Approach Considerations
The cornerstones of atrial fibrillation (AF) management are rate control and anticoagulation [1, 19] and rhythm control for those symptomatically limited by AF. [19] The clinical decision to use a rhythm-control or rate-control strategy requires an integrated consideration of several factors, including degree of symptoms, likelihood of successful cardioversion, presence of comorbidities, and candidacy for AF ablation (eg, catheter-based pulmonary vein electric isolation or surgical ablation).
Restoration of sinus rhythm with regularization of the heart's rhythm improves cardiac hemodynamics and exercise tolerance. By maintaining the atrial contribution to cardiac output, symptoms of heart failure and overall quality of life can improve. As AF contributes to pathologic atrial and ventricular remodeling, restoration of sinus rhythm can slow or, in some cases, reverse atrial dilatation and left ventricular dysfunction. For these reasons, most clinicians focus initially on restoration and maintenance of sinus rhythm in patients with new-onset AF and opt for a rate-control strategy only when rhythm control fails.
However, several randomized controlled trials have demonstrated that a strategy aimed at restoring and maintaining sinus rhythm neither improves survival nor reduces the risk of stroke in patients with AF.
In the AFFIRM study (Atrial Fibrillation Follow-up Investigation of Rhythm Management), an insignificant trend toward increased mortality was noted in the rate control group, and importantly, no evidence suggested that the rhythm-control strategy protected patients from stroke. In the study, 4060 subjects aged 65 years or older whose AF was likely to be recurrent and who were at risk for stroke were randomized to a strategy of rhythm control (cardioversion to sinus rhythm plus drugs to maintain sinus rhythm) versus a strategy of rate control (in which no attempt was made to restore or maintain normal sinus rhythm). [36] Clinically silent recurrences of AF in the rhythm-control group are theorized to be responsible for the increased rates of thromboembolic events and mortality noted in this cohort. This underscores the importance of anticoagulation in both rhythm-control and rate-control patients.
New developments aimed at curing AF are being explored actively. By reducing the critical mass required to sustain AF through either surgical or catheter-based compartmentalization of the atria (ie, maze procedure), fibrillatory wavelets collide with fixed anatomic obstacles, such as suture lines or complete lines of ablation, thus eliminating or reducing the development of permanent AF. One concern is that an extensive maze procedure can render the atrial severely hypocontractile, which may elevate the risk of embolic stroke even if AF is substantively suppressed. Some patients with focal origins of their AF also may be candidates for catheter ablation. Simple electric isolation of the origins of the pulmonary veins has proven roughly up to 80% successful in substantially reducing frequency and duration of AF in patients who do not tolerate AF well.
AF ablation methods continue to be studied and modified and thus may be considered as a work in progress rather than a mature primary therapy. Go to Catheter Ablation for complete information on this topic.
2019 ACC/AHA/HRS updated guidelines
A focused update of the American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Clinical Practice Guidelines and the Heart Rhythm Society (HRS) 2014 guidelines for the management of patients with atrial fibrillation (AF) was released in January 2019. [57, 58, 59]
Selecting an anticoagulant regimen
For patients with AF and an elevated CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years [doubled], diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism [doubled], vascular disease, age 65-74 years, sex category) score of 2 or greater in men or 3 or greater in women, oral anticoagulants are recommended.
Female sex, in the absence of other AF risk factors (CHA2DS2-VASc score of 0 in males and 1 in females), carries a low stroke risk that is similar to males. Adding female sex to the CHA2DS2-VASc score matters for age >65 years or ≥2 non–sex-related stroke risk factors.
Non-vitamin K oral anticoagulants (NOACs) (dabigatran, rivaroxaban, apixaban, and edoxaban) are recommended over warfarin in NOAC-eligible patients with AF (except those with moderate-to-severe mitral stenosis or a mechanical heart valve).
In patients with AF (except those with moderate-to-severe mitral stenosis or a mechanical heart valve), the CHA2DS2-VASc score is recommended for assessment of stroke risk. For patients with AF who have mechanical heart valves, warfarin is recommended.
Renal and hepatic function should be evaluated before initiation of a NOAC, and both should be reevaluated at least annually.
Aspirin is no longer recommended for patients with low CHA2DS2-VASc scores. For patients with AF (except those with moderate-to-severe mitral stenosis or a mechanical heart valve) and a CHA2DS2-VASc score of 1 in men or 2 in women, clinicians may consider prescribing an oral anticoagulant to reduce the risk of thromboembolic stroke.
Interruption and bridging anticoagulation
Idarucizumab is recommended for dabigatran reversal in the event of life-threatening bleeding or an urgent procedure. Andexanet alfa can be useful for rivaroxaban and apixaban reversal in the event of life-threatening or uncontrolled bleeding.
Percutaneous approaches to occlude the LAA
Percutaneous left atrial appendage (LAA) occlusion may be considered in patients with AF at an increased risk of stroke who have contraindications to long-term anticoagulation.
Prevention of thromboembolism
For patients with AF or atrial flutter of at least 48 hours, or when the AF duration is unknown, anticoagulation with warfarin (international normalized ratio [INR] 2.0-3.0), a factor Xa inhibitor, or direct thrombin inhibitor is recommended for at least 3 weeks before and at least 4 weeks after cardioversion, regardless of the CHA2DS2-VASc score or the method (electrical or pharmacologic) used to restore sinus rhythm.
Catheter ablation in HF
AF catheter ablation may be reasonable in selected patients with symptomatic AF and heart failure (HF) with reduced left ventricular (LV) ejection fraction (HFrEF) to potentially lower the mortality rate and reduce hospitalization for HF.
AF complication ACS
In patients with AF at increased risk of stroke (based on CHA2DS2-VASc risk score of ≥2) who have undergone percutaneous coronary intervention (PCI) with stenting for acute coronary syndrome (ACS), the following is reasonable to reduce the risk of bleeding as compared with triple therapy (oral anticoagulant, aspirin, and P2Y12 inhibitor):
-
Double therapy with a P2Y12 inhibitor (clopidogrel or ticagrelor) and dose-adjusted vitamin K antagonist.
-
Double therapy with P2Y12 inhibitors (clopidogrel) and low-dose rivaroxaban 15 mg daily.
-
Double therapy with a P2Y12 inhibitor (clopidogrel) and dabigatran 150 mg twice daily.
If triple therapy is prescribed for patients with AF who are at an increased risk of stroke (based on CHA2DS2-VASc risk score of ≥2) and who have undergone PCI with stenting (drug eluting or bare metal) for ACS, clinicians may consider a transition to double therapy (oral anticoagulant and P2Y12 inhibitor) at 4-6 weeks.
Weight loss in patients with AF
For overweight and obese patients with AF, weight loss, combined with risk factor modification, is recommended.
Risk-Management Decisions
One of the major management decisions in atrial fibrillation (AF) (and atrial flutter) is determining the risk of stroke and appropriate anticoagulation regimen for low-, intermediate-, and high-risk patients. For each anticoagulant, the benefit in terms of stroke reduction must be weighed against the risk of clinically significant bleeding.
Overall, approximately 15-25% of all strokes in the United States (75,000/y) can be attributed to AF. Known risk factors for stroke in patients with AF include advancing age, female sex, hypertension, diabetes, heart failure, prior history of stroke/transient ischemic attack (TIA)/thromboembolism, coronary artery disease, peripheral arterial disease, and valvular heart disease (rheumatic valvular disease). [1]
At least four large clinical trials have clearly demonstrated that anticoagulation with warfarin decreases the risk of stroke by 50-80%. In relatively recent trials, the newer oral anticoagulants (dabigatran, rivaroxaban, apixaban, and edoxaban) have proven to be similarly effective (dabigatran 110 mg, rivaroxaban, or edoxaban) or superior (dabigatran 150 mg or apixaban) to warfarin for prevention of stroke and thromboembolism. [60] However, although anticoagulants reduce 30-day mortality from ischemic stroke, these agents increase intracranial hemorrhage–related mortality. [61] If warfarin is chosen for anticoagulation, a target international normalized ratio (INR) of 2-3 is traditionally used in this cohort, as this limits the risk of hemorrhage while providing protection against thrombus formation. Warfarin is also superior to clopidogrel or a combination of clopidogrel and aspirin in the prevention of embolic events in higher-risk patients.
