Abstract
Unwanted self-discharge of LFP/AG and NMC811/AG cells can be caused by in situ generation of a redox shuttle molecule after formation at elevated temperature with common alkyl carbonate electrolyte. This study investigates the redox shuttle generation for several electrolyte additives, e.g., vinylene carbonate and lithium difluorophosphate, by measuring the additive reduction onset potential, first cycle inefficiency and gas evolution during formation at temperatures between 25 and 70 °C. After formation, electrolyte is extracted from pouch cells for visual inspection and quantification of redox shuttle activity in coin cells by cyclic voltammetry. The redox shuttle molecule is identified by GC-MS and NMR as dimethyl terephthalate. It is generated in the absence of an effective SEI-forming additive, according to a proposed formation mechanism that requires residual water in the electrolyte, catalytic quantities of lithium methoxide generated at the negative electrode and, surprisingly, polyethylene terephthalate tape within the cell.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Lithium-ion batteries play an important role in the world's transition to sustainable energy. Since they are used at an increasing rate for large-scale stationary storage of renewable energy, the ability of lithium-ion cells to hold their charge over extended periods of time at various temperatures is crucial. Lithium iron phosphate (LiFePO4 or LFP) is a more sustainable and lower cost alternative to layered transition metal oxide cathodes (e.g., LiNi0.8Mn0.1Co0.1O2 or NMC811) and especially suited for stationary applications. However, Logan et al. showed that storage of LFP/artificial graphite (AG) cells at 60 °C with lithium hexafluorophosphate (LiPF6) conducting salt dissolved in common alkyl carbonate can lead to full self-discharge in only 500 h unless effective electrolyte additives are used. 1 Buechele et al. then performed systematic open circuit storage experiments with LFP/AG and NMC811/AG cells and assigned the capacity loss during storage to irreversible and reversible self-discharge. 2 They found that reversible self-discharge accounts for the majority of capacity loss in cells without electrolyte additives, that it correlates strongly with formation temperature, is lower with lithium bis(fluorosulfonyl)imide (LiFSI) conducting salt, and is suppressed if vinylene carbonate (VC) is used as electrolyte additive. 2 Buechele et al. also showed that a voltage hold at 4.2 V after formation is effective at reducing reversible self-discharge in LFP/AG cells without electrolyte additives. 2 The sensitivity of reversible self-discharge to cell voltage correlates with the observation that low voltage LFP/AG cells without electrolyte additives showed significantly more reversible self-discharge than higher voltage NMC811/AG cells without electrolyte additives. 2 The large currents needed to explain the rapid self-discharge at elevated temperature pointed towards a reversible shuttle that is generated in the battery cells. 2 Other groups also ascribed reversible self-discharge to a shuttle reaction. 3,4 Similarly, Logan et al. found that small inefficiencies of long-lived LFP/AG cells detected by ultra-high precision coulometry (UHPC) and microcalorimetry could only be explained by a reversible shuttle reaction. 5
Boulanger et al. were able to measure the redox shuttle activity in electrolytes extracted from LFP/AG and NMC811/AG pouch cells after high temperature formation by cyclic voltammetry (CV). 6 The authors found a correlation between formation temperature and stable shuttling currents in slow CV sweeps. Electrolyte extracted from cells with VC did not show shuttling currents, which agrees with the self-discharge data by Buechele et al. 2 Since VC forms a well-passivating solid-electrolyte interphase (SEI) on graphite, 7 it also indicates that the shuttle species could be generated at the negative electrode. Boulanger et al. demonstrated that the shuttle species gives rise to a strong electrolyte discoloration, which was not due to dissolved transition metals, but they were unable to identify the shuttle species. 6
This study will use LFP/AG and NMC811/AG pouch cells with alkyl carbonate electrolyte and LiPF6 conducting salt formed at temperatures between 25 and 70 °C to explore the effect of electrolyte additives on redox shuttle generation. We will quantify the additive reduction onset potential, first cycle inefficiency and gas evolution as important formation metrics that will allow to assess the level of passivation of the negative electrode. The additives will be divided into SEI-forming and non-SEI-forming additives, in order to help understand the conditions needed for redox shuttle generation. Electrolyte extracted from pouch cells will be subject to visual inspection and various chemical analysis techniques to finally identify the redox shuttle molecule and propose a formation mechanism.
