findings
Results of this meta-analysis combining data from 22 studies support the hypothesis that exposure to Agent Orange is associated with a statistically significant increase in the risk of birth defects, with a significant heterogeneity of effects across study populations. The result complements a previous finding5 that the risk of spina bifida, a specific birth defect, was elevated with Agent Orange exposure.
The strength of the association between exposure to Agent Orange/dioxin and birth defects in the Vietnamese population was substantially greater than that in the non-Vietnamese populations. This observation is consistent with the finding that higher dioxin concentrations were found in the Vietnamese population in affected areas1,44,47 than in US Vietnam veterans.43,50 Moreover, sub-group meta-analyses of four differentially exposed groups also demonstrated that the magnitude of the association increased with greater degrees of exposure to Agent Orange, rated on intensity and duration of exposure, and dioxin concentrations measured in human blood or adipose tissues. This finding suggests a pattern of a dose–response relationship between studies. In addition, the results were also consistent with the fact that in the Vietnamese civilian studies, women and men were both exposed so that effects could be both teratogenic and mutagenic, whereas in the studies of North Vietnam and Ranch Hand veterans only mutagenesis was possible since the exposed were males.
The estimate of association is stronger in cohort studies than in case–control studies particularly among non-Vietnamese studies. This was probably due to the fact that out of six non-Vietnamese cohort studies, three were conducted on Ranch Hand veterans who were actually exposed to Agent Orange while all three non-Vietnamese case–control studies considered non-Ranch Hand veterans involved in the Vietnam war who had minimal or no exposure as the exposed. Among the Vietnamese studies in which there is no discrepancy in exposure by study type, cohort studies did not show a higher estimate of risk than case–control studies.
The presence of heterogeneity across studies is hardly surprising, given the wide range of study designs, populations under investigation, methods of ascertainment of outcome, and levels of exposure. However, sub-group analyses defined by degrees of exposure have almost resolved heterogeneity. Except for studies of North Vietnam veterans, studies of international non-Ranch Hand veterans, Ranch Hand veterans, and Vietnamese sprayed civilians appeared to be homogeneous and, thus, measuring the same underlying risk of birth defects associated with exposure to Agent Orange/dioxin. The studies of North Vietnam veterans become homogeneous after removing the study by Can et al.,31 which is methodologically different from other studies of these veterans. The difference is that exposure assessment was made based on history of military service of North Vietnam veterans reported by their wives. After removing this study, the summary RR increased slightly from 2.61 (95% CI 1.72–3.95) to 2.75 (95% CI 2.17–3.49) (Table 1).
Strengths and weaknesses of the study
This study is the first to have incorporated unpublished data from Vietnamese veterans and sprayed civilians, which allows a better delineation of associations and evaluation of a dose–response relationship across different populations. The strength of this meta-analysis is to increase statistical power over individual studies and examine the variability between studies. Given that any single study is unlikely to unequivocally confirm or refute the association between Agent Orange exposure and birth defects, a systematic combination of data from all available sources to arrive at a more reliable estimate is preferable to any individual study.
The limitation of this study is the inability to perform sub-group meta-analyses for specific categories of birth defects except for spina bifida, which will be presented in another paper. This is because the data on specific birth defects was only available in a small number of studies, and, where it was available, only based on a small number of defects for each category. Furthermore, the way in which the birth defects were categorized was not always consistent across studies. A new teratogen or mutagen may cause a single type of malformation or may cause a specific combination of them.51 Therefore, it is possible that many of birth defects may not be related to Agent Orange exposure, and their inclusion can cause dilution of RRs for those that are related.
Some of the included studies were unable to obtain data on known risk factors for birth defects such as maternal and paternal age, ethnicity, parental tobacco intake and alcohol consumption, maternal diabetes, folic acid intake, and infection and fever during pregnancy. Thus, it was impossible to examine the effects of these factors on the summary RR except in as much as most non-Vietnamese investigators adjusted their ORs or RRs for confounding from some or all of these risk factors.