Most clinicians agree that the risk-benefit ratio of anticoagulants in low-risk patients with AF is not advantageous. The appropriate treatment regimen for patients with AF at intermediate risk is controversial. In this population, clinicians should assess risk factors for thromboembolic disease, patient preference, risk of bleeding, risk of falls or trauma, and likelihood of medication adherence. [62]
Note that treatment risks exist with concomitant antiplatelet therapy with oral anticoagulation in patients with AF. A study analyzing concomitant use of aspirin and its association with clinical outcomes among AF patients treated with oral anticoagulation found a significantly increased risk for bleeding among those receiving both therapies. [63] Hospitalizations for bleeding events were also increased in the group treated with this treatment combination.
Of the 7347 AF patients on oral anticoagulation therapy who participated in the study, 2543 (35%) also received aspirin. [63] Among the patients treated with aspirin, 39% did not have a history of atherosclerotic disease and 17% had elevated ATRIA bleeding risk scores. Compared with patients receiving oral anticoagulation alone, those receiving concomitant aspirin had a significantly higher risk of major bleeding (adjusted hazard ratio [HR] 1.53, 95% confidence interval [CI] 1.20-1.96) and bleeding hospitalizations (adjusted HR 1.52, 95% CI 1.17-1.97). [63]
Results from a retrospective study by Sjalander et al of 115,185 Swedish patients with AF indicated that aspirin as monotherapy not only did not protect against stroke, but it was also associated with an increased risk of ischemic stroke and thromboembolic events in elderly patients, as compared with no antithrombotic treatment. [64] In the study, 58,671 patients received aspirin monotherapy, whereas 56,514 did not receive any antithrombotic treatment at baseline; mean follow-up was 1.5 years.
Several risk factor assessment algorithms have been developed to aid the clinician on decisions on anticoagulation for patients with AF. The CHADS2 index (Cardiac failure, Hypertension, Age ≥75 years, Diabetes, Stroke or transient ischemic attack [TIA]) was widely used previously [65] ; however, multiple more recent studies have proven the superiority of the CHA2DS2-Vasc score over the CHADS2 score in predicting the risk of thromboembolism in patients with AF, particularly for participants with low to intermediated CHADS2 scores (0-1). [44, 66]
The CHA2DS2-Vasc score uses a point system to determine yearly thromboembolic risk. Two points are assigned for a history of stroke or TIA, thromboembolism, or age of 75 years or older, and one point is given for age 65-74 years or a history of hypertension, diabetes, heart failure, arterial disease (coronary artery disease, peripheral arterial disease, or aortic plaque), or female sex. The predictive value of this scoring system was evaluated in 90,490 elderly patients with nonvalvular AF who were taking warfarin therapy. [67] An increase in CHA2 DS2-VASc score was associated with serial increase in the risk of stroke (see Table 1 below).
Table 1. Stroke Rate in Patients with Nonvalvular Atrial Fibrillation not Treated with Anticoagulation [67] (Open Table in a new window)
CHA2 DS2-VASc Score |
Unadjusted Stroke Rate (%/y) |
0 |
0.2 |
1 |
0.6 |
2 |
2.2 |
3 |
3.2 |
4 |
4.8 |
5 |
7.2 |
6 |
9.7 |
7 |
11.2 |
8 |
10.8 |
9 |
12.2 |
Recommendations on anticoagulation for patients with nonvalvular AF have been based on the 2014 American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS) task force guidelines on the management of patients with AF (see Table 2 below). [1]
Table 2. Recommendations for Antithrombotic Therapy in Patients with Nonvalvular Atrial Fibrillation (Open Table in a new window)
CHA2 DS2-VASc Score |
Recommended Therapy |
| 0 | No therapy |
| 1 | No therapy, or aspirin 81-325 mg daily, or anticoagulation therapy (eg, warfarin [international normalized ratio (INR) goal 2-3], dabigatran, rivaroxaban, apixaban, edoxaban) |
≥2 |
Anticoagulation therapy (eg, warfarin [INR goal 2-3], dabigatran, rivaroxaban, apixaban, edoxaban) |
Management of New-Onset AF
Results from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study and similar findings from the smaller Rate Control Versus Electrical Cardioversion (RACE) trial [68] led to the development of consensus guidelines that recommend an initial rate-control strategy for the majority of asymptomatic patients with atrial fibrillation (AF).
Rate control
Regardless of the long-term management strategy chosen, control of ventricular rate is a critical component of management of new-onset AF. The main determinants of the ventricular rate during AF are those intrinsic and extrinsic factors that influence atrioventricular (AV) conduction. Foremost among these are the intrinsic AV nodal conduction properties. Underlying sympathetic and parasympathetic tone also influences AV nodal conduction. Rate-controlling agents act primarily by increasing AV nodal refractoriness.
Beta-blockers and calcium channel blockers are first-line agents for rate control in AF. These drugs can be administered either intravenously or orally. They are effective at rest and with exertion. Intravenous diltiazem or metoprolol are commonly used for AF with a rapid ventricular response. Caution should be exercised in patients with reactive airway disease who are given beta-blockers.
Digoxin can be used in the acute setting but does little to control the ventricular rate in active patients. As such, it is rarely used as monotherapy. Caution should be exercised in elderly patients and those with renal failure receiving digoxin. Digoxin is indicated in patients with heart failure and reduced left ventricular function.
A large study of elderly persons with nonvalvular AF or atrial flutter indicated that digoxin therapy can increase the risk that a patient will die within approximately 3 years by more than 20%. [69, 70] The study, The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF), involved more than 122,000 elderly US veterans (mean age 72 years) with newly diagnosed AF or atrial flutter, almost a quarter of whom underwent early therapy with digoxin. After a follow-up period of about 3 years, the multivariate-adjusted hazard ratio for patient mortality was calculated to be 1.26 in the digoxin group. According to the investigators, the increased mortality risk was not associated with drug adherence, concomitant treatment, comorbid cardiovascular disorders, or renal function. [69, 70]
Amiodarone has a class IIa recommendation from the 2014 American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS) for use as a rate-controlling agent for patients who are intolerant of or unresponsive to other agents, such as patients with congestive heart failure (CHF) who may otherwise not tolerate diltiazem or metoprolol. [1] Caution should be exercised in those who are not receiving anticoagulation, as amiodarone can promote cardioversion.
Extreme care must be taken in patients with preexcitation syndrome and AF. Blocking the AV node in some of these patients may lead to AF impulses that are transmitted exclusively down the accessory pathway, and this can result in ventricular fibrillation. (If this happens, the patient will require immediate defibrillation.) Beta-blockers, non-dihydropyridine calcium channel blockers, digoxin, and intravenous amiodarone are contraindicated in these patients; flecainide or amiodarone can be used instead. [1, 71]
Anticoagulation
One of the most important considerations in the acute management of atrial fibrillation is the need for anticoagulation (see the image below). Acute cardioversion for AF carries a risk of thromboembolism unless anticoagulation therapy is initiated prior to the procedure and continued post procedure. Risk of thromboembolism is similar in patients undergoing either pharmacologic or electrical cardioversion. The risk of thromboembolic events is greatest when AF has been present for longer than 48 hours.
Transesophageal echocardiography (TEE) is a good predictor of acute risk. If no thrombus is seen in the cardiac chambers, particularly the left atrial appendage, and dense spontaneous echo contrast is not seen, cardioversion has low acute risk of stroke. Effective anticoagulation in patients with AF reduces the risk of stroke 3-fold after 4-6 weeks.
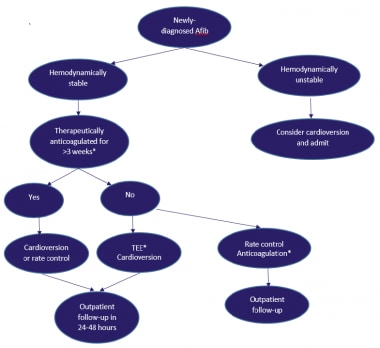 Patient management for newly diagnosed atrial fibrillation (Afib). *Therapeutic anticoagulation implies either treatment with warfarin with a therapeutic international normalized ratio (INR) (2-3) or with newer oral anticoagulants (dabigatran, rivaroxaban, apixaban, or edoxaban). Transesophageal echocardiography (TEE)/cardioversion should be performed with an anticoagulation strategy using either low molecular-weight heparin (LMWH) 1 mg/kg twice daily as a bridge, with initiation of warfarin (INR 2-3) or newer oral anticoagulants.
Patient management for newly diagnosed atrial fibrillation (Afib). *Therapeutic anticoagulation implies either treatment with warfarin with a therapeutic international normalized ratio (INR) (2-3) or with newer oral anticoagulants (dabigatran, rivaroxaban, apixaban, or edoxaban). Transesophageal echocardiography (TEE)/cardioversion should be performed with an anticoagulation strategy using either low molecular-weight heparin (LMWH) 1 mg/kg twice daily as a bridge, with initiation of warfarin (INR 2-3) or newer oral anticoagulants.