Experimental
Electrolyte preparation
All electrolytes in this study use 1.5 M LiPF6 as conducting salt. Control electrolyte (CTRL) refers to 1.5 M LiPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) at a weight ratio of 3:7. CTRL + 1 wt% lithium difluorophosphate is referred as 1LFO. Similarly, CTRL + 2 wt% VC is referred as 2VC. The other electrolyte formulations are abbreviated analogously and contain succinonitrile (SN), fluoroethylene carbonate (FEC), ethylene sulfate (DTD) or prop-1-ene-1,3-sultone (PES). If dimethyl carbonate is used as single solvent the electrolyte is simply referred to as DMC. All electrolyte solvents, additives and the lithium salt were used as-received (<20 ppm water, Shenzhen Capchem, China) and mixed in an Ar-filled glovebox. Other molecules explored in this study are lithium methoxide (LiOMe), dimethyl terephthalate (DMT), ferrocene (Fc), iron trifluoromethanesulfonate (iron triflate or FeOTf), trifluoromethanesulfonate (manganese triflate or MnOTf, all from Sigma-Aldrich, USA) and trimethyl phosphate (TMP). Figure 1 shows the chemical structures of these molecules.
Figure 1. Chemical structures of the molecules explored in the electrolytes of this study. DMC and EC are used as electrolyte solvents, LiPF6 as lithium salt. LFO, VC, FEC, DTD, PES and SN are electrolyte additives. Other molecules explored in this study are LiOMe, DMT, TMP, Fc, FeOTf and MnOTf.
Download figure:
Standard image High-resolution imagePouch cell assembly
402035-size lithium-ion pouch cells with LFP (nominal capacity 220 mAh to 3.65 V) or NMC811 (nominal capacity 175 mAh to 4.06 V) positive electrodes and AG negative electrodes were obtained vacuum sealed without electrolyte (LiFUN Technologies, China). The pouch cells were dried under vacuum at 120 °C for 14 h, filled with 2 ml (LFP/AG) or 1.7 ml electrolyte (NMC811/AG), and vacuum sealed at −90 kPa gauge pressure. This is double the amount of electrolyte needed, so that after formation, the electrolyte can be easily extracted for further experiments. The LFP/AG cells receive more electrolyte because their active electrode area is larger than that of the NMC811/AG cells (85.0 cm2 for LFP/AG, 52.3 cm2 for NMC811/AG).
Formation and storage protocol
All cells were charged to 1.5 V and held at constant voltage for 16 h to ensure proper wetting of the electrode pores with electrolyte while avoiding dissolution of the anode current collector. The cells then completed a single C/20 formation cycle and a C/10 recharge to 50% state of charge (SOC) on a Maccor Series 4000 test system at different formation temperatures, TF = 25, 40, 55 or 70 °C. LFP/AG cells were formed between 2.5 and 3.65 V and NMC811/AG cells between 3.0 and 4.06 V. After formation, all cells were stored at 25 °C for 1 week. A minimum storage time of 1 week is necessary to ensure a uniform composition of the electrolyte inside and outside the jellyroll and to obtain reliable results in electrolyte analysis. 8,9
Ex-situ gas measurements
The volume of gas evolved in the pouch cell during formation was measured using Archimedes' principle. For this purpose, the weight of the pouch cell suspended in deionized water (18 MΩ) was measured at room temperature before and after formation using a hook attached to the bottom of a Shimadzu balance (AUW200D). By using the density of the deionized water,  the acceleration due to gravity,
the acceleration due to gravity,  and the change in weight,
and the change in weight,  the change in volume can be calculated:
the change in volume can be calculated:
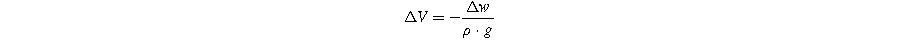
Electrolyte extraction and visual inspection
After formation and storage, the pouch cells were cut open in an Ar-filled glovebox to squeeze the electrolyte into dried polypropylene vials. The vials were capped, wrapped with parafilm, photographed, and kept in non-transparent bags in the glovebox, i.e., excluded from light and air, for later use. The electrolytes were then used for further experiments as soon as possible after their extraction date. The effect of the storage duration on the redox shuttle behavior is unknown.
Cyclic voltammetry (CV) in coin cells
CVs were recorded for electrolytes either in the pristine state or extracted from pouch cells after formation and storage. 0.1 ml electrolyte were added to 2325-size coin cells consisting of a 12.75 mm diameter aluminum foil (working-electrode), a metallic lithium foil (counter-electrode) and two pieces of Celgard 2320 as separator. Three CV cycles were measured using a VMP3 potentiostat (Bio-Logic, France) at 0.1 mV s−1 and 25 °C between 2.6 and 3.75 V vs Li+/Li. The second and third cycles are shown in the CV plots.
