Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia
Abstract
Background
The efficacy and safety of tofacitinib, a Janus kinase inhibitor, in patients who are hospitalized with coronavirus disease 2019 (Covid-19) pneumonia are unclear.
Methods
We randomly assigned, in a 1:1 ratio, hospitalized adults with Covid-19 pneumonia to receive either tofacitinib at a dose of 10 mg or placebo twice daily for up to 14 days or until hospital discharge. The primary outcome was the occurrence of death or respiratory failure through day 28 as assessed with the use of an eight-level ordinal scale (with scores ranging from 1 to 8 and higher scores indicating a worse condition). All-cause mortality and safety were also assessed.

Results
A total of 289 patients underwent randomization at 15 sites in Brazil. Overall, 89.3% of the patients received glucocorticoids during hospitalization. The cumulative incidence of death or respiratory failure through day 28 was 18.1% in the tofacitinib group and 29.0% in the placebo group (risk ratio, 0.63; 95% confidence interval [CI], 0.41 to 0.97; P=0.04). Death from any cause through day 28 occurred in 2.8% of the patients in the tofacitinib group and in 5.5% of those in the placebo group (hazard ratio, 0.49; 95% CI, 0.15 to 1.63). The proportional odds of having a worse score on the eight-level ordinal scale with tofacitinib, as compared with placebo, was 0.60 (95% CI, 0.36 to 1.00) at day 14 and 0.54 (95% CI, 0.27 to 1.06) at day 28. Serious adverse events occurred in 20 patients (14.1%) in the tofacitinib group and in 17 (12.0%) in the placebo group.
Conclusions
Among patients hospitalized with Covid-19 pneumonia, tofacitinib led to a lower risk of death or respiratory failure through day 28 than placebo. (Funded by Pfizer; STOP-COVID ClinicalTrials.gov number, NCT04469114.)
Coronavirus disease 2019 (Covid-19) is a viral disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Despite the rapid development of vaccines, a large part of the world population remains at risk for Covid-19. Therefore, effective, safe, and easy-to-administer therapies for hospitalized patients with Covid-19 are needed.
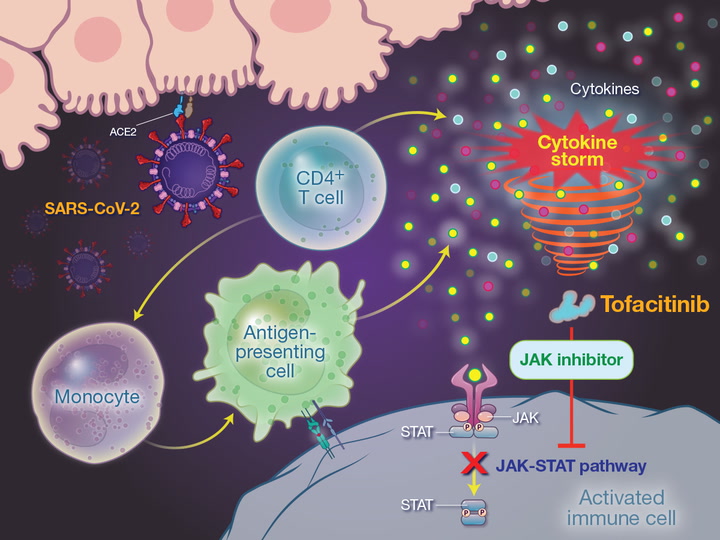
Severe manifestations of SARS-CoV-2 infection are associated with an exaggerated immune response driven by interleukin-6, tumor necrosis factor α, and other cytokines in a pattern called a cytokine storm.1 Tofacitinib is an orally administered selective inhibitor of Janus kinase (JAK) 1 and JAK3, with functional selectivity for JAK2, that blocks intracellular transduction pathways after a cytokine is bound to its receptor. As a consequence, no cellular response is triggered, and cytokine production is indirectly suppressed.2-5 Tofacitinib also modulates the action of interferons and interleukin-6, decreasing the release of cytokines by type 1 and type 17 helper T cells, which are implicated in the pathogenesis of the acute respiratory distress syndrome.6-8 Thus, the action of tofacitinib on multiple critical pathways of the inflammatory cascade may ameliorate progressive, inflammation-driven lung injury in hospitalized patients with Covid-19. We conducted a multicenter, randomized, double-blind, placebo-controlled trial to investigate the efficacy and safety of tofacitinib in hospitalized patients with Covid-19 pneumonia who were not receiving noninvasive or invasive ventilation.
Methods
Trial Design and Oversight
In the Study of Tofacitinib in Hospitalized Patients with Covid-19 Pneumonia (STOP-COVID), we compared tofacitinib with placebo in patients with Covid-19 pneumonia. The trial protocol (available with the full text of this article at NEJM.org) was approved by the institutional ethics board at participating sites. The trial was conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki.