There was double counting in Ranch Hand studies. The total number of veterans in all three Ranch Hand studies8,22,26 is 2491, almost two times higher than 1250—the estimated total of these veterans.52 After a close examination of quality of these three studies, we decided to exclude the study by Lathrop,22 because it has major methodological flaws. These flaws include exposure assessment based on aggregate measure, ascertainment of birth defects based on self-reports without validation and lack of control for potential confounders. In contrast, the two other studies8,26 based exposure assessment on levels of parental serum dioxin, ascertained birth defects from hospital records, and made appropriate adjustment for potential confounders. Furthermore, the total of Ranch Hand veterans in these two studies is 1245; the number does not exceed the estimated total of these veterans. Thus, double counting was unlikely. After excluding the Lathrop study, the summary RR of non-Vietnamese studies decreases slightly, but is still statistically significant (RR = 1.28; 95% CI 1.02–1.61), and the summary RR of Ranch Hand studies is almost unchanged (RR = 1.20; 95% CI 1.08–1.34 compared with RR = 1.18; 95% CI 1.05–1.32) (Table 4).
Potential non-causal explanations
As in all meta-analyses, particularly meta-analyses of observational studies, potential alternative explanations for the observed association need to be addressed. These include publication bias, selection and measurement bias, and confounding.
A funnel plot and the Egger's test only suggest the presence of publication bias among Vietnamese studies, indicating the absence of small studies with ORs or RRs <1. However, 11 of 13 Vietnamese studies were unpublished in formal databases; many of them were not identified in earlier reviews. Furthermore, it is unlikely that there were Vietnamese studies that found an OR or RR <1 if Agent Orange exposure is truly associated with elevated birth defects, because exposed Vietnamese have experienced higher levels of exposure to Agent Orange/dioxin, both in intensity and duration. Therefore, the potential of publication bias affecting the summary RR is unlikely.
The assessment of exposure and outcomes in some cohort studies were made largely through interviewing parent(s) at the time of data collection, which generally occurred 10 years or more after exposure. This approach is known to miss those who died in both exposed and non-exposed groups, and could introduce survival bias. The mortality studies of US Vietnam-era veterans consistently found an excess of deaths among US-Vietnam veterans relative to US non-Vietnam veterans for the first 5 years.53–56 Also, studies in the Vietnamese population consistently showed that Vietnamese exposed to Agent Orange often reported more health complications and had a shorter life expectancy than non-Agent Orange exposed Vietnamese.39 By removing the most highly exposed individuals from these cohorts, this type of survival bias may lower the risk of birth defects associated with exposure to Agent Orange/dioxin.
Ecological assessment of exposure could bias the association between exposure to Agent Orange and risk of birth defects. With the exception of some of the US researchers measured individual exposure, using biomarkers8,26 or an Agent Orange Exposure Opportunity Index,21 the assessment of exposure was made based on groups of study participants according to their residential location in sprayed areas (Vietnamese civilians) or military history in Vietnam documented in military records (international veterans). Except for Ranch Hand veterans and Vietnamese civilians who were truly exposed, it is possible that a substantial number of international veterans presumed to be exposed on the basis of Vietnam military service had either minimal or no actual exposure. To the extent that non-exposed individuals were misclassified as the exposed, this type of bias would have underestimated, not overestimated, the risk of congenital anomalies associated with Agent Orange exposure.
The ascertainment of birth defects in most Vietnamese studies and two non-Vietnamese studies22,27 was done through direct interviews or questionnaires administered to parent(s) without verification. Some other Vietnamese studies identified only living children with observable defect(s) reported by their parents and then verified by physicians who were blind to study groups. These methods of data collection inevitably excluded malformed children, who died prior to the time of data collection or children with a minor defect that may not be known by their parents. This could explain a very low incidence of birth defects in the Vietnamese cohort studies (Table 2) as compared with the average estimated frequency of birth defects of 3–5% in the general population.57 This under-ascertainment of birth defects could be differential, because exposed parents, under the influence of media coverage, may have been more motivated to remember and report their children with a birth defect than those not exposed. As a result, the association between Agent Orange exposure and birth defects might have been overestimated.
In examining quality of included studies, we found that although Vietnamese studies were unlikely to have exposure misclassification, most of them appeared weak under some methodological criteria relative to the non-Vietnamese studies. These weaknesses include inadequate statistical power, limited validation of self-reported birth defects, and lack of adjustment for confounding variables. In contrast, most non-Vietnamese studies had adequate statistical power, reliable methods of data collection and study design, verification of self-reported birth defects, and control for major confounding variables. After, removing the Vietnamese studies from our meta-analysis, we observed a lower summary RR that remained statistically significant, 1.29, 95% CI 1.04–1.59, compared with the summary RR including the Vietnamese studies, 1.95, 95% CI 1.59–2.39. Given the lower levels of Agent Orange exposure in non-Vietnamese studies, the reduction in the magnitude of the effect after removing the Vietnamese studies is not surprising.