Patients with newly diagnosed AF and patients awaiting electrical cardioversion can be started on intravenous heparin (activated partial thromboplastin time [aPTT] of 45-60 seconds) or low-molecular-weight heparin (LMWH) (1 mg/kg twice daily [BID]).
Patients can be started concomitantly on warfarin in an inpatient setting while awaiting a therapeutic international normalized ratio [INR] value (2-3). Many practices have developed specialized anticoagulation clinics to monitor INR values closely. Newer oral anticoagulants are attractive alternatives to warfarin in patients with nonvalvular AF. These agents include dabigatran, rivaroxaban, apixaban, and edoxaban, and they can be started without the need for an anticoagulation bridge with heparin or LMWH before cardioversion.
Cardioversion
Cardioversion may be performed electively or emergently to restore sinus rhythm in patients with new-onset AF. Cardioversion is most successful when initiated within 7 days after onset of AF. The need for cardioversion may be acute when AF is responsible for hypotension, heart failure, or angina.
Pharmacologic agents or direct current energy can be used to cardiovert patients with AF. Pharmacologic cardioversion has the advantage of not requiring sedation or anesthesia, but the major disadvantage is the risk of ventricular tachycardia and other serious arrhythmias.
Long-Term Management
Long-term management of atrial fibrillation (AF) is focused on reducing the likelihood of AF recurrence, reducing AF-related symptoms, control of ventricular rate, and reducing stroke risk. As discussed previously, AF is often the result of established cardiovascular risk factors. Appropriate management of these risk factors will reduce the likelihood of future episodes of AF and AF-related morbidity and mortality. Anticoagulation with either aspirin or warfarin should be initiated for all individuals with AF, except in those with contraindications. Selection of the appropriate antithrombotic regimen for a given patient should be balanced between the risk of stroke and the risk of bleeding. Antiarrhythmic therapy can aid in maintenance of sinus rhythm in certain patients but requires close monitoring.
Optimal long-term strategies for AF management should be based on a thoroughly integrated consideration of patient-specific factors and likelihood of success. As a rule, younger patients with more severe symptoms and fewer comorbidities tend to derive greater benefit from a long-term focus on rhythm control. Older patients with structural heart disease (eg, left ventricular hypertrophy, prior myocardial infarction, depressed ejection fraction, atrial dilatation) are less likely to remain in sinus rhythm and are more likely to have serious side effects from antiarrhythmic drugs. In this cohort, most clinicians focus on long-term rate control.
Because of the electrophysiologic and structural remodeling caused by AF, many patients with paroxysmal AF will progress to persistent and long-standing persistent AF. The degree to which this reflects the continuing influence of underlying cardiovascular risk factors as opposed to a direct effect of AF is unknown. Regardless, clinicians need to reevaluate their management strategies frequently, as AF burden and comorbidities increase with time.
Anticoagulation
The goal of long-term anticoagulation in AF is to reduce the risk of thromboembolism. Patients in AF have a risk of stroke or peripheral embolism that is approximately five times that of individuals in sinus rhythm. Recommendations for anticoagulation for patients with nonvalvular AF are based on guidelines from a 2014 American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS) task force on the management of patients with AF. [1] Currently approved anticoagulants include warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban.
Warfarin
Anticoagulation therapy with warfarin is significantly more effective than antiplatelet therapy (relative risk of 40%) if the international normalized ratio (INR) is adjusted. The INR goal in AF is usually between 2 and 3, except in patients who are at a significant risk for stroke (eg, patients with artificial valves, those with rheumatic heart disease, and those at a high risk for AF with recurrent prior strokes), in whom the INR should be maintained between 2.5 and 3.5. A lower INR goal (1.8-2) may be considered in elderly patients who are at high risk for a fall.
Anticoagulation clinics have shown more success and a lower complication rate than primary care physicians in controlling patients’ INR. In addition, one study reported that patients who used an Internet-based program for patient self-management of oral anticoagulant therapy achieved a higher mean time in the therapeutic range than patients whose INR was controlled by an established anticoagulation clinic. [72] Similar programs alone or in combination with regular care provided by anticoagulation clinics may improve the mean time that patients are in the therapeutic range and may further reduce the risk of stroke.
As patients with AF age, the relative efficacy of oral anticoagulation appears not to decrease, whereas the efficacy of antiplatelet therapy does appear to decrease. [73] A mutation in coagulation factor IX may cause spontaneous bleeding, even with an INR in the therapeutic range. Adverse effects of warfarin therapy are not limited to bleeding, however; other important side effects include skin necrosis within the first few days of therapy and cholesterol embolization to the skin or visceral organs in the first few weeks of therapy.
Several scoring systems have been developed to estimate risk-benefit for warfarin use in AF (summarized below).
The major adverse effect of anticoagulation therapy with warfarin is bleeding. Factors that increase this risk include the following:
-
History of bleeding (the strongest predictive risk factor)
-
Age older than 75 years
-
Liver or renal disease
-
Malignancy
-
Thrombocytopenia or aspirin use
-
Hypertension
-
Diabetes mellitus
-
Anemia
-
Prior stroke
-
Fall risk
-
Genetic predisposition
-
Supratherapeutic INR
Several risk models have been introduced. The risk model called HEMORR2HAGES assigns points to risk factors, as follows [74] :
-
History of bleeding (2 points)
-
Hepatic or renal disease (1 point)
-
Alcohol abuse (1 point)
-
Malignancy (1 point)
-
Older age (>75 years) (1 point)
-
Reduced platelet count or function, including aspirin therapy (1 point)
-
Hypertension (1 point)
-
Anemia (1 point)
-
Genetic predisposition (1 point)
-
Excessive fall risk (1 point)
-
Stroke (1 point)
Using this scoring, the risks of a major bleeding event per 100 patient-years of warfarin therapy are as follows:
-
0 points: 1.9%
-
1 point: 2.5%
-
2 points: 5.3%
-
3 points: 8.4%
-
4 points: 10.4%
-
5 or more points: 12.3%
When the bleeding risk outweighs the benefit, avoidance of anticoagulation therapy in AF should be considered. In addition, because of its teratogenic effects, anticoagulation with warfarin is contraindicated in pregnant women, especially in the first trimester.
Dabigatran
Dabigatran (Pradaxa) is a direct oral thrombin inhibitor. The RE-LY study evaluated the efficacy and safety of two different doses of dabigatran relative to warfarin in more than 18,000 patients with AF. Patients were randomized to one of three arms: (1) adjusted-dose warfarin, (2) dabigatran 110 mg twice daily (BID), or (3) dabigatran 150 mg BID. Dabigatran 110 mg was noninferior to warfarin for the primary efficacy endpoint of stroke or systemic embolization, whereas dabigatran 150 mg was significantly more effective than warfarin or dabigatran 110 mg. Major bleeding occurred significantly less often with dabigatran 110 mg than with warfarin; dabigatran 150 mg had similar bleeding to that of warfarin. [75, 76]
A meta-analysis by Uchino and Hernandez evaluated the risk of myocardial infarction or acute coronary syndrome (ACS) with the use of dabigatran. The results suggest the risk of myocardial infarction or ACS was similar when using revised RE-LY trial results. Dabigatran is associated with an increased risk of myocardial infarction or ACS in an extensive range of patients when tested against different controls. [77]
A different meta-analysis involving more than 1000 patients found that major bleeding complications were generally less critical and more manageable in patients being treated with dabigatran than in those on warfarin therapy. For example, in patients treated with dabigatran, the worst major bleeds tended to be gastrointestinal, whereas in patients treated with warfarin, most of the worst bleeds were intracranial and therefore more difficult to treat. In addition, among patients with major bleeds, the dabigatran patients spent less time in intensive care and had a lower mortality rate than did the warfarin patients. [78, 79]
The US Food and Drug Administration (FDA) has approved the 150 mg BID dose—but not the 110 mg BID dose—of dabigatran for the management of patients with AF. The 75 mg BID dose has also been approved for patients with moderate renal failure (creatinine clearance of 15-29 mL/min). Patients with AF who are not candidates for dabigatran include those with prosthetic heart valves or hemodynamically significant valve disease, severe renal failure (creatinine clearance ≤15 mL/min), or advanced liver disease.