The trial was sponsored by Pfizer and was designed and led by a steering committee that included academic investigators and representatives from Pfizer. The trial operations and statistical analyses were conducted by the Academic Research Organization of the Hospital Israelita Albert Einstein in São Paulo. An independent data and safety monitoring board reviewed unblinded patient-level data for safety on an ongoing basis during the trial. Pfizer provided the entire trial budget, which covered all trial-related expenses including but not limited to investigator fees, costs related to investigational product suppliers and importation, insurance, applicable taxes and fees, and funding to support the activities of the data and safety monitoring board.
All the authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. The trial committee members and participating investigators are listed in the Supplementary Appendix, available at NEJM.org.
Trial Population
The trial included patients 18 years of age or older who had laboratory-confirmed SARS-CoV-2 infection as determined on reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay before randomization, who had evidence of Covid-19 pneumonia on radiographic imaging (computed tomography or radiography of the chest), and who had been hospitalized for less than 72 hours. Information regarding the timing of the qualifying RT-PCR assay in relation to symptom onset is provided in Section S3.1 in the Supplementary Appendix. High-flow devices constituted the maximum oxygen support that was allowed for trial inclusion.
The main exclusion criteria were the use of noninvasive or invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) on the day of randomization, a history of thrombosis or current thrombosis, known immunosuppression, and any current cancer for which the patient was receiving active treatment. Details of the eligibility criteria are provided in Section S3.2. Written informed consent was obtained from each patient or from the patient’s legally authorized representative if the patient was unable to provide informed consent.
Randomization, Interventions, and Follow-up
Eligible patients were randomly assigned in a 1:1 ratio to receive either tofacitinib or placebo. Randomization, with stratification according to site, was performed with the use of a central concealed, Web-based, automated randomization system. Patients received either oral tofacitinib at a dose of 10 mg or placebo twice daily for up to 14 days or until hospital discharge, whichever was earlier. If a participant underwent intubation before the end of the 14-day treatment period (or before discharge), they continued to receive tofacitinib or placebo if it was considered to be clinically appropriate by the treating physicians. A reduced-dose regimen of 5 mg of tofacitinib (or matching placebo) twice daily was administered in patients with an estimated glomerular filtration rate of less than 50 ml per minute per 1.73 m2 of body-surface area, in those with moderate hepatic impairment, and in those with concomitant use of a strong CYP3A4 inhibitor or a combination of a moderate CYP3A4 inhibitor and a strong CYP2C19 inhibitor. The rationale for the tofacitinib dosage is provided in Section S3.3.
All the patients were treated according to local standards of care for Covid-19, which could have included glucocorticoids, antibiotic agents, anticoagulants, and antiviral agents. Concomitant use of other JAK inhibitors, biologic agents, potent immunosuppressants, interleukin-1 inhibitors, interleukin-6 inhibitors, or potent CYP450 inducers was prohibited. Patients were assessed daily (up to day 28) while hospitalized. Follow-up visits occurred on day 14 and on day 28 for participants who were discharged before day 14 or 28. Prespecified reasons for permanent discontinuation of the trial intervention are described in Section S3.4.
Outcomes
The primary outcome was death or respiratory failure during the 28 days of follow-up. Death or respiratory failure was determined to occur if participants met the criteria for category 6 (status of being hospitalized while receiving noninvasive ventilation or ventilation through high-flow oxygen devices), 7 (status of being hospitalized while receiving invasive mechanical ventilation or ECMO), or 8 (death) on the eight-level National Institute of Allergy and Infectious Diseases (NIAID) ordinal scale of disease severity (on a scale from 1 to 8, with higher scores indicating a worse condition) (Table S1 in the Supplementary Appendix). Patients who were enrolled in the trial while they were receiving oxygen through high-flow devices (category 6) were considered to have met the criteria for the primary outcome if they presented with clinical worsening to category 7 or 8. The occurrence of the primary outcome was adjudicated by an independent clinical-events classification committee, whose members were unaware of the group assignments. The protocol and statistical analysis plan used an inverted ordinal scale, which was reversed in this report to be consistent with previous studies.
Secondary efficacy outcomes were the cumulative incidence of death through day 28, the scores on the NIAID ordinal scale of disease severity at day 14 and at day 28, the status of being alive and not using mechanical ventilation or ECMO at day 14 and day 28, the status of being alive and not hospitalized at day 14 and day 28, cure (defined as resolution of fever and cough and no use of ventilatory or oxygen support), the duration of stay in the hospital, and the duration of stay in the intensive care unit (ICU). The occurrence and severity of adverse events were evaluated and coded according to the Medical Dictionary for Regulatory Activities, version 23.1. Details of adverse event reporting, including the reporting of prespecified adverse events of special interest, are described in Section S3.5.