Gas chromatography mass spectrometry (GC-MS)
GC-MS samples were prepared by diluting 0.1 ml of electrolyte in 5 ml of organic CH2Cl2 and 1 ml of deionized water. The samples were then shaken to ensure complete extraction of LiPF6 into the aqueous layer and subsequently left for 5 min to allow the organic and aqueous layers to separate properly. A pipette was used to inject the bottom, organic layer into the GC-MS machine (Agilent 7890 gas chromatograph coupled to an Agilent 5977B single-quadrupole mass spectrometer with 70 eV ion source). The values presented in this work are reported as relative intensity with respect to the highest peak in the specific measurement, i.e., the DMC peak.
Nuclear magnetic resonance (NMR)
The NMR samples were prepared by filling 0.6 ml electrolyte into to a glass NMR tube (Wilmad 506-PP-8) and capped with a gas-tight NMR cap (Wilmad WG-3891–100) in an Ar-filled glovebox. 1H (16 scans), 19F (32 scans) and 31P (16 scans) NMR spectra were recorded on a Bruker Avance 500 spectrometer.
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR)
ATR-FTIR spectra were collected using a Cary 630 FTIR (Agilent Technologies) equipped with a germanium crystal attenuated total reflectance (ATR) accessory. Sixteen scans were collected for each background and sample measurement at a resolution of 4 cm−1 using MicroLab PC software. All measurements were performed in an Ar-filled glovebox.
Results
Linking differential capacity to SEI formation for LFP/AG and NMC811/AG pouch cells
Figure 2 shows the differential capacity as a function of voltage for the beginning of the formation cycle at formation temperature TF = 25 °C (blue lines), 40 °C (black lines), 55 °C (orange lines) and 70 °C (red lines) for LFP/AG (see Fig. 2a) and NMC811/AG (see Fig. 2b) pouch cells with eight different electrolytes. CTRL electrolyte is used in Figs. 2a-1 and 2b-1, DMC electrolyte is used in Figs. 2a-4 and 2b-4, and additive containing electrolytes are used in the other panels.
Figure 2. Differential capacity vs voltage (dQ/dV vs V) at the beginning of the formation cycle as a function of formation temperature, TF, for (a) LFP/AG and (b) NMC811/AG pouch cells with CTRL electrolyte (a-1, b-1), 1 wt% LFO (a-2, b-2), 1 wt% SN (a-3, b-3), DMC (a-4, b-4), 2 wt% VC (a-5, b-5), 2 wt% FEC (a-6, b-6), 1 wt% DTD (a-7, b-7), and 2 wt% PES (a-8, b-8). Cells were formed at C/20 from 2.5 to 3.65 V for LFP/AG and 3.0 to 4.06 V for NMC811/AG.
Download figure:
Standard image High-resolution imageFor all cell types and electrolyte formulations, the differential capacity curves shift to lower cell voltages, i.e., higher graphite potentials, the higher the formation temperature, TF. At TF = 70 °C, the onset of electrolyte reduction, indicated by a rise in the dQ/dV vs V curve, begins significantly earlier than at lower formation temperature, e.g., TF = 25 °C. For the LFP/AG CTRL cells in Fig. 2a-1 the initial dQ/dV feature can be interpreted as the reduction onset of EC, starting at ∼2.5 V for TF = 70 °C. 10 EC reduction leads to the buildup of an SEI on the negative electrode. 11 The addition of 1 wt% LFO or 1 wt% SN results in almost the exact same dQ/dV feature for all TF (compare Figs. 2a-2 and 2a-3 with 2a-1). There is no dQ/dV feature associated with these additives in the respective voltage region. This is in good agreement with literature reports that suggest LFO and SN act on the positive side rather than to build an SEI on the negative electrode. 12–15 The LFP/AG DMC cells in Fig. 2a-4 show no EC reduction peak at all, since they do not contain any EC. Since DMC alone does not form an effective SEI layer, these cells are expected to have a non-passivated negative electrode. 16,17 LFP/AG cells with VC, FEC, DTD or PES (see Figs. 2a-5 to 2a-8) consistently show dQ/dV peaks at lower cell voltages than CTRL, indicating additive reduction before EC reduction. The dQ/dV peak attributed to EC reduction is either suppressed or completely absent in these cells, indicating the formation of a primarily additive-derived SEI layer. These four additives have been described as effective SEI-formers in the literature. 17–20 At TF = 70 °C the additive reduction started at 2.4 V for 2VC (see Fig. 2a-5), 2.2 V for 2FEC (see Fig. 2a-6), 2.1 V for 1DTD (see Fig. 2a-7) and 2.05 V for 2PES (see Fig. 2a-8).