Rivaroxaban
Rivaroxaban (Xarelto) was approved by the FDA in November 2011 for nonvalvular AF. [80] It is a highly selective direct factor Xa inhibitor with high oral bioavailability, and with rapid onset of action. Clinical trial data have shown that it allows predictable anticoagulation with no need for dose adjustments and routine coagulation monitoring. [81]
Approval of rivaroxaban was based on the ROCKET-AF multinational, double-blind trial, in which the risk of major bleeding was similar for rivaroxaban and warfarin, but a significantly lower risk of intracranial hemorrhage and fatal bleeding was seen with rivaroxaban when compared with warfarin. [82] The study included over 14,000 patients who were randomized to either rivaroxaban or warfarin; rivaroxaban 20 mg once daily was used for patients with normal renal function and 15 mg once daily for patients with mild renal failure (creatinine clearance of 30-49 mL/min). In the primary analysis of this study, rivaroxaban was found to be noninferior to warfarin for prevention of stroke or systemic embolism in patients with nonvalvular AF. [82] During the approval process, there was concern expressed over the amount of time the warfarin-treated patients spent at the optimal INR during the study (57.8%), which was lower than in other trials with warfarin (eg, RE-LY trial for Dabigatran). [75] Also, the participants of the ROCKET-AF trial had higher mean CHADS2 scores (3.67) when compared to those of the RE-LY trial (2.1).
Apixaban
Another factor Xa inhibitor, apixaban (Eliquis), was approved by the FDA in December 2012. Approval was based on two clinical trials: ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in AF) and AVERROES (Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in AF Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment). (Patients with serum creatinine of 2.5 mL/dL or greater were excluded from both apixaban trials.)
The ARISTOTLE trial compared apixaban with warfarin for the prevention of stroke or systemic embolism in 18,201 patients with AF and found that apixaban was superior to warfarin in preventing stroke or systemic embolism, caused less bleeding, and resulted in lower mortality. [83, 84, 85]
The AVERROES trial, which compared apixaban with aspirin in 5599 patients with AF for whom warfarin therapy was considered unsuitable, was stopped early (after 1.1 year) after an interim analysis because apixaban showed a significant reduction in stroke and systemic embolism compared with aspirin. [86] A modest increase of major bleeding was observed with apixaban compared with aspirin. [86]
Edoxaban
Edoxaban (Savaysa) was approved for the prevention of thromboembolism in AF by the FDA in January 2015 on the basis of results from the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction Study 48) trial. [87] This double-blind, noninferiority trial randomized 21,105 patients with nonvalvular AF to high-dose edoxaban (60 mg daily), low-dose edoxaban (30 mg daily), or warfarin (creatinine clearance up to 30 mL/min was an exclusion criterion). Mean CHADS2 score for the subjects in this trial was 2.8. In intention-to-treat analyses, both doses of edoxaban were noninferior to warfarin for prevention of the stroke and systemic embolic events; however, there was a trend toward superiority for high-dose edoxaban (embolic risk of 1.57% with high-dose edoxaban compared to 1.8% with warfarin; P = 0.08). [87]
Of note, in participants with a creatinine clearance of 95 mL/min or greater, the hazard ratios (HRs) for developing embolic events were similar between the high-dose edoxaban and the warfarin groups. [87] Consequently, the FDA recommends avoiding edoxaban in patients with a creatinine clearance of 95 mL/min. [88] Both doses of edoxaban were reported to be superior to warfarin for all types of bleeding, except gastrointestinal bleeding wherein low-dose edoxaban was superior (HR: 0.67 (ie, 33% lower risk of bleeding); P< 0.001), whereas high-dose edoxaban was inferior to warfarin (HR: 1.23 [ie, 23% higher risk of bleeding]; P = 0.03).
A meta-analysis of four randomized trials involving 42,411 patients who received newer anticoagulants and 29,272 who received warfarin showed that, in patients with AF, the newer oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban protected against stroke or systemic embolism better than warfarin and had comparable safety profiles. [87, 89, 90, 91]
The newer anticoagulants also significantly reduced the incidence of all-cause mortality and intracranial hemorrhage, but increased gastrointestinal bleeding. Median follow-up periods ranged from 1.8 years to 2.8 years. The risk of stroke or systemic embolic events was reduced by 19% with the newer anticoagulants compared with warfarin; hemorrhagic strokes accounted for a large proportion of the reduction. Compared with warfarin, low-dose new anticoagulant regimens showed similar overall reductions in stroke or systemic embolic events and a more favorable bleeding profile, but significantly more ischemic strokes. [87, 89, 90, 91]
Newer oral anticoagulants versus warfarin
There are several advantages of using the newer oral anticoagulants over warfarin, including the following:
-
Predictable pharmacologic profiles with fewer drug–drug interactions, and dietary effects
-
Lower risk of intracranial bleeding
-
Rapid onset and offset of action, with no need for bridging with parenteral anticoagulant therapy during initiation or after interruption
-
No need for periodic INR testing
-
Superiority to warfarin for reducing the risk of thromboembolic events with dabigatran 150 mg BID and apixaban
Disadvantages of the newer oral anticoagulants include the following:
-
Requires strict compliance, because missing even a single dose could result in a period without anticoagulation
-
Limited safety profile data for patients with severe kidney failure
-
No data for their use in the presence of mechanical heart valves (dabigatran was associated with increased risk of thromboembolic complications in patients with mechanical heart valves in the RE-ALIGN trial) or valvular AF, due to hemodynamically significant mitral stenosis
-
No data for their use in pregnant or lactating women, in children, or in patients with a recent stroke (≤7-14 days), reversible causes of AF, severe increase in blood pressure, and significant liver disease
-
Lack of reliable blood tests to ascertain therapeutic effect or toxicity
Reversal of anticoagulation
In the presence of acute major bleeding, emergent reversal of anticoagulation is required. Fresh frozen plasma is often utilized to reverse the effects of warfarin, but it takes 6-24 hours to achieve compete reversal. In more emergent settings, prothrombin complex concentrates (PCCs) can be used, because they provide complete reversal of anticoagulation in 15-20 minutes. [94]
For patients taking newer oral anticoagulants, several reversal agents have been developed; however, it should be noted that these newer anticoagulants have short half-lives (5-17 hours), and reversal is rarely indicated. Idarucizumab (Praxbind) is a monoclonal antibody fragment which binds with high affinity to dabigatran. Its efficacy was studied in the RE-VERSE AD trial (Reversal Effects of Idarucizumab on Active Dabigatran) in which 90 patients who were taking dabigatran and presented with serious bleeding or had a need for an urgent invasive procedure (< 8 hours) were given two doses of idarucizumab 15 minutes apart. As measured by laboratory testing, idarucizumab completely normalized coagulation parameters in 90% of patients within the first 10-30 minutes. Five thrombotic events and 18 deaths were reported, but there was no control group to compare the relative risk of thrombosis and death. [92]
Andexanet alfa targets and sequesters factor Xa inhibitors (rivaraoxaban, apixaban, edoxaban). This agent is currently under clinical trials and is not FDA approved. [93, 95]
Recommendations from the American Academy of Neurology (AAN)
In 2014, the AAN released level B and C recommendations on the prevention of stroke in patients with nonvalvular AF. The level B recommendations included the following [96, 97] :
-
Patients with nonvalvular AF should be informed that the potential benefit of antithrombotic treatment in reducing stroke risk must be weighed against an increased risk for major bleeding from such therapy.
-
Patients with nonvalvular AF and a history of transient ischemic attack (TIA) or stroke should routinely be offered anticoagulation therapy.
-
Dabigatran, rivaroxaban, or apixaban, which are associated with a lower risk of intracranial hemorrhage than warfarin, should be administered to patients with a higher intracranial bleeding risk.
-
Dabigatran, rivaroxaban, or apixaban should also be administered to patients who refuse or are unable to undergo frequent periodic testing of their INR.
-
Oral anticoagulants should routinely be offered to elderly patients (aged >75 years) with nonvalvular AF who do not have a history of recent, unprovoked bleeding or intracranial hemorrhage.
-
Patients with nonvalvular AF who have dementia or who suffer occasional falls can be offered oral anticoagulation, but patients or their families/caregivers should be informed that the risk-benefit ratio of such therapy is uncertain in patients who have moderate to severe dementia or who suffer very frequent falls.
-
In developing countries, where newer anticoagulants may be unavailable or too expensive, the guidelines state that in patients who have a moderate stroke risk, the use of the antiplatelet agent triflusal 600 mg/day in combination with moderate anticoagulation (INR 1.25-2.0) with acenocoumarol is probably more effective in reducing stroke risk than is the use of acenocoumarol alone at the higher INR (2.0-3.0).