Possible mechanism and public health implications
Although it is not possible to make inferences on the mechanism of the association between Agent Orange exposure and birth defects in this study, the results are consistent with previous animal studies. Indeed, the detrimental effect of dioxin on congenital malformations has been documented in animal studies in which dioxin was shown to act as either a teratogen or mutagen.58–63 For example, maternal exposure to dioxin resulted in cleft palate and hydronephrosis in mice and hamsters, intestinal haemorrhage and renal abnormalities in rats,61 extra ribs in rabbits, and spontaneous abortions in monkeys.62 There is evidence that mutagenic effects of dioxin can take place at the genetic level.63 Dioxin was found to cause chromosomal anomalies in the bone marrow cells of some specific strains of rats and mice, and stimulate RNA synthesis in rat liver.63 Evidence from animal studies indicates that the observed association between Agent Orange/dioxin and birth defects in human seems biologically plausible.
The evidence from this meta-analysis indicates that among populations exposed to Agent Orange, an elevated incidence of birth defects may have occurred. Babies born with congenital malformations often require extensive surgical and medical care, and many have life-long disabilities and handicaps. Congenital abnormalities also have a major impact on their families and communities. This accentuates the need for effective and feasible intervention strategies at local, national, and international levels to relieve physical and emotional sufferings of disabled children and their families as the victims of Agent Orange. In addition, dioxin concentration is still found to be high in some parts of Vietnam where heavy spraying was conducted during the war1,3; therefore, cleaning of the environment should be undertaken as soon as possible to prevent further occurrence of congenital malformations in the local population.
Conclusion and recommendations for future research
Findings from this meta-analysis support the hypothesis that exposure to Agent Orange is associated with a statistically significant increase in risk of birth defects. The biological mechanism of this association and methodological limitations of Vietnamese studies warrant the consideration of conducting a large-scale and well-designed study in heavily sprayed regions of Vietnam to further elucidate the aetiology of the Agent Orange and birth defects relationship. Future studies need to include biological measures of exposure. The long half-life of dioxin makes this possible even now.
The association between Agent Orange or dioxin and birth defects is controversial owing to inconsistent data.
Synthesis of data across studies showed that parental exposure to dioxin or Agent Orange increased the risk of birth defects.
While the increased risk was present in both American ex-servicemen and Vietnamese; the latter had a more pronounced increase, probably due to high levels of exposure.
Considerable variability in effect sizes was found among studies considered.
We thank the 10-80 Committee (Vietnam) for providing unpublished data. This work has been conducted while the first author was a postgraduate student at the University of Sydney's School of Public Health, under the support of an Australian Development Scholarship (ADS). We also express special thanks to Dr Patricia Dolan Mullen at the School of Public Health, University of Texas at Houston for her helpful comments on revising the manuscript.
References
Dwernychuk LW, Cau HD, Hatfield CT et al. Dioxin reservoirs in southern Viet Nam—a legacy of Agent Orange.
Stellman J, Stellman S, Christian R, Weber T, Tomasallo C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam.
Dwernychuk LW, Cau HD. Aerial applications of Agent Orange vs. military installations-patterns of human exposure to TCDD in Southern Vietnam. The abstract presented at the third International Conference of Agent Orange/dioxin. Hanoi, 2001. Available at: http://www.niehs.nih.gov/external/usvcrp/conf2002/abstract.htm (Accessed April, 25,
Buckingham W. Operation ranch hand—herbicides in Southeast Asia 1969–1971. Available at: http://cpcug.org/user/billb/ranchhand/ranchhand.htm (Accessed August 14,
Institute of Medicine. Veterans and Agent Orange—Update. Washington DC: National Academy Press,
Wolfe WH, Michalek JE, Miner JC et al. Paternal serum dioxin and reproductive outcomes among veterans of Operation Ranch Hand.
Field B, Kerr C. Reproductive behavior and consistent patterns of abnormalities in offspring of Vietnam veterans.