Statistical Analysis
We estimated that the assignment of 260 patients, with randomization performed in a 1:1 ratio, would provide the trial with 80% power to detect a between-group difference of 15 percentage points in the incidence of the primary outcome, assuming that 15% of the participants in the tofacitinib group and 30% of those in the placebo group would have an event (death or respiratory failure through day 28). The hypothesis of superiority was tested at a two-tailed alpha level of 5%. The efficacy analyses included all the participants who underwent randomization. Safety analyses included all the participants who underwent randomization and took at least one dose of tofacitinib or placebo.
The results for the primary efficacy outcome were analyzed by means of binary regression with Firth correction, with trial group and antiviral therapy for Covid-19 as covariates, and are expressed as a risk ratio. The antiviral treatments on day 1 were used in the statistical model. Dichotomous secondary outcomes were analyzed in a manner similar to that used for the primary outcome. The effect of the intervention on death through day 28 is expressed as a hazard ratio derived from Cox regression. For ordinal data, a proportional-odds model with adjustment for baseline antiviral therapy was used. An odds ratio of less than 1.0 represents a clinical improvement as assessed on the ordinal scale. Odds proportionality was assessed with the use of the method of Pulkstenis–Robinson.9 We created Kaplan–Meier survival curves to express the time until the occurrence of the primary outcome, both overall and stratified according to the use of supplemental oxygen at baseline, and the occurrence of death through 28 days.
As a sensitivity analysis, results for the primary outcome were analyzed by means of binary regression with Firth correction, with use of glucocorticoids and antiviral agents at baseline as covariates. In addition, results for the primary outcome were analyzed by means of logistic regression with Firth correction, with adjustment for baseline antiviral therapy. Prespecified subgroup analyses were performed according to age, sex, concomitant use of antiviral therapy, concomitant use of glucocorticoids, and time from symptom onset to randomization.
For the primary outcome, a two-sided P value of less than 0.05 was considered to indicate statistical significance. The 95% confidence intervals were estimated for all effect measures. The widths of the 95% confidence intervals for the secondary outcomes were not adjusted for multiple comparisons, so the intervals should not be used to infer definitive treatment effects. All the analyses were performed with the use of SAS software, version 9.4 (SAS Institute), and R software, version 3.6.3 (R Foundation for Statistical Computing). Additional details about the statistical analysis are provided in Section S3.6.
Results
Patients
From September 16 through December 13, 2020, a total of 289 consecutive patients from 15 sites in Brazil were enrolled; 144 patients were randomly assigned to receive tofacitinib and 145 to receive placebo (Figure 1). Of these patients, 142 in each group received tofacitinib or placebo as assigned. All the patients in both groups completed the 28-day follow-up or died. No patient was lost to follow-up or withdrew consent.
Figure 1

Enrollment, Randomization, and Follow-up.
The primary analysis population included all the patients who underwent randomization. Patients may have been excluded from the trial for more than one reason. A total of 36 patients were excluded owing to “other medical conditions,” which meant that the patient had another medical or psychiatric condition including recent (within the past year) or active suicidal ideation or had a behavior abnormality or an abnormal laboratory test result that might have increased the risk associated with trial participation or that, in the investigator’s judgment, would have made the participant inappropriate for inclusion in the trial. A total of 289 patients underwent randomization, and all of them had data that could be evaluated. Covid-19 denotes coronavirus disease 2019, ECMO extracorporeal membrane oxygenation, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
The baseline characteristics of the patients were well balanced between the groups (Table 1). The mean age of the patients was 56 years, and 34.9% of the patients were women. The median time from symptom onset to randomization was 10 days, and the median time from the diagnosis of Covid-19 to randomization was 5 days. The median body-mass index (the weight in kilograms divided by the square of the height in meters) was 29.7. A total of 50.2% of the patients had hypertension, and 23.5% had diabetes mellitus. At baseline, 75.4% of the patients were receiving supplemental oxygen, 78.5% were being treated with glucocorticoids, 77.9% were receiving prophylactic anticoagulation, and 20.8% were receiving therapeutic anticoagulation.