-
A risk-stratification scheme should be used by clinicians to help them decide which patients with nonvalvular AF would particularly benefit from anticoagulation therapy, but it should not be the definitive means of making such decisions.
Postoperative and postdischarge anticoagulation therapy
Anticoagulation prior to and during an elective surgery may be continued or stopped depending on the patient’s risk of bleeding and risk of thromboembolism. If the risk of thromboembolism is high (stratified by the CHA2DS2-Vasc score) and the risk of bleeding is low, anticoagulation should be continued with the INR in the low therapeutic range. However, a high risk of bleeding during the procedure should prompt discontinuation of warfarin for 3-5 days prior to surgery. These patients should then be treated with heparin prior to and following the operation to allow discontinuation of anticoagulation if bleeding occurs. Newer anticoagulants can generally be discontinued 1-2 days before the surgery and do not require bridging with heparin or low molecular-weight heparin (LMWH).
In general, patients who develop AF only postoperatively do not need anticoagulation. Administration of preoperative and postoperative beta-blockers is usually sufficient, as postoperative AF is usually paroxysmal and tends to terminate spontaneously. The Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) AF Substudy found that the administering of colchicine appears to be safe and efficacious in the reduction of postoperative AF, which could potentially halve the complication and reduce the time a patient stays in the hospital. [98]
Research has shown that the administration of colchicine in patients who underwent pulmonary vein isolation helped to prevent early recurrences of paroxysmal AF. [99, 100] This process appeared to be mediated through a postablation reduction in inflammation.
A large cohort study in Denmark compared the bleeding risk of anticoagulants prescribed upon hospital discharge for AF: During mean follow-up (3.3 years), 11.4% of patients experienced a nonfatal or fatal bleeding episode. [101] The highest incidence for bleeding was observed for dual therapy with warfarin and clopidogrel and for triple therapy with warfarin, aspirin, and clopidogrel (3-fold higher risk) compared with single agent use. [101]
Omega-3 fatty acids
Several small trials have suggested that treatment for paroxysmal AF with prescription omega-3 fatty acids may provide a safe and effective treatment option. However, no benefit has been found to date. [102, 103]
Angiotensin-converting enzyme (ACE) inhibitors and ACE-receptor blockers (ARB)
Trials examining the incidence of AF in patients with heart failure who are treated with ACE inhibitors or ARBs have demonstrated a potential beneficial effect on AF recurrence. This recurrence is thought to be mediated by blocking the rennin-angiotensin-aldosterone system and the downstream effects on atrial mechanical and electrical remodeling. [104, 105, 106]
A study by Yusuf et al examined the effects of irbesartan in patients with permanent AF or at least two episodes of paroxysmal AF in the previous 6 months. [107] Irbesartan did not demonstrate a benefit in patients with AF who were already receiving an ACE inhibitor or patients in sinus rhythm. No reduction in cardiovascular death, stroke, or myocardial infarction was noted in the patient population studied.
Rate control
As discussed previously, several trials have validated the noninferiority of an initial rate-control strategy. Many clinicians believe, however, that an attempt at a rhythm-control strategy should be made in most patients. Older patients with comorbid cardiovascular disease have a lower likelihood of successful long-term rhythm control, and thus, these patients are often managed using a rate-control strategy. Some patients managed initially with a rhythm-control strategy will progress to recurrent or persistent AF. Clinicians often switch to a rate-control strategy as the AF burden increases.
Effectiveness of rate control should be assessed both at rest and with exertion, especially in patients who experience primarily exertional AF-related symptoms. Twenty-four hour Holter monitoring or exercise-treadmill testing can be helpful in evaluating heart rate variability.
Adequate rate control was previously defined as a heart rate of 60-80 bpm at rest and 90-115 bpm with moderate exercise. However, the ACC/AHA/HRS guidelines on the management of AF now advise that there is no benefit in achieving strict heart rate control (< 80 bpm at rest, < 110 bpm after a 6-minute walk) relative to more lenient rate control (< 110 bpm at rest). Strict rate control in patients with stable ventricular function is no longer recommended. [1]
AV nodal blocking medications are the cornerstone of rate control in long-standing AF. In the absence of an accessory pathway, oral beta-blockers, non-dihydropyridine calcium channel blockers, and digoxin are effective. Generally, coadministration of beta-blockers and calcium channel blockers is reserved for patients in whom adequate rate control cannot be achieved with a single agent.
Digoxin can be effective in sedentary patients (especially in those with heart failure) but requires close monitoring of drug levels, serum electrolytes (potassium, magnesium), and renal function. Combinations of rate-control medications (eg, beta-blocker and digoxin) may be superior to individual agents in some patients.
Amiodarone may contribute to ventricular rate control. However, antiarrhythmic agents may organize AF to a potentially life-threatening atrial flutter with 1:1 AV conduction. Particularly with class IC agents, maintenance of effective AV nodal rate control is essential in most patients. Therefore, administration of a beta-blocker or calcium channel blocker is recommended before class IC drugs are initiated.
In the presence of tachycardia-mediated cardiomyopathy or inadequate ventricular rate control despite drug therapy, AV nodal ablation and permanent pacemaker implantation may be considered.
Rhythm control
Maintenance of sinus rhythm requires treatment of cardiovascular risk factors and any underlying disorder (eg, hyperthyroidism, sleep apnea) that may have triggered AF. As mentioned previously, several antiarrhythmic drugs (flecainide, propafenone, dofetilide, amiodarone) have an established efficacy in the pharmacologic conversion of AF to sinus rhythm. The noncardiac adverse effects and contraindications of each drug should be checked prior to administration.
Amiodarone, as a part of a strategy to achieve sinus rhythm, appears to be safe and effective in patients with persistent AF, according to Doyle and Ho. However, in their study, intolerable adverse effects were more common with amiodarone than with placebo or rate-control drugs. [108] Nevertheless, in patients with cardiac disease such as coronary artery disease or systolic or diastolic heart failure, amiodarone becomes the drug of choice because of its decreased proarrhythmic effects compared with other antiarrhythmic drugs. [71]
Amiodarone was also found to be more effective at maintaining sinus rhythm than other drugs in the Canadian Trial of Atrial Fibrillation (CTAF) and the Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T). [109, 110]
Dronedarone is structurally similar to amiodarone, but it lacks amiodarone's iodine moieties. Although the lack of iodine moieties reduces the incidence of adverse events, dronedarone is less effective for rhythm control than amiodarone. [111] Dronedarone has been found to be associated with increased mortality in patients with permanent AF. The randomized, double-blind, phase III Permanent Atrial fibriLLation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS) trial was halted following a preliminary review that revealed that dronedarone was associated with a 2-fold rise in risk of death. [112] Two-fold increases in two other endpoints, stroke and hospitalization for heart failure, were also noted when compared with placebo.
The FDA advises healthcare professionals not to prescribe dronedarone to patients with permanent AF. A separate study by Connolly et al also found that dronedarone increased rates of heart failure, stroke, and death from cardiovascular causes in patients with permanent AF who were at risk for major vascular events; the authors of that study suggested that dronedarone should not be used in this group of patients. [113] The 2014 ACC/AHA/HRS guidelines for the management of AF advise against using dronedarone for patients with New York Heart Association (NYHA) class III and IV heart failure or for patients who have had an episode of decompensated heart failure in the past 4 weeks. [1]
Several distinct agents, most notably sotalol, are used for the long-term maintenance of sinus rhythm. Sotalol is efficacious, but as with other class III drugs, it requires close monitoring of the QT interval and serum electrolyte levels. Sotalol is associated with the risk of QT interval prolongation and torsade de pointes. The proarrhythmic effect of sotalol is increased in patients with congestive heart failure (unlike dofetilide and amiodarone), so it is generally contraindicated in such patients or in those with a prolonged QT interval. Hypokalemia should be corrected and monitored prior to administration of sotalol because it may also prolong the QT interval. Sotalol can be used in patients with coronary artery disease. [71]
In a study of 99 consecutive patients with persistent AF, atrial flutter, or both, patients whose AF responded to chemical cardioversion with dofetilide were particularly vulnerable to proarrhythmias. [114, 115] Of the 99 patients, 46 had successful cardioversion after an average of 2.2 doses of dofetilide, and 53 required electrical cardioversion after an average of 4.7 doses. Of the 21 patients who chemically converted with only one dose of dofetilide, 15 developed QT prolongation and had to either adjust their dose or discontinue treatment. In contrast, only one patient in the electrical conversion group had to discontinue treatment because of QT prolongation. In all, 2% of the patients in the electrical conversion group and 17% of those in the dofetilide-sensitive group had to discontinue treatment because of QT prolongation (P = 0.007). [114, 115]
Class III agents (sotalol, amiodarone) also have some beta-blocking effect and should be used with caution in patients with a history of bradycardia.