Sweeney A. Reproductive epidemiology of dioxins. In: Schecter A (ed.). Dioxin and Health. New York and London: Plenum Press,
Institute of Medicine. Reproductive effects. In: Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides, Institute of Medicine (ed.). Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington DC: National Academy Press,
Environmental Protection Agency. Draft exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds 2000. Available at: http://www.epa.gov/NCEA/pdfs/dioxin/index.htm (Accessed July 20,
Egger M, Smith GD. Principles and procedures for systematic reviews. In: Egger M, Smith GD, Altman DG (eds). Systematic Reviews in Health Care, Meta-analysis in Context. London: BMJ,
Pettiti D. Meta Analysis, Decision Analysis and Cost Effectiveness Analysis. New York: Oxford University Press,
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease.
DerSimonian R, Laird N. Meta-analysis in clinical trials.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis.
Egger M, Davey Smith G, Schneider M, Minder CE. Bias in meta-analysis detected by a simple, graphical test. BMJ
Sterne JAC, Bradburn MJ, Egger M. Meta-analysis in Stata. In: Egger M, Smith GD, Altman DG (eds). Systematic Reviews in Health Care: Meta-analysis in Context. London: BMJ,
Erickson J, Mulinare J, McClain P et al. Vietnam veterans’ risks for fathering babies with birth defects.
Lathrop GD, Wolf WH, Albanese RA, Moynahan PM. An Epidemiological Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. Baseline Morbidity Study Results. TX, US: Air Force School of Aerospace Medicine, Aerospace Medical Division,
Donovan J, MacLennan R, Adena M. Vietnam service and the risk of congenital anomalies. A case–control study.
Centers for Disease Control (CDC). Health Status of Vietnam Veterans. III. Reproductive Health.
Aschengrau A, Monson R. Paternal military service in Vietnam and the risk of late adverse pregnancy outcomes.
Wolfe WH, Michalek JE, Miner JC, Rahe AJ. An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. TX, US: Epidemiology Research Division, Armstrong Laboratory, Human System Division,
Kang H, Mahan C, Lee K, Magee C, Mather S, Matanoski G. Pregnancy outcomes among US women Vietnam veterans.
Khoa ND. Some biological parameters collected on the groups of people in an area affected by chemicals. In: Proceedings of the first International Conference of Agent Orange/dioxin, Ho Chi Minh City,
Can N, Xiem NT, Tong NK, Duong DB. A case–control survey of congenital defects in My Van District, Hai Hung Province. In: Proceedings of the first International Conference of Agent Orange/dioxin, Hanoi,
Phuong NTN, Huong LTD. Consequence of chemical warfare on reproductive outcomes—an epidemiological survey in two localities in the South of Vietnam. In: Proceedings of the first International Conference of Agent Orange/dioxin, Ho Chi Minh City,
Can N, Xiem NT, Hong TT, Tong NK, Duong DB. An Epidemiological Survey of Pregnancies in the North of Vietnam. In: Proceedings of the first International Conference of Agent Orange/dioxin, Ho Chi Minh City,
Lang TD, Tung TT, Van DD. Mutagenic Effects on the First Generation After Exposure to Agent Orange. In: Proceedings of the first International Conference of Agent Orange/dioxin, Ho Chi Minh City,
Tanh V, Chi HTK, Thai NTP. Cohort Study on Reproductive Anomalies in Two Villages in Song Be Province, Vietnam. In: Proceedings of the second International Conference of Agent Orange/dioxin, Hanoi,
Phuong NTN, Thuy TT, Phuong PK. An estimate of differences among women giving birth to deformed babies and among those with hydatidiform mole seen at the ob-gyn hospital of Ho Chi Minh city in Vietnam.