The NMC811/AG cells in Fig. 2b show the same trends as the LFP/AG cells in Fig. 2a. However, the reduction peaks occur "later," i.e., at 0.1 to 0.2 V higher cell voltages. For example, the EC reduction for NMC811/AG CTRL at TF = 70 °C starts at 2.6 V (see Fig. 2b-1), whereas the EC reduction for LFP/AG CTRL at TF = 70 °C begins at 2.5 V (see Fig. 2a-1). Generally, the reduction process starts when the potential of the negative electrode is sufficiently low to allow electron transfer to the solvent or additive molecule and is independent of the potential at the positive electrode. That means, the EC reduction starts at the same negative electrode potential (vs Li+/Li) for the LFP/AG and NMC811/AG cells in Figs. 2a-1 and 2b-1, respectively. However, Fig. 2 shows the full cell voltage, i.e., the difference between the positive and negative electrode potential. Thus, the difference in the LFP and NMC811 voltage curves can explain the slight offset in full cell reduction onset voltages. 21 Irrespective of that, the correlation between reduction onset voltage and formation temperature holds and is likely due to facilitated charge-transfer kinetics at higher temperature.
First cycle inefficiency (FCIE or 1-FCE) of LFP/AG and NMC811/AG pouch cells
Figure 3 shows the FCIE for formation at TF = 25 °C (blue bars), 40 °C (black bars), 55 °C (orange bars) and 70 °C (red bars) for the LFP/AG (see Fig. 3a) and NMC811/AG (see Fig. 3b) pouch cells presented in Fig. 2. The LFP/AG cells in Fig. 3a show the lowest FCIE for TF = 40 °C and the highest for TF = 70 °C. Typically, higher formation temperatures lead to more electrolyte reduction, a thicker SEI layer, and more lithium loss, which explains the rise in FCIE towards high TF. However, a moderate temperature of TF = 40 °C seems to improve the SEI buildup compared to TF = 25 °C and results in lower FCIE (see Fig. 3a). 22 The LFP/AG DMC cells in Fig. 3a-4 are an exception, since they have their lowest FCIE of 0.17 at TF = 25 °C and show a linear increase to 0.28 with temperature. However, already at TF = 25 °C the FCIE is higher than for all other LFP/AG cells, which indicates poor passivation of the negative electrode. This is in good agreement with the results of Fig. 2, where DMC cells showed a lack of dQ/dV features associated with SEI buildup.
Figure 3. First cycle inefficiency (FCIE or 1-FCE) as a function of formation temperature, TF, for (a) LFP/AG and (b) NMC811/AG pouch cells with CTRL electrolyte (a-1, b-1), 1 wt% LFO (a-2, b-2), 1 wt% SN (a-3, b-3), DMC (a-4, b-4), 2 wt% VC (a-5, b-5), 2 wt% FEC (a-6, b-6), 1 wt% DTD (a-7, b-7), and 2 wt% PES (a-8, b-8). Cells were formed at C/20 from 2.5 to 3.65 V for LFP/AG and 3.0 to 4.06 V for NMC811/AG.
Download figure:
Standard image High-resolution imageThe NMC811/AG cells at TF = 25 and 40 °C in Fig. 3b show FCIE values about twice as high as their LFP/AG counterparts in Fig. 3a. For TF = 55 °C the FCIE is approximately 1.5 times that of LFP/AG cells. A possible explanation is the irreversible capacity loss (ICL) of layered oxide cathode materials like NMC811 due to kinetic hindrance for full re-lithiation, especially at low temperatures, shown for example by Phattharasupakun et al. 23 This means not all Li+-ions that leave the NMC811 electrode on first charge can be re-intercalated on discharge unless the discharge rates are very slow. 24 The ICL is usually overcompensated by the lithium loss for SEI buildup, but this is not the case for the NMC811/AG cells in this study. Since the ICL would be especially high at low TF, it could explain the unusually high FCIE of ≥0.12 for NMC811/AG at TF = 25 °C (see Fig. 3b).
Gas evolution during formation of LFP/AG and NMC811/AG pouch cells
Figure 4 shows the total gas volume generated during formation of the LFP/AG (see Fig. 4a) and NMC811/AG (see Fig. 4b) pouch cells presented in Figs. 2 and 3. The LFP/AG cells with SEI-building additives like VC, FEC and PES show less gas generation (see Figs. 4a-5, 4a-6 and 4a-8) than the cells without SEI-building additives (see Figs. 4a-1 to 4a-4). Only LFP/AG 1DTD in Fig. 4a-7 is an exception and shows significant gas evolution comparable to LFP/AG CTRL in Fig. 4a-1. lFP/AG DMC cells show the highest gas evolution, which is in good agreement with the typical assumption that a poorly passivating SEI leads to strong gas evolution. 7,16
Figure 4. Total gas volume generated during formation as a function of formation temperature, TF, for (a) LFP/AG and (b) NMC811/AG pouch cells with CTRL electrolyte (a-1, b-1), 1 wt% LFO (a-2, b-2), 1 wt% SN (a-3, b-3), DMC (a-4, b-4), 2 wt% VC (a-5, b-5), 2 wt% FEC (a-6, b-6), 1 wt% DTD (a-7, b-7), and 2 wt% PES (a-8, b-8). Cells were formed at C/20 from 2.5 to 3.65 V for LFP/AG and 3.0 to 4.06 V for NMC811/AG.