Class Ic drugs (flecainide, propafenone) increased the mortality risk in patients with coronary artery disease during the Cardiac Arrhythmia Suppression Trial (CAST) and therefore should not be used in these patients. [116]
Class Ic drugs increased the mortality risk in patients with coronary artery disease during the Cardiac Arrhythmia Suppression Trial (CAST) and therefore should not be used in these patients. [110]
Ablation (catheter based, surgical, or hybrid) for AF can also be performed for achieving rhythm-control. The ACC/AHA/HRS guidelines recommend catheter ablation in the following settings [1] :
-
It is useful for patients with symptomatic paroxysmal AF who are intolerant of, or whose condition is refractory to, at least one class I or III antiarrhythmic medication when a rhythm-control strategy is desired (class I, level of evidence [LOE]: A).
-
It is reasonable as a treatment for certain patients with symptomatic persistent AF who are intolerant of, or whose condition is refractory to, at least one class I or III antiarrhythmic medication (class IIa, LOE: A).
-
It is a reasonable initial strategy for rhythm control prior to using antiarrhythmic drug therapy for patients with recurrent symptomatic paroxysmal AF (class IIa, LOE: B).
Surgical ablation of AF is also an option for patients with AF undergoing other cardiac surgery and for those patients in whom pharmacologic and catheter-based procedures are ineffective or contraindicated. AF ablation may be superior to AV nodal ablation and biventricular pacing in heart failure patients but is technically difficult and demanding, and the widespread applicability of ablation in this population of patients is uncertain.
In the first randomized clinical trial comparing the efficacy and safety of catheter ablation versus minimally invasive surgical ablation during a 12-month follow-up, Boersma et al found that patients with AF who had a dilated left atrium and hypertension or who failed prior AF catheter ablation, surgical ablation was superior in achieving freedom from left atrial arrhythmias after 12 months of follow-up; however, the procedural adverse event rate was found to be significantly higher with surgical ablation than for catheter ablation, primarily postoperative pneumothorax, major bleeding, and an increased need for permanent pacing. [117]
Go to Catheter Ablation for complete information on this topic.
New medical and device-based rhythm-control therapies are being explored actively. Experimental and clinical data suggest that renin-angiotensin system (RAS) antagonists and HMG-CoA-reductase inhibitors (statins) may decrease the incidence of AF and increase the likelihood of successful cardioversion. [118, 119, 120, 121] Device-based therapies under investigation include single- and dual-site atrial pacemakers to prevent AF, as well as atrial defibrillators to rapidly restore sinus rhythm. Invasive (surgical and catheter-based) therapies to compartmentalize the atria and localize focal triggers (in the pulmonary veins) are being evaluated and refined.
Electrical cardioversion
Patients who are hemodynamically unstable, who have severe dyspnea or chest pain with AF, or who have preexcited AF should undergo urgent cardioversion. [71] In stable patients with symptomatic new-onset AF, the rate-control strategy may be considered first to control the ventricular rate. If rate-control treatment does not elicit a response or if echocardiography does not reveal any valvular or functional abnormality of the heart, cardioversion is indicated.
Direct current (DC) cardioversion is the delivery of electrical current that is synchronized to the QRS complexes; it can be delivered in monophasic or biphasic waveforms. The required energy for cardioversion is usually 100-200 J (sometimes higher energy is required) for monophasic waveforms and less for biphasic waveforms. The patient should be sedated. In patients with AF of relatively short duration in whom the left atrium is not significantly large, the success rate of cardioversion exceeds 75% (ie, the size of the left atrium and the duration of AF inversely correlate with the success rate of cardioversion).
Embolization is the most important complication of cardioversion. Accordingly, thrombus in the heart should be ruled out with transesophageal echocardiography (TEE), or anticoagulation should be provided for 3-4 weeks before cardioversion is performed. Stunning of the atria and stasis can occur after cardioversion, and this can lead to thrombus formation even though the patient is in sinus rhythm. Therefore, the patient should receive anticoagulants for at least 4 weeks following the procedure.
Other complications of electrical cardioversion may include pulmonary edema, hypotension, myocardial dysfunction, and skin burns, which may be avoided with the use of steroid cream and proper technique. Electrical cardioversion is also associated with some ST- and T-wave changes on electrocardiography (ECG) and may elevate levels of serum cardiac biomarkers. Synchronization prevents serious ventricular arrhythmias.
Placement of pads or paddle positions include anterior-lateral (ventricular apex and right infraclavicular) and anterior-posterior (sternum and left scapular), with at least one study suggesting increased efficacy with the anterior-posterior (AP) method.
Biphasic waveforms are proved to convert AF at lower energies and higher rates than monophasic waveforms. Strategies include dose escalation (70, 120, 150, 170 J for biphasic or 100, 200, 300, 360 J for monophasic) versus beginning with single high energy/highest success rate for single shock delivered. Patients who are stable and/or awake and can tolerate sedation should be pretreated, with typical regimens involving midazolam, fentanyl, and propofol.
Cardioversion of patients with implanted pacemakers and defibrillator devices is safe when appropriate precautions are taken. Keeping the cardioversion pads in an AP orientation ensures that the shocks are not directly over the generator. Alteration in pacer-programmed data has been reported, as well as heart block and elevated enzymes if the current is conducted through a pacer lead.
Pharmacologic cardioversion
Although pharmacologic cardioversion may be used as the first-line strategy, it is used mainly if DC cardioversion fails or, in some cases, as a precardioversion strategy.
Out-of-hospital self-administration of either flecainide 300 mg or propafenone 600 mg (weight-based dosages if >70 kg) was determined to be successful in terminating AF in 94% of episodes (mean time to symptom resolution of 133 minutes) by Alboni et al. The investigators studied outpatient treatment of AF with a “pill-in-the-pocket” approach in 268 patients with little or no structural heart disease presenting to the emergency department with symptomatic AF. [122]
Pretreatment with amiodarone, flecainide, ibutilide, propafenone, or sotalol has been shown to increase the success rate of DC cardioversion. [4] This strategy is also recommended when DC cardioversion fails and prior to repeat DC cardioversion. [4] Intravenous amiodarone is typically given as a 150-mg bolus over 10-15 minutes, followed by a continuous infusion of 1 mg/min for 6 hours and then 0.5 mg/min.
Hemodynamically unstable patients (eg, those with hypotension) may not tolerate antiarrhythmic drugs, and the adverse effects and contraindications of each antiarrhythmic drug should be considered carefully before administration. Because of possible proarrhythmic adverse effects of antiarrhythmic drugs, these patients should be monitored for at least 24 hours, requiring hospitalization in most cases.
The FDA mandates inpatient monitoring for dofetilide initiation. Patients who start sotalol usually require inpatient monitoring (for torsade de pointes), although patients with no heart disease, with a QT interval less than 450 msec, and with normal electrolyte levels should be started on outpatient medications.
Special considerations
Postoperative AF is common, and perioperative beta-blockers are recommended in all patients undergoing cardiac surgery unless contraindicated. [123] Preoperative administration of amiodarone and sotalol may reduce the incidence of AF in patients undergoing cardiac surgery. As such, these agents may be used as prophylactic therapy in those at high risk for postoperative AF.
Postoperative AF was reduced by treatment with landiolol hydrochloride. [124] Amelioration of ischemia, an anti-inflammatory effect, and inhibition of sympathetic hypertonia by landiolol presumably reduced the occurrence of AF. Hypotension or bradycardia did not develop in any of the patients, indicating the safety of this beta-blocker. These findings suggest that landiolol hydrochloride could be useful in the perioperative management of patients undergoing cardiac surgery. [124]
Retrospective data suggest that atrial-based pacing (AAI, DDD modes) reduces the risk of developing AF and increases the interval between episodes in patients with sick sinus syndrome. [125]
Overview of Surgical and Catheter Ablation
The goal of catheter ablation and surgical treatment of atrial fibrillation (AF) is to disconnect triggers and/or to modify the substrate for AF. Mapping and radiofrequency (RF) ablation of AF is one of the most complex ablation procedures. Numerous approaches are used depending on the expertise of the cardiac electrophysiologist and characteristics of the AF.
Paroxysmal AF is usually caused by triggered and ectopic activity in pulmonary veins, and ablation around the veins terminates the arrhythmia. In persistent AF, triggering foci and reentry circuits may coexist in the atrial tissue, requiring more extensive mapping and ablation to terminate the AF; this yields a lower success rate than ablation used to treat paroxysmal AF.