Table 1

| Characteristic | Tofacitinib (N=144) |
Placebo (N=145) |
Total (N=289) |
|---|---|---|---|
| Age | |||
| Mean age — yr | 55±14 | 57±14 | 56±14 |
| Distribution — no. (%) | |||
| <45 yr | 39 (27.1) | 30 (20.7) | 69 (23.9) |
| 45–64 yr | 64 (44.4) | 67 (46.2) | 131 (45.3) |
| ≥65 yr | 41 (28.5) | 48 (33.1) | 89 (30.8) |
| Female sex — no. (%) | 50 (34.7) | 51 (35.2) | 101 (34.9) |
| Race — no. (%)† | |||
| White | 118 (81.9) | 123 (84.8) | 241 (83.4) |
| Black | 12 (8.3) | 13 (9.0) | 25 (8.7) |
| Multiracial | 11 (7.6) | 7 (4.8) | 18 (6.2) |
| Other or unknown | 3 (2.1) | 2 (1.4) | 5 (1.7) |
| Median body-mass index (IQR) | 29.4 (26.8–33.2) | 29.7 (26.4–32.7) | 29.7 (26.7–32.9) |
| Median time from symptom onset to randomization (IQR) — days‡ | 10 (7–12) | 9 (7–11) | 10 (7–11) |
| Median time from Covid-19 diagnosis to randomization (IQR) — days | 5 (2–8) | 4 (2–8) | 5 (2–8) |
| Hospitalization in the ICU at randomization — no. (%) | 28 (19.4) | 26 (17.9) | 54 (18.7) |
| Medical history — no. (%) | |||
| Hypertension | 67 (46.5) | 78 (53.8) | 145 (50.2) |
| Diabetes | 34 (23.6) | 34 (23.4) | 68 (23.5) |
| Dyslipidemia | 30 (20.8) | 20 (13.8) | 50 (17.3) |
| Current or former smoking§ | 41 (28.5) | 41 (28.3) | 82 (28.4) |
| Score on NIAID ordinal scale — no. (%)¶ | |||
| 4: Hospitalized, not receiving supplemental oxygen but receiving ongoing medical care, Covid-19–related or otherwise | 34 (23.6) | 37 (25.5) | 71 (24.6) |
| 5: Hospitalized, receiving supplemental oxygen through low-flow devices | 91 (63.2) | 90 (62.1) | 181 (62.6) |
| 6: Hospitalized, receiving supplemental oxygen through high-flow devices | 19 (13.2) | 18 (12.4) | 37 (12.8) |
| Treatment — no. (%) | |||
| Glucocorticoid | 114 (79.2) | 113 (77.9) | 227 (78.5) |
| Antiviral agent‖ | 20 (13.9) | 18 (12.4) | 38 (13.1) |
| Prophylactic anticoagulation | 113 (78.5) | 112 (77.2) | 225 (77.9) |
| Therapeutic anticoagulation | 28 (19.4) | 32 (22.1) | 60 (20.8) |
Characteristics of the Patients at Baseline.*
*
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. Covid-19 denotes coronavirus disease 2019, ICU intensive care unit, and IQR interquartile range.
†
Race was determined by the investigator and recorded on the case-report form.
‡
The time from symptom onset to randomization was missing for two patients (one in each group).
§
History of tobacco use was missing for one patient in the placebo group.
¶
Scores on the National Institute of Allergy and Infectious Diseases (NIAID) ordinal scale range from 1 to 8, with higher scores indicating a worse condition.
‖
In all cases, the antiviral agent used was oseltamivir.
Details concerning adherence to the trial regimen and the use of other medications are presented in Tables S2 and S3. Overall, 14.1% of the patients in the tofacitinib group and 10.6% of those in the placebo group permanently discontinued the assigned regimen before the end of the trial. The median number of days that tofacitinib or placebo was used was 5 days in the tofacitinib group and 6 days in the placebo group. Overall, 89.3% of the patients (88.9% in the tofacitinib group and 89.7% in the placebo group) received glucocorticoids during hospitalization. Antiviral therapy was received by 20 patients in each group; in all cases, the antiviral agent used was oseltamivir. Major protocol deviations are presented in Table S4.
Primary Outcome
Results for the primary and secondary outcomes are shown in Table 2. Death or respiratory failure through day 28 occurred in 18.1% of the patients in the tofacitinib group and in 29.0% of those in the placebo group (risk ratio, 0.63; 95% confidence interval [CI], 0.41 to 0.97; P=0.04) (Figure 2). Sensitivity analyses for the primary outcome, including the analysis that was adjusted for use of glucocorticoids, showed results similar to those of the main analysis (Table S5). The results were also consistent regardless of patients’ baseline scores on the ordinal scale (Table S6 and Figs. S1 through S3). Results for the primary outcome were generally consistent across prespecified subgroups (Figure 3).
Figure 2

Cumulative Incidence of the Primary Outcome.
The primary outcome was death or respiratory failure through day 28. The risk ratio and P value for the primary outcome were calculated by means of binary regression with Firth correction, with trial group and inclusion of antiviral therapy for Covid-19 as covariates. The inset shows the same data on an expanded y axis.