Download figure:
Standard image High-resolution imageFigure 4b shows that most NMC811/AG cells have a somewhat lower gas evolution than LFP/AG cells in Fig. 4a. The gas volume of NMC811/AG DMC cells in Fig. 4b-4 is 20%–30% lower than that of LFP/AG DMC cells in Fig. 4a-4. Similar trends are observed for CTRL and 1SN. Considering that the NMC811/AG cells have a 20% lower capacity than the LFP/AG cells and use less electrolyte, lower amounts of formation gas are expected. The NMC811/AG cells with SEI-building additives VC, FEC and PES (see Figs. 4b-5, 4b-6, and 4b-8) show low formation gas, indicating good passivation of the negative electrode. 7,16 This would normally also lead to a low FCIE, further suggesting that the high FCIE observed in Figs. 3b-5, 3b-6 and 3b-8 is indeed due to the ICL of NMC811.
Visual inspection of electrolyte extracted from LFP/AG and NMC811/AG pouch cells
Figure 5 shows photographs of electrolytes after extraction from the LFP/AG (see Fig. 5a) and NMC811/AG (see Fig. 5b) pouch cells presented in Figs. 2, 3, and 4. CTRL, 1SN and DMC electrolytes from LFP/AG and NMC811/AG cells show a color gradient from transparent or yellow at TF = 25 °C to dark red/brown at TF = 70 °C (see Figs. 5a-1, 5a-3, 5a-4, 5b-1, 5b-3 and 5b-4). This is in agreement with the electrolyte color change observed by Boulanger et al., for LFP/AG and NMC811/AG cells formed at the same temperatures. 6
Figure 5. Visual inspection of electrolytes extracted from LFP/AG and NMC811/AG pouch cells after formation at different temperatures, TF. The cells contain CTRL electrolyte (a-1, b-1), 1 wt% LFO (a-2, b-2), 1 wt% SN (a-3, b-3), DMC (a-4, b-4), 2 wt% VC (a-5, b-5), 2 wt% FEC (a-6, b-6), 1 wt% DTD (a-7, b-7), and 2 wt% PES (a-8, b-8). Cells were formed at C/20 from 2.5 to 3.65 V for LFP/AG and 3.0 to 4.06 V for NMC811/AG. After formation, they were stored for 1 week at 25 °C before electrolyte extraction to allow for electrolyte equilibration.
Download figure:
Standard image High-resolution imageElectrolyte from LFP/AG and NMC811/AG cells with SEI-building additives, e.g., 2VC, 1DTD and 2PES, are transparent for all TF (see Figs. 5a-5, 5a-7, 5a-8, 5b-5, 5b-7 and 5b-8). 2FEC shows a minor pink discoloration for TF = 25 and 40 °C (see Figs. 5a-6 and 5b-6). Another exception is 1LFO which is transparent for all TF, even though LFO it is not considered to have SEI-building properties (see Figs. 5a-2 and 5b-2). In general, however, it can be stated that effective SEI-formers prevent electrolyte discoloration, confirming previous results by Boulanger et al. with 2VC. 6 Boulanger et al. proposed the discoloration is in part caused by the in situ generation of a redox shuttle molecule. 6 The trends in discoloration shown in Fig. 5 would suggest, that SEI-building additives are capable of suppressing the redox shuttle generation.
Cyclic voltammetry with Ferrocene (Fc) containing electrolytes
In the following, Al/Li coin cells will be used to probe extracted electrolytes for the presence of a redox shuttle. Details on the coin cell setup can be found in the article by Boulanger et al. 6 Before testing extracted electrolytes, however, it is important to demonstrate that the coin cell setup can be used for quantitative shuttling current measurements. Figure 6 shows CVs for pristine electrolytes consisting of EC:DMC 3:7 with 1.5 M LiPF6 and (a) 0.88 mM, (b) 4.4 mM or (c) 8.8 mM Fc. All CVs are recorded at 0.1 mV s−1 and 25 °C. Fc is a known redox shuttle molecule with a redox potential of 3.2 V vs Li+/Li. 25 As soon as the voltage is increased beyond that threshold, Fc can be oxidized to Fc+ at the Al electrode and the current increases (see Fig. 6). It is important to realize that this is not a classical three-electrode setup where oxidation and reduction of Fc happens at the working electrode and symmetrical CV peaks are obtained. 25 Instead, in our setup Fc+ can easily diffuse through the separator to the Li electrode, which is so low in chemical potential that Fc+ can be reduced back to Fc at all times. Thus, a relatively constant diffusion limited current is observed in Fig. 6 once the voltage passes the threshold of 3.2 V vs Li+/Li.