Antiarrhythimic drug treatment for 6 weeks after ablation of paroxysmal AF was shown to be well tolerated, to reduce the incidence of clinically significant atrial arrhythmias, and to reduce the need for cardioversion or hospital admission during that period, according to Roux et al. [126] Class IC drugs were used as the first line of therapy, and sotalol was the most commonly used drug in cases of left ventricular dysfunction or coronary artery disease. Measured outcomes included atrial arrhythmias lasting more than 24 hours; atrial arrhythmias associated with severe symptoms that required hospitalization, cardioversion, or initiation/change of antiarrhythmic drug therapy; and intolerance to antiarrhythmic agent requiring drug cessation. [126]
Hussein et al performed a registry study that examined controls and patients with mitral valve replacement who underwent AF ablation. [127] No cases of catheter entrapment or stroke were reported. Although most patients required more than one ablation, at last follow-up, 69% were arrhythmia-free and no longer taking antiarrhythmic medications. This provides evidence that AF ablation is safe in this group of patients. Of note, many patients had flutter and creation of a flutter line was one of the keys to success. [127]
Compartmentalization of the Atria
Two approaches to compartmentalization of the atria are surgical, by which multiple cuts are made to the atria, and radiofrequency ablation (RFA).
Surgical compartmentalization of the atria (maze procedure)
Since its inception, surgical compartmentalization of the atria, or the “maze” procedure, has evolved as an exciting approach with the potential to cure atrial fibrillation (AF). The procedure involves making a series of small endocardial incisions in the right and left atria to isolate the pulmonary veins and interrupt potential reentrant pathways required for AF maintenance. Early experience showed that atrial transport is restored postoperatively and that long-term anticoagulation is not required.
The downside remains the need for an open chest procedure; however, thoracoscopic approaches have been developed which reduce the duration of hospitalization and recovery times. The maze procedure remains an attractive procedure for patients with AF who are undergoing concomitant mitral valve procedures. Its role as a primary therapy for AF is doubtful. The role of lesion sets on outcome after maze procedure was studied; the addition of right-sided ablation was found to improve clinical and electrophysiologic results after maze procedure. [128]
Compartmentalization of the atria with continuous ablation lines of blockage
As a parallel to the maze procedure, electrophysiologists have attempted to mimic surgical suture lines with radiofrequency (RF) lesions. The procedures tend to last many hours, and success rates have been somewhat disappointing (50-60%), with the occurrence of left atrial reentrant tachycardias and left atrial flutters (requiring further ablation procedures). [129]
Researchers are uncertain which areas of the atria are necessary to sustain AF. Purely right-sided lesions are not sufficient to eliminate AF, making left atrial procedures necessary. In addition, gaps in linear lesions can be difficult to find.
Research currently focuses on catheter design to deliver linear continuous lesions. Additionally, alternative energy sources (eg, cryotherapy, laser, ultrasonography) may improve the ability to deliver transmural lesions in the left atrium.
Catheter Ablation of Focal Triggers of AF
In some patients, atrial fibrillation (AF) appears to be triggered by electrically active pulmonary vein foci. [130] These patients typically have an abundance of ectopic atrial beats noted on 24-hour Holter monitoring. Electrical isolation of individual pulmonary veins, and thus the ectopic foci, is performed successfully at many centers, and patient selection is key to success.
In a study by Santangeli and colleagues, 59% of patients with paroxysmal AF who underwent a single pulmonary vein antrum isolation (PVAI) procedure were arrhythmia free by 10-year follow-up. [131] The study involved 513 adult patients with drug-refractory paroxysmal AF, all of whom underwent catheter ablation extended to the posterior wall between the pulmonary veins.
Among those patients who underwent multiple procedures for recurrent arrhythmia, Santangeli et al reported that 87% were arrhythmia free by the 10-year mark and that the rate of late recurrence of AF was lower than those reported for segmental and less-extensive antral isolation procedures. [131] However, nonpulmonary vein triggers causing very late recurrence of atrial arrhythmia developed in a significant number of patients.
In a follow-up study, these researchers reported similar findings: 58.7% of patients with paroxysmal AF who underwent the single procedure remained arrhythmia free survival after 12 years, with the highest rate of recurrent arrhythmia in the year 1 (21%) and the lowest rate between years 6 and 12 of follow-up (5.3%). [132] Nearly three quarters of the patients (74%) required repeat procedures, with nearly one third of these (31%) undergoing reconnection in the pulmonary vein antrum after a single procedure and none after two procedures, and another approximate 14% who developed recurrent owing to new non-pulmonary vein triggers. Overall, after multiple procedures, 87% of patients achieved freedom from recurrent AF/atrial tachycardia. [132]
Two major catheter-based modalities for isolating pulmonary venous triggers currently exist: radiofrequency ablation (RFA) and cryoballoon ablation. Cryoballoon ablation offers significantly shorter fluoroscopy and procedures times with similar efficacy as RFA in patients with paroxysmal AF. [133] Patients with persistent AF often require left atrial compartmentalization and ablation of nonpulmonary vein triggers; RFA is preferred in these scenarios.
Chest computed tomography (CT) scanning or magnetic resonance imaging (MRI) can be used to recreate 3-dimensional anatomy in the left atrium, thus aiding in mapping and creating contiguous lines in the left atrium.
The AF cure rate after pulmonary vein isolation may be influenced by sinus node function in both the early and late stages. Although further examinations are required, pulmonary vein isolation may be an adequate treatment for persistent/permanent AF in patients with normal sinus node function. [134]
Patients with paroxysmal AF in whom antiarrhythmic drug therapy does not elicit a response are potential candidates for ablation of AF. The threshold for catheter ablation has fallen over the years and is likely to continue to fall. Ablation of persistent AF is more complex and yields lower success rates. Therefore, RFA is generally considered only if antiarrhythmic drugs fail in patients with persistent AF who remain severely symptomatic despite adequate ventricular rate control. [135]
The success rate of catheter ablation in the treatment of AF varies depending on the type and duration of AF (ie, paroxysmal vs persistent), structural remodeling of the heart, and the technique and expertise of the cardiac electrophysiologist, but it usually ranges from 60-80% over 1-2 years of follow-up.
Patients opting for AF ablation should be told to expect to undergo repeat ablations because these are not uncommon and they improve overall success. [136] In a randomized, clinical trial, a repeat pulmonary vein isolation procedure was more effective than the use of antiarrhythmic drugs in preventing recurrences of paroxysmal AF. [137, 138] The results of the trial further suggested that switching to antiarrhythmic drugs may give the AF time to worsen.
In this study, 154 patients with a 4- to 5-year history of symptomatic AF before the first ablation were randomized to antiarrhythmics or to repeat pulmonary vein isolation. [138] By 3 months, the AF burden was significantly lower in the repeat pulmonary vein isolation group than in the antiarrhythmics group (1.9% vs 3.3%). The AF burden then began to rise in the antiarrhythmics group, reaching 18.8% by 36 months. In contrast, the AF burden did not begin to rise in the reablation group until 15 months after the procedure, reaching just 5.6% at 36 months.
Complications are rarely seen with catheter ablation of AF, but they can include cardiac perforation, pericardial effusion, cardiac tamponade, vascular access complications (bleeding, pseudoaneurysms), pulmonary vein stenosis, thromboembolism, atrioesophageal fistula, left atrial flutter/tachycardia, and phrenic nerve injury (which is more common with cryoballoon ablation). Pulmonary vein stenosis develops in about 6% of patients and may cause dyspnea, chest pain, cough, and hemoptysis. [4] If pulmonary vein stenosis is suspected following catheter ablation, further diagnostic workup with transesophageal echocardiography (TEE), spiral CT scanning, or MRI is recommended. MRI is the most accurate test in diagnosing this complication. Patients with pulmonary vein stenosis should undergo percutaneous angioplasty, which can significantly improve pulmonary blood flow and the patient's symptoms.
Go to Catheter Ablation for complete information on this topic.
AV Node Modification and Permanent Pacemakers
Atrioventricular (AV) node modification may be an alternative in patients with persistent atrial fibrillation (AF) and an uncontrolled ventricular response despite aggressive medical therapy. Catheter ablation of the AV junction permanently interrupts conduction from the atria to the ventricles.