Figure 3

Subgroup Analyses of Death or Respiratory Failure through Day 28.
In the subgroup analyses, the risk ratios for death or respiratory failure through day 28 (primary outcome) were calculated by means of binary regression with Firth correction, with trial group and antiviral therapy for Covid-19 as covariates. For the analysis according to use of antiviral therapy at baseline, in all cases, the antiviral agent used was oseltamivir. Time from symptom onset was analyzed in two ways: according to thirds (the prespecified analysis) and above versus below or equal to the median (10 days; post hoc analysis). Two patients (one in the tofacitinib group and one in the placebo group) did not have data on the date of symptom onset. The size of the boxes is proportional to the number of patients and events, and arrows indicate that the boundary of the 95% confidence interval is outside the graphed area.
Table 2

| Outcome | Tofacitinib (N=144) |
Placebo (N=145) |
Measure of Effect (95% CI) |
P Value |
|---|---|---|---|---|
| number (percent) | ||||
| Primary outcome: death or respiratory failure through day 28† | 26 (18.1) | 42 (29.0) | 0.63 (0.41–0.97) | 0.04 |
| Secondary outcomes | ||||
| Death through day 28‡ | 4 (2.8) | 8 (5.5) | 0.49 (0.15–1.63) | |
| Score on NIAID ordinal scale§ | ||||
| At day 14 | 0.60 (0.36–1.00) | |||
| 1 | 110 (76.4) | 96 (66.2) | ||
| 2 | 11 (7.6) | 15 (10.3) | ||
| 3 | 1 (0.7) | 2 (1.4) | ||
| 4 | 5 (3.5) | 6 (4.1) | ||
| 5 | 7 (4.9) | 6 (4.1) | ||
| 6 | 1 (0.7) | 6 (4.1) | ||
| 7 | 7 (4.9) | 9 (6.2) | ||
| 8 | 2 (1.4) | 5 (3.4) | ||
| At day 28 | 0.54 (0.27–1.06) | |||
| 1 | 129 (89.6) | 119 (82.1) | ||
| 2 | 5 (3.5) | 10 (6.9) | ||
| 3 | 0 | 1 (0.7) | ||
| 4 | 0 | 2 (1.4) | ||
| 5 | 4 (2.8) | 1 (0.7) | ||
| 6 | 1 (0.7) | 0 | ||
| 7 | 1 (0.7) | 4 (2.8) | ||
| 8 | 4 (2.8) | 8 (5.5) | ||
| Status at day 14¶ | ||||
| Alive and not using mechanical ventilation or ECMO | 135 (93.8) | 131 (90.3) | 1.04 (0.97–1.12) | |
| Alive and not hospitalized | 121 (84.0) | 111 (76.6) | 1.11 (0.99–1.24) | |
| Status at day 28¶ | ||||
| Alive and not using mechanical ventilation or ECMO | 139 (96.5) | 133 (91.7) | 1.06 (1.00–1.12) | |
| Alive and not hospitalized | 134 (93.1) | 129 (89.0) | 1.05 (0.97–1.13) | |
| Cured‖ | 134 (93.1) | 132 (91.0) | 1.03 (0.95–1.10) | |
Primary and Secondary Efficacy Outcomes.*
*
CI denotes confidence interval.
†
The risk ratio and P value for the primary outcome were calculated by means of binary regression with Firth correction, with trial group and inclusion of antiviral therapy for Covid-19 as covariates.
‡
The effect of the intervention on death until day 28 is expressed as hazard ratio derived from Cox regression.
§
For ordinal data, a proportional-odds model with adjustment for inclusion of antiviral therapy at baseline was used. The assumption of proportional odds was met with the use of the method of Pulkstenis–Robinson (the P value for the 14-day analysis was 0.63 and for the 28-day analysis was 0.14). In this case, a P value higher than 0.05 was considered to indicate that the proportional-odds assumption was met. An odds ratio of less than 1 represents a clinical improvement with tofacitinib, as compared with placebo, as assessed on the ordinal scale. Scores on the eight-category NIAID ordinal scale range from 1 to 8, with higher scores indicating a worse condition. A score of 1 indicates that the patient was not hospitalized, with no limitations on activities; 2, was not hospitalized but had limitation on activities or was receiving supplemental oxygen at home; 3, was hospitalized, without use of supplemental oxygen and no longer required ongoing medical care; 4, was hospitalized and not receiving supplemental oxygen but receiving ongoing medical care (Covid-19–related or otherwise); 5, was hospitalized and receiving supplemental oxygen through low-flow devices; 6, was hospitalized and receiving oxygen through noninvasive ventilation or high-flow devices; 7, was hospitalized and receiving invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); and 8, died.