Figure 6 shows a quantitative correlation between Fc concentration and CV shuttling current. The electrolyte with 0.88 mM has a shuttling current of ∼2 μA (see Fig. 6a), whereas the ten times higher Fc concentration of 8.8 mM leads to a shuttling current of ∼20 μA (see Fig. 6c). For reference, 0.88 mM Fc corresponds to 0.016 wt% Fc in the electrolyte given Fc's molecular weight of 186.04 g mol−1. Thus, a typical redox shuttle current of 1 μA can be generated from concentrations as low as ∼0.33 mM Fc or 0.006 wt% Fc. This concentration will not be the same for an in situ generated redox shuttle molecule with different molecular weight, diffusivity and charge transfer kinetics at the interfaces, however, it is likely that the concentration of such a molecule would also be relatively small. Calculations that link shuttle concentration, diffusion time and shuttling current, as well as a schematic of the shuttling process can be found in the article by Boulanger et al. 6
Figure 6. CVs for pristine electrolytes consisting of EC:DMC 3:7 with 1.5 M LiPF6 and (a) 0.88 mM, (b) 4.4 mM or (c) 8.8 mM Fc. All CVs were recorded in Al/Li coin cells at 0.1 mV s−1 and 25 °C.
Download figure:
Standard image High-resolution imageCVs of electrolytes extracted from LFP/AG and NMC811/AG pouch cells
Figure 7 shows CVs for electrolytes extracted from LFP/AG (see Fig. 7a) and NMC811/AG (see Fig. 7b) pouch cells after formation at different TF, recorded in Al/Li coin cells at 0.1 mV s−1 and 25 °C. The measurements are done with the exact electrolytes shown in Fig. 5. As demonstrated in the previous section, this method can detect the presence of a redox shuttle in the extracted electrolytes and should also provide an idea of the relative concentration. 2,6
Figure 7. CVs for electrolytes extracted from (a) LFP/AG and (b) NMC811/AG pouch cells after formation at different temperature, TF. The cells contain CTRL electrolyte (a-1, b-1), 1 wt% LFO (a-2, b-2), 1 wt% SN (a-3, b-3), DMC (a-4, b-4), 2 wt% VC (a-5, b-5), 2 wt% FEC (a-6, b-6), 1 wt% DTD (a-7, b-7), and 2 wt% PES (a-8, b-8). Cells were formed at C/20 from 2.5 to 3.65 V for LFP/AG and 3.0 to 4.06 V for NMC811/AG. After formation, they were stored for 1 week at 25 °C before electrolyte extraction to allow for electrolyte equilibration. All CVs were recorded in Al/Li coin cells at 0.1 mV s−1 and 25 °C. In some instances, the extracted electrolyte volume was insufficient to record CVs.
Download figure:
Standard image High-resolution imageElectrolytes without SEI-building additives, e.g., CTRL, 1SN and DMC, show higher redox shuttle activity the higher the formation temperature, TF (see Figs. 7a-1, 7a-3, 7a-4, 7b-1, 7b-3 and b-4). CTRL electrolyte from NMC811/AG cells formed at TF = 70 °C shows shuttling currents up to 8 μA (see Fig. 7b-1). These currents are of similar magnitude as the shuttling currents previously found by Boulanger et al. for LFP/AG and NMC811/AG. 6 DMC electrolytes show larger shuttling currents especially at TF = 55 and 70 °C. The fact that 1LFO electrolyte does not generate significant shuttling currents is in agreement with the absence of color change. In general, all electrolytes that showed discoloration in Fig. 5 also show shutting currents in Fig. 7.