Because the result is permanent AV block, a permanent ventricular pacemaker is required. AF may still be present, but the pacemaker governs the ventricular response. The risk of thromboembolism is unchanged, and patients still require anticoagulation; however, most patients are relieved of their symptoms. During the first 1-3 months, the pacing rate must be programmed in the 80- to 90-beat range to prevent torsade de pointes, which presumably occurs because of slow ventricular rates and early after-depolarizations. In patients with ventricular dysfunction (left ventricular ejection fraction < 50%) and permanent ventricular pacing, a biventricular device may be appropriate. [139] Improvements in left ventricular size and function, functional class, and quality-of-life scores have been demonstrated. [140]
Left Atrial Appendage Percutaneous Closure
The majority of embolic stroke in patients with nonvalvular atrial fibrillation (AF) are associated with left atrial appendage (LAA) thrombi. LAA closure may be a suitable alternative to long-term warfarin therapy for stroke prophylaxis in patients with nonvalvular AF. [141] Currently available devices for LAA closure/ligation include the WATCHMAN device, WAVECREST device, AMPLATZER cardiac plug (ACP) or amulet, and LARIAT endocardial/epicardial suture. [142]
Two randomized trials have assessed the efficacy and safety of LAA closure using the WATCHMAN device. The PROTECT-AF (Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) trial randomized 707 patients with nonvalvular AF and at least one additional risk factor for stroke to either warfarin or LAA closure. [141] Patients who received the WATCHMAN device received 45 days of warfarin and aspirin therapy after implantation. If there was an adequate seal (ie, no leaks >5 mm around the device on TEE performed 45 days after implantation), patients were transitioned to aspirin and clopidogrel for 6 months, followed by lifelong aspirin .
The WATCHMAN device was found to be noninferior to warfarin therapy for the composite primary end-point of stroke, systemic embolism, and cardiovascular or unexplained death. [141] Furthermore, the risk of hemorrhagic stroke was significantly lower in group implanted with the WATCHMAN device compared to the group who received warfarin therapy. However, up to 5% of patients who received the WATCHMAN device developed serious pericardial effusions.
Due to this safety concern, the PREVAIL (Prospective Randomized Evaluation of the Watchman LAA Closure Device in Patients with Atrial Fibrillation Versus Long-Term Warfarin Therapy) trial was undertaken, in which only 2.2% of the participants developed pericardial effusion. [143] This trial randomized 407 patients to receive WATCHMAN device or warfarin therapy, wherein the presence of more than one risk factor for stroke was required, the mean CHADS2 score for the participants was 2.6, and 25% of the operators had to be new operators.
In a patient-level meta-analysis utilizing data from the PROTECT and the PREVAIL studies, as well as follow-up registry data (2406 patients with average duration of follow-up 2.7 years), Holmes et al reported that the WATCHMAN device was associated with a nearly 80% reduction in the risk of hemorrhagic stroke, and a 50% reduction in the risk of cardiovascular/unexplained death, when compared to warfarin therapy. [144] However, the risk of ischemic stroke with the WATCHMAN device was significantly higher than with warfarin therapy.
On the basis of the published trial data, the WATCHMAN device implantation seems to be reasonable alternative to warfarin therapy when there is contraindications for long-term anticoagulation with warfarin. Large clinical trials for the WAVECREST and AMPLATZER devices are under way.
As compared to the other three percutaneous LAA closure devices, LARIAT is an endocardial/epicardial suturing system for ligation of LAA. An epicardial approach is utilized to deliver a pretied suture over a snare, and this is facilitated by an endocardial magnetic-tip guide wire. In a multicenter series, major bleeding was reported in 9% of the patients who underwent LARIAT procedure. [145] However, unlike with the WATCHMAN device, there is no need for postprocedure anticoagulation. Larger studies with long-term follow-up to assess the efficacy and safety of LARIAT device are also under way.
Consultations
Consultation with a cardiac electrophysiologist or knowledgeable clinician is recommended prior to antiarrhythmic drug initiation in patients with atrial fibrillation (AF).
A cardiologist may be consulted emergently if complicating factors are present or if the patient is experiencing ongoing cardiac ischemia or infarction not treatable with direct current (DC) cardioversion, rate-reduction measures, and standard chest pain protocols. [146] A patient with acute myocardial infarction (AMI) and new-onset AF who is stable may benefit from simple rate-control measures (eg, intravenous beta-blockers) while being prepared for the catheterization laboratory and while intravenous nitrates, heparin, and aspirin are initiated. In the patient with an ST elevation MI (STEMI), the main emphasis, however, is to minimize door-to-open-artery time.
A patient's cardiologist plays a vital role in determining the most appropriate long-term strategy for a patient with AF and provides crucial follow-up care.
Long Term Monitoring
Catheter ablation of atrial fibrillation (AF)
Patients who undergo AF catheter ablation should be monitored for the signs and symptoms of potential complications, such as the following:
-
Cardiac perforation
-
Pericardial effusion
-
Cardiac tamponade
-
Vascular access complications
-
Pulmonary vein stenosis
-
Thromboembolism
-
Atrioesophageal fistula
-
Left atrial flutter/tachycardia
-
Phrenic nerve palsy
In addition, AF can recur and most episodes are asymptomatic. Therefore, it is important to monitor for signs and symptoms of recurrent AF in follow-up visits and to administer appropriate diagnostic tests if recurrence is suspected. In a prospective study (2011-2014) that evaluated conventional intermittent Holter and electrocardiographic (ECG) monitoring for recurrent AF following surgical ablation with continuous monitoring via an implantable loop recorder (ILR) in 47 patients, compliance at 12 months was 93% for IRL, 76% for Holter monitoring, and 85% for ECG monitoring. [147] Moreover, detection of atrial tachyarrhythmias was equivalent between continuous monitoring with ILR and intermittent Holter and ECG monitoring. However, the investigators cautioned that these data were limited for broad use of continuous monitoring owing to a high rate of false-positive results (54%) and a limited number of events available for review (11%). [147]
Further outpatient care
Assessment and reassessment of thromboembolic risk is necessary, and periodic ECG monitoring (especially when taking antiarrhythmic agents) and Holter monitoring are often necessary to assess for paroxysmal AF and/or rate control.
Deterrence/prevention
Experimental and clinical data suggest that renin-angiotensin system (RAS) antagonists and HMG-CoA reductase inhibitors (statins) may decrease the incidence of AF and increase the likelihood of successful cardioversion. [118, 119, 120, 121]
In addition, treatment of underlying cardiovascular risk factors such as hypertension, coronary artery disease (CAD), valvular heart disease, obesity, sleep apnea, diabetes, and heart failure is likely to decrease the incidence of AF. Fish oil preparations have also been shown to reduce ventricular arrhythmias in at-risk populations (CAD) and may also protect against AF.
-
Ventricular rate varies from 130-168 beats per minute. Rhythm is irregularly irregular. P waves are not discernible.
-
Classification scheme for patients with atrial fibrillation (AF).
-
Patient management for newly diagnosed atrial fibrillation (Afib). *Therapeutic anticoagulation implies either treatment with warfarin with a therapeutic international normalized ratio (INR) (2-3) or with newer oral anticoagulants (dabigatran, rivaroxaban, apixaban, or edoxaban). Transesophageal echocardiography (TEE)/cardioversion should be performed with an anticoagulation strategy using either low molecular-weight heparin (LMWH) 1 mg/kg twice daily as a bridge, with initiation of warfarin (INR 2-3) or newer oral anticoagulants.
-
Antiarrhythmic drug algorithm for the medical management of sinus rhythm in patients with atrial fibrillation.
-
The image on the right is a reconstructed 3-dimensional image of the right atrium in a patient undergoing atrial fibrillation ablation. The figure on the left was created with a mapping catheter using Endocardial Solutions mapping technology. It represents the endocardial shell of the right atrium and is used as the template during left atrial ablation procedures.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Risk-Management Decisions
- Management of New-Onset AF
- Long-Term Management
- Overview of Surgical and Catheter Ablation
- Compartmentalization of the Atria
- Catheter Ablation of Focal Triggers of AF
- AV Node Modification and Permanent Pacemakers
- Left Atrial Appendage Percutaneous Closure
- Consultations
- Long Term Monitoring
- Show All
- Guidelines
- Atrial Fibrillation Classification
- Stroke Risk Assessment
- Antithrombotic Therapy
- Rate Control
- Cardioversion
- Maintaining Sinus Rhythm
- Catheter Ablation
- Supraventricular Arrhythmias Guidelines
- 2019 ESC/AEPC Guidelines for the Management of Supraventricular Tachycardia
- 2017 EHRA Consensus Document on the Management of Supraventricular Arrhythmias
- Resources
- Show All
- Medication
- Questions & Answers
- Media Gallery
- Tables
- References