Phong DN, Thu NT. A cohort approach on dioxin-induced risk on pregnancies of wives, offspring (F1) and grand children (F2) of the Second Indochina War's veterans at Thanh Tri, Hanoi. In: Proceedings of the second International Conference of Agent Orange/dioxin, Hanoi, Vietnam,
Phuong NTN, Thang TM. Survey on long-term effects of defoliations and herbicides on human reproduction at U Minh District (Minh Hai province). In: Proceedings of the second International Conference of Agent Orange/dioxin, Hanoi,
Dai LC, Quynh HT, Phong DN, Hien NTM. An investigation on the reproductive abnormalities in family of North Vietnam veterans exposed to herbicides during wartime. In: Proceedings of the second International Conference of Agent Orange/dioxin, Hanoi,
Dai B, Gia Q, Dai LC, Thuy B. Survey on diseases and reproductive abnormalities among soldiers deployed in chemicals sprayed areas in comparison with unexposed controls. In: Proceedings of the second International Conference of Agent Orange/dioxin, Hanoi, Vietnam,
10/80 Committee, Hatfield Consultant Ltd. Retrospective Epidemiological Survey in four Communes - Aso, Huong Lam, Hong Thuong and Hong Van. In: 10-80 Committee and Hatfield Consultant Ltd (ed.). Study of Impact and Development of Mitigation Strategies Related to the Use of Agent Orange Herbicides in the A Luoi Valley—Thua Thien Hue Province. Hanoi: 10-80 Committee,
Hung TM, Cuc PTK, Cau HD. Spina Bifida investigated by Spinal X-Ray among children of veterans exposed to defoliant in the war. In: 10-80 Committee (ed.). Consequences of Chemicals Used in Vietnam War 1961–1971. Hanoi: 10-80 Committee,
Schecter AJ, Constable JD. Elevated level of 2,3,7,8-Tetrachlorodibenzodioxin in adipose tissue of certain U.S. veterans of the Vietnam war.
Centers for Disease Control. Serum 2,3,7,8-Tetrachlorodibenzo-p-dioxin Levels in US Army Vietnam-are veterans.
Kang HK, Watanabe KK, Breen J et al. Dioxins and dibenzofurans in adipose tissue of US Vietnam veterans and controls.
Schecter AJ, Dai LC, Thuy LT et al. Agent Orange and the Vietnamese: the persistence of elevated dioxin levels in human tissues.
Schecter AJ, McGee H, Stanley JS, Boggess K, Brandt-Rauf P. Dioxins and dioxin-like chemicals in blood and semen of American Vietnam veterans from the state of Michigan.
Michalek JE, Rahe AJ, Kulkarni PM, Tripathi RC. Levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin in 1,302 unexposed Air Force Vietnam-era veterans.
Schecter AJ, Dai LC, Papke O et al. Recent dioxin contamination from Agent Orange in residents of a southern Vietnam city.
Kang HK, Dalager NA, Needham LL et al. US Army Chemical Corps Vietnam veterans health study: preliminary results.
Schecter AJ, Pavuk M, Constable JD, Dai LC, Papke O. A follow-up: high level of dioxin contamination in Vietnamese from Agent Orange, three decades after the end of spraying.
Kahn PC, Gochfeld M, Nygren M, Hansson M et al. Dioxins and dibenzofurans in blood and adipose tissue of Agent Orange-exposed Vietnam veterans and matched controls.
Schardein JL. Principles of teratogenesis applicable to human exposure to drugs and chemicals. In: DiCarlo FJ, Oehme FW (ed.). Chemically Induced Birth Defects. New York: Marcel Dekker, Inc.,
Institute of Medicine. Exposure assessment. In: Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides, Institute of Medicine (ed.). Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Washington DC: National Academy Press,
Watanabe KK, Kang HK. Mortality patterns among Vietnam veterans: A 24-year retrospective analysis.
Dalager NA, Kang HK. Mortality among Army Chemical Corps Vietnam veterans.
Centers for Disease Control. Post-service mortality among Vietnam veterans . The Centers for Disease Control Vietnam Experience Study.
Boehmer TK, Flanders WD, McGeehin MA, Boyle C, Barrett DH. Post-service mortality in Vietnam veterans, 30-year follow up.
Edmonds LD, James LM. Temporal trends in the birth prevalence of selected congenital malformations in the Birth Defects Monitoring Program/Commission on Professional and Hospital Activities, 1979–1989.
Nessel CS, Gallo MA. Dioxins and related compounds. In: Lippmann M (ed.). Environmental Toxicants. New York: Van Nostrand Reinhold,
DeVito MJ, Birnbaum LS. Dioxins: model chemicals for assessing receptor-mediated toxicity.
Bryant PL, Reid LM, Schmid JE, Buckalew AR, Abbott BD. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on fetal mouse urinary tract epithelium in vitro.
Couture LA, Harris MW. Characterization of the peak period of sensitivity for the induction of hydronephrosis in C57BL/6N mice following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Theobald HM, Peterson RE. Developmental and reproductive toxicity of dioxins and other ah receptor agonists. In: Schecter A (ed.). Dioxin and Health. New York and London: Plenum Press,