¶
Risk ratios for these secondary outcomes were calculated in a manner similar to that for the primary outcome. A risk ratio of more than 1 represents a clinical benefit of tofacitinib as compared with placebo. A status of alive and not using mechanical ventilation or ECMO represents a score on the NIAID ordinal scale of 1 to 6. A status of alive and not hospitalized represents a score of 1 or 2.
‖
Cure referred to resolution of fever and cough and no use of ventilatory or oxygen support. A total of 2 patients did not have information on the date of symptom start but had ordinal scale data at day 28. They were considered to have met the criteria for being cured given that they were not using ventilatory or oxygen support at day 28.
Secondary Outcomes
Death from any cause through day 28 occurred in 2.8% of patients in the tofacitinib group and in 5.5% of those in the placebo group (hazard ratio, 0.49; 95% CI, 0.15 to 1.63) (Fig. S4). As compared with placebo, the proportional odds of having a worse score on the eight-level ordinal scale with tofacitinib was 0.60 (95% CI, 0.36 to 1.00) at day 14 and 0.54 (95% CI, 0.27 to 1.06) at day 28. The assumption of proportional odds was met. The distributions of patients’ scores on the eight-level ordinal scale at 14 days and 28 days are shown in Figure S5. Sensitivity analyses for the secondary outcomes are shown in Table S7. The median duration of hospital stay and ICU stay were similar in the two groups (Table S8).
Safety Outcomes
Adverse events occurred in 26.1% of the patients in the tofacitinib group and in 22.5% of those in the placebo group (Table S9). Serious adverse events occurred in 20 patients (14.1%) in the tofacitinib group and in 17 (12.0%) in the placebo group (Table S10). Among the adverse events of special interest, deep-vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis occurred in 1 patient each in the tofacitinib group; hemorrhagic stroke and cardiogenic shock occurred in 1 patient each in the placebo group. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group. Adverse events other than death that led to the discontinuation of the trial regimen occurred in 11.3% of the patients in the tofacitinib group and in 3.5% of those in the placebo group (Table S11); the most common such events were an increase in aminotransferase levels (in 4.2% of the patients in the tofacitinib group and in 0.7% of those in the placebo group) and lymphopenia (in 2.8% and 1.4%, respectively).
Discussion
In this randomized, double-blind, placebo-controlled trial involving hospitalized patients with Covid-19 pneumonia, tofacitinib was superior to placebo in reducing the incidence of death or respiratory failure through day 28. These effects were consistent regardless of sex, age, duration of symptoms, and use of glucocorticoids at baseline; they were also consistent across different levels of supplemental oxygen use at baseline. Although STOP-COVID was not powered to detect a difference in mortality or in the incidence of other secondary outcomes between the two groups, the direction of effects favored tofacitinib. Finally, tofacitinib was not associated with a higher risk of secondary infection or thromboembolic events.
The effects of JAK inhibition in patients with Covid-19 have been assessed previously.10-13 In the second stage of the Adaptive Covid-19 Treatment Trial (ACTT-2), combination treatment with baricitinib and remdesivir was superior to remdesivir treatment alone in shortening the time to recovery, particularly among patients receiving high-flow oxygen or noninvasive mechanical ventilation.14 In addition, patients in the combination-treatment group had a higher likelihood of improved clinical status at day 15 than those who received only remdesivir.
In ACTT-2, only approximately 22% of the participants received glucocorticoid therapy during the trial. In our trial, the majority (89.3%) of patients were treated with glucocorticoids during hospitalization. The Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial and a subsequent meta-analysis showed that the use of glucocorticoids reduced mortality among hospitalized patients with Covid-19 receiving oxygen therapy.15,16 On the basis of these results, glucocorticoids are recommended by current guidelines as part of the standard care for this patient population.17 Our findings show that tofacitinib, when added to standard care including glucocorticoids, led to a lower risk of clinical events among patients hospitalized with Covid-19 pneumonia than placebo.
The first stage of the Adaptive Covid-19 Treatment Trial (ACTT-1) showed that treatment with the antiviral agent remdesivir was superior to use of placebo in shortening the time to recovery in patients with Covid-19.18 Given these results, remdesivir was approved by the Food and Drug Administration as a standard-of-care treatment for Covid-19.19 Remdesivir was not available in Brazil during the conduct of our trial; the only antiviral agent used was oseltamivir, which has not been shown to be effective in patients with Covid-19. Therefore, our trial does not offer evidence of a benefit of tofacitinib treatment in addition to established antiviral therapy.