Electrolytes with SEI-building additives, e.g., 2VC, 2FEC, 1DTD and 2PES show merely capacitive currents even at high TF (see Figs. 6a-5 to 6a-8 and 6b-5 to 6b-8). Boulanger et al. found the same result for LFP/AG 2VC cells and it can now be generalized to several SEI-forming additives. 6
CVs of pristine and heated electrolytes with and without candidate shuttle molecules
Figure 8 shows CVs for pristine electrolytes consisting of EC:DMC 3:7 with 1.5 M LiPF6 (CTRL, see Fig. 8a-1) and 2 wt% VC (see Fig. 8a-2), 8.8 mM LiOMe (see Fig. 8b-1), 0.3 M LiOMe (see Fig. 8b-2), 8.8 mM FeOTf (see Fig. 8c-1) or 8.8 mM MnOTf (see Fig. 8c-2). The electrolytes were either used at 25 °C (black curves) or after 65 h at 70 °C (red curves). The electrolytes were stored and/or heated in Al bottles. All CVs were recorded in Al/Li coin cells at 0.1 mV s−1 and 25 °C. None of the electrolytes in Fig. 8 show redox shuttle behavior, instead only capacitive currents <1 μA can be seen. Storing the electrolytes for 65 h at 70 °C—the typical formation period—leads to somewhat larger capacitive currents in some cases but clearly does not yield the large shuttling currents observed previously (see Fig. 7).
Figure 8. CVs for pristine electrolytes consisting of (a-1) EC:DMC 3:7 with 1.5 M LiPF6 (CTRL) and (a-2) 2 wt% VC, (b-1) 8.8 mM LiOMe, (b-2) 0.3 M LiOMe, (c-1) 8.8 mM FeOTf or (c-2) 8.8 mM MnOTf. Electrolyte were either used at 25 °C (black curves) or after 65 h at 70 °C (red curves). The electrolytes were stored and/or heated in Al bottles. All CVs were recorded in Al/Li coin cells at 0.1 mV s−1 and 25 °C.
Download figure:
Standard image High-resolution imageThe CVs with pristine CTRL and 2VC electrolytes in Fig. 8a show no shuttling current even after exposure to 70 °C for 65 h (red curves). This shows that heating alone does not lead to redox shuttle generation and instead electrochemical reactions during the formation are needed. It is likely that charge transfer reactions between the negative electrode and electrolyte molecules as well as potential follow-up reactions are needed to generate the redox shuttle. A well passivating SEI layer can supress such reactions and DMC as a single solvent seems to be sufficient to generate the redox shuttle.
Figure 8b shows that addition of LiOMe, a known DMC reduction product, 16,26 does not cause any shuttling current. Changing the concentration or heating the electrolyte has no effect on the shuttling current (see Fig. 8b-2). It is valid to assume that other lithium alkoxides, which could be created in lithium-ion cells, would also show no shuttling behavior. 16
Figure 8c shows that addition of transition metal salts like FeOTf or MnOTf does not cause redox shuttle currents. This experiment should simulate transition metal dissolution from Fe or Mn containing positive electrode materials like LFP or NMC811. 27 The absence of shuttling current is in agreement with Buechele et al. who showed that the magnitude of self-discharge observed in LFP/AG and NMC811/AG cells, cannot be explained by transition metal dissolution. 2
At this point, the following interim summary can be given about redox shuttle generation: (i) it likely originates from the negative electrode since it is found in LFP and NMC811 cells alike; (ii) it can be supressed by effective SEI-forming additives; (iii) it is particularly strong in non-passivating DMC electrolyte; (iv) it scales with formation temperature; (v) temperature alone is not sufficient for redox shuttle generation, instead electrochemical reactions are needed; and (vi) LiOMe and transition metals can be ruled out as shuttle molecules.
The subsequent experiments shall identify the shuttle molecule by chemical analysis of the extracted electrolytes. To facilitate the shuttle identification, these experiments use only DMC electrolyte. This reduces the complexity of electrolyte analysis by GC-MS and NMR. If both, EC and DMC, are present in the electrolyte molecules like dimethyl-2,5-dioxahexane carboxylate (DMOHC) can be formed, which makes the search for the shuttle molecule more difficult. 6,28,29
Redox shuttle identification in GC-MS spectra of extracted electrolytes
Figure 9 shows the GC-MS spectra of electrolytes extracted from LFP/AG (see. Fig. 9b) and NMC811/AG pouch cells (see Fig. 9c) initially filled with DMC electrolyte after formation at TF = 40 or 70 °C. Note that the CVs of these exact electrolytes are shown in Figs. 7a-4 and 7b-4. Pictures of these electrolytes are shown in Figs. 5a-4 and 5b-4.
Figure 9. GC-MS spectra of (a) pristine DMC + 1.5 M LiPF6 electrolyte with and without 0.1 M DMT and DMC electrolyte extracted from (b) LFP/AG and (c) NMC811/AG pouch cells. Cells did formation at TF = 40 or 70 °C and were stored for 1 week at 25 °C before electrolyte extraction to allow for electrolyte equilibration.