On the basis of current evidence, antiviral therapy is most likely to be effective in early Covid-19. The hyperinflammatory responses are thought to drive the clinical symptoms in later stages of the disease.20 Therefore, antiinflammatory interventions are needed in hospitalized patients with mild, moderate, or severe Covid-19.21 Glucocorticoids are likely to be most effective in severely ill patients. Taken together, the results of ACTT-2 and STOP-COVID provide evidence that JAK inhibition represents an additional therapeutic option for treating Covid-19 pneumonia in patients who are not yet receiving invasive mechanical ventilation. These agents are orally administered and have few drug–drug interactions. Ongoing trials with tofacitinib (ClinicalTrials.gov numbers, NCT04415151 and NCT04750317) and baricitinib (NCT04390464, NCT04640168, NCT04421027, and NCT04381936) may provide further insights regarding the effects of JAK inhibitors in patients with Covid-19.
Several other specific immune modulators are being tested in patients with Covid-19 pneumonia, and the results have been mixed.22 These include anticytokines such as interleukin-1 and interleukin-6 receptor antagonists (e.g., anakinra, tocilizumab, sarilumab, and siltuximab), tumor necrosis factor inhibitors (e.g., adalimumab and infliximab), and granulocyte–macrophage colony-stimulating factors (e.g., gimsilumab, lenzilumab, and namilumab).23-29 In STOP-COVID, the use of these agents was prohibited. Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with Covid-19 remains to be determined.
In this trial, among hospitalized adult patients with Covid-19 pneumonia, tofacitinib led to a lower risk of death or respiratory failure through day 28 than placebo.
Notes
This article was published on June 16, 2021, and updated on July 29, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Supported by Pfizer.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Supplementary Material
Research Summary (nejmoa2101643_research-summary.pdf)
- Download
- 220.21 KB
Protocol (nejmoa2101643_protocol.pdf)
- Download
- 1.50 MB
Supplementary Appendix (nejmoa2101643_appendix.pdf)
- Download
- 576.08 KB
Disclosure Forms (nejmoa2101643_disclosures.pdf)
- Download
- 551.42 KB
Data Sharing Statement (nejmoa2101643_data-sharing.pdf)
- Download
- 69.93 KB
References
1.
Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020;383:2255-2273.
2.
Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723-1736.
3.
Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390:457-468.
4.
Dowty ME, Lin TH, Jesson MI, et al. Janus kinase inhibitors for the treatment of rheumatoid arthritis demonstrate similar profiles of in vitro cytokine receptor inhibition. Pharmacol Res Perspect 2019;7(6):e00537-e00537.
5.
Gadina M, Johnson C, Schwartz D, et al. Translational and clinical advances in JAK-STAT biology: the present and future of jakinibs. J Leukoc Biol 2018;104:499-514.
6.
Maeshima K, Yamaoka K, Kubo S, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-γ and interleukin-17 production by human CD4+ T cells. Arthritis Rheum 2012;64:1790-1798.
7.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033-1034.
8.
Boor PPC, de Ruiter PE, Asmawidjaja PS, Lubberts E, van der Laan LJW, Kwekkeboom J. JAK-inhibitor tofacitinib suppresses interferon alfa production by plasmacytoid dendritic cells and inhibits arthrogenic and antiviral effects of interferon alfa. Transl Res 2017;188:67-79.
9.
Pulkstenis E, Robinson TJ. Goodness-of-fit tests for ordinal response regression models. Stat Med 2004;23:999-1014.
10.
Titanji BK, Farley MM, Mehta A, et al. Use of baricitinib in patients with moderate to severe coronavirus disease 2019. Clin Infect Dis 2021;72:1247-1250.
11.
Cantini F, Niccoli L, Nannini C, et al. Beneficial impact of baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect 2020;81:647-679.
12.
Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, Garcia-Gomez C, Jimenez-Vizuete JM, Martinez-Alfaro E. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology (Oxford) 2021;60:399-407.
13.
Petrone L, Petruccioli E, Alonzi T, et al. In-vitro evaluation of the immunomodulatory effects of baricitinib: implication for COVID-19 therapy. J Infect 2021;82:58-66.
14.
Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med 2021;384:795-807.
15.
The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693-704.
16.
The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324:1330-1341.
17.
Rochwerg B, Siemieniuk RA, Agoritsas T, et al. A living WHO guideline on drugs for covid-19. BMJ 2020;370:m3379-m3379.
18.
Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 2020;383:1813-1826.
19.
Rubin D, Chan-Tack K, Farley J, Sherwat A. FDA approval of remdesivir — a step in the right direction. N Engl J Med 2020;383:2598-2600.
20.
Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med 2020;383:1757-1766.
21.
Goletti D, Cantini F. Baricitinib therapy in Covid-19 pneumonia — an unmet need fulfilled. N Engl J Med 2021;384:867-869.
22.
Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN. Pharmaco-immunomodulatory therapy in COVID-19. Drugs 2020;80:1267-1292.
23.
Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021;384:20-30.
24.
Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med 2021;384:1503-1516.
25.
Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020;383:2333-2344.
26.
Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:24-31.
27.
Hermine O, Mariette X, Tharaux P-L, Resche-Rigon M, Porcher R, Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:32-40.
28.
The CORIMUNO-19 Collaborative group. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med 2021;9:295-304.
29.
Lescure F-X, Honda H, Fowler RA, et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021;9:522-532.
Information & Authors
Information
Published In
Copyright
Copyright © 2021 Massachusetts Medical Society. All rights reserved.
History
Published online: June 16, 2021
Published in issue: July 29, 2021
Topics
Authors
Affiliations
From the Hospital Israelita Albert Einstein (P.O.G., R.H.F., D.D.F.M., B.S.S., S.R.L.A., R.V.P.S., L.P.A.P., K.C., R.G.R.A.P.M., F.M., H.P.G., L.V.R., O.B.), the Heart Institute, InCor, University of São Paulo Medical School (R.H.F., R.K.F., V.M.J.), BP Mirante–A Beneficência Portuguesa de São Paulo (A.M.S.), BP–A Beneficência Portuguesa de São Paulo (V.C.V.), and Pfizer (M.D., M.R.T.P.), São Paulo, Centro Integrado de Pesquisa, Hospital de Base, São José do Rio Preto Medical School, São José do Rio Preto (L.N.M.), Pontifícia Universidade Católica de Campinas, Campinas (J.F.S.), Hospital Universitário São Francisco de Assis na Providência de Deus and Irmandade do Senhor Bom Jesus dos Passos da Santa Casa de Misericórida de Bragança Paulista, Bragança Paulista (M.O.A.), Hospital São Vicente de Paulo, Passo Fundo (A.P.T.), and Hospital Estadual Jayme dos Santos Neves, Vila Velha (P.A.M.) — all in Brazil; Pfizer, Collegeville, PA (D.Q.); Pfizer, Lima, Peru (D.P.L.); Pfizer, Istanbul, Turkey (L.M.G.); Pfizer, Rotterdam, the Netherlands (J.J.D.); and Pfizer, New York (T.K.).
Metrics & Citations
Metrics
Altmetrics
Citations
Export citation
Select the format you want to export the citation of this publication.
Cited by
- Tofacitinib in Patients Hospitalized With Moderate and Severe COVID-19: Not Just Another Kinase Inhibitor, Cureus, (2024).https://doi.org/10.7759/cureus.52725
- Paradigm of immune dysregulation in coronavirus disease-2019 infection, Exploration of Immunology, (1-33), (2024).https://doi.org/10.37349/ei.2024.00126
- Agranulocytosis and secondary infection related to JAK inhibitors and IL-6 receptor blockers: a disproportionality analysis using the US Food and drug administration adverse event reporting system, Frontiers in Pharmacology, 14, (2024).https://doi.org/10.3389/fphar.2023.1323240
- Corticosteroids induce an early but limited decrease in IL-6 dependent pro-inflammatory responses in critically ill COVID-19 patients, Minerva Anestesiologica, 90, 3, (2024).https://doi.org/10.23736/S0375-9393.23.17765-0
- Shared Immune Associations Between COVID-19 and Inflammatory Bowel Disease: A Cross-Sectional Observational Study in Shanghai, China, Journal of Inflammation Research, Volume 17, (1929-1940), (2024).https://doi.org/10.2147/JIR.S449746
- Statistical analyses of ordinal outcomes in randomised controlled trials: a scoping review, Trials, 25, 1, (2024).https://doi.org/10.1186/s13063-024-08072-2
- Protective role of the HSP90 inhibitor, STA-9090, in lungs of SARS-CoV-2-infected Syrian golden hamsters, BMJ Open Respiratory Research, 11, 1, (e001762), (2024).https://doi.org/10.1136/bmjresp-2023-001762
- Clinical trials of pharmacological interventions for SARS‐CoV‐2 published in leading medical journals report adherence but not how it was assessed, British Journal of Clinical Pharmacology, 90, 4, (1130-1141), (2024).https://doi.org/10.1111/bcp.15992
- Guidance for prevention and management of COVID-19 in children and adolescents: A consensus statement from the Pediatric Infectious Diseases Society Pediatric COVID-19 Therapies Taskforce, Journal of the Pediatric Infectious Diseases Society, 13, 3, (159-185), (2024).https://doi.org/10.1093/jpids/piad116
- Investigational pharmacological agents for the treatment of ARDS, Expert Opinion on Investigational Drugs, 33, 3, (243-277), (2024).https://doi.org/10.1080/13543784.2024.2315128
- See more
Loading...