Download figure:
Standard image High-resolution imageFigure 9b shows clear peaks for DMC at 5.5 min, TMP at 11.8 min, EC at 12.2 min and DMT at 18.2 min retention time for the electrolyte extracted from an LFP/AG cell after formation at TF = 70 °C (red line). Apart from the DMC peak these peaks cannot be found in the electrolyte extracted from an LFP/AG cell that did formation at TF = 40 °C (see Fig. 9b black dotted line). There are minor peaks between 14 and 16 min retention time for TF = 70 °C, that are also absent for TF = 40 °C.
Figure 9c shows the same peaks that were found for electrolyte from an LFP/AG cell after formation at TF = 70 °C in electrolyte extracted from an NMC811/AG cell after formation at TF = 70 °C (red line). There is one additional peak at 11.8 min, which stems from a carbonate species. Like the LFP/AG cell, also the NMC811/AG cell after formation at TF = 40 °C shows no significant peaks besides the DMC peak (see Fig. 9c black line).
For reference, Fig. 9a shows the GC-MS spectra of pristine DMC electrolyte and DMC + 0.1 M DMT. As expected, no significant peaks show up in the GC-MS spectra for the pristine DMC electrolyte, besides the DMC peak itself at 5.5 min retention time (see Fig. 9a black line). Pristine DMC electrolyte with 0.1 M DMT shows an additional DMT peak around 18.2 min retention time (see Fig. 9a green line), just like the TF = 70 °C electrolytes in Figs. 9b and 9c (red lines). This shows that the electrolytes used for this study are free of major contaminants and that the species found after formation at TF = 70 °C are generated in situ. The control injection proves that the peak at 18.2 min retention time is correctly identified at DMT.
The CVs of DMC electrolyte from cells formed at TF = 70 °C show much higher redox shuttle currents than for cells formed at TF = 40 °C (compare red and black lines in Figs. 7a-4 and 7b-4). This correlates well with the presence of TMP, EC, and DMT peaks at TF = 70 °C and their absence at TF = 40 °C. Hence, these species are possible redox shuttle candidates. 2,6 Since EC is commonly used as co-solvent in battery electrolytes, it can be ruled out as shuttle molecule. The formation of TMP has been reported before after high temperature formation. 30 A formation mechanism was proposed by Weber et al. and will be reviewed in the Discussion section. 31 TMP is used by some as a non-flammable additive in lithium-ion cells due to its high oxidation stability. 32–34 Therefore, it can also be ruled out as shuttle molecule. Lastly, the carbonate species at 11.8 min in electrolyte from the NMC811/AG cell at TF = 70 °C is highly unlikely to be the redox shuttle molecule since it is absent in LFP/AG cells. Thus, the following NMR experiments will focus on DMT as the primary shuttle candidate.
Redox shuttle identification in 1H NMR spectra of extracted electrolytes
Figure 10 shows the 1H NMR spectra of the electrolytes measured in Fig. 9. Since we were not able to extract enough electrolyte from the TF = 40 °C pouch cells for all experiments, the following NMR experiments were done with electrolytes after formation at TF = 70 and 25 °C.
Figure 10. 1H NMR spectra of (a) pristine DMC + 1.5 M LiPF6 electrolyte, (b) the same electrolyte with 0.1 M DMT and DMC electrolyte extracted from (c, d) LFP/AG and (e, f) NMC 811/AG pouch cells after formation at TF = 25 or 70 °C. All cells were stored for 1 week at 25 °C before electrolyte extraction to allow for electrolyte equilibration.
Download figure:
Standard image High-resolution imageFigure 10a shows a reference 1H NMR spectrum for pristine DMC electrolyte with no peaks besides DMC around 4.25 ppm. This shows that the electrolyte in this study is free of major contaminants. Adding 0.1 M DMT leads to two additional peaks around 4.45 and 8.6 ppm (see Fig. 10b), which correspond to the 6 protons and the 4 protons that share the same chemical environment, respectively. The integrated peak areas are in the expected ratio of 6 to 4.
Figures 10c and 10e show 1H NMR spectra for LFP/AG TF = 25 °C electrolyte and NMC811/AG TF = 25 °C electrolyte, respectively. While the former does not show DMC satellite peaks (likely due to an error in the measurement), the latter serves as a suitable reference spectrum for electrolyte after formation at moderate temperature showing the DMC peak around 4.25 ppm and only one small peak at 5.1 ppm corresponding to EC (see Fig. 10e).
Figure 10d shows a 1H NMR spectra for LFP/AG TF = 70 °C electrolyte with peaks for DMC around 4.25 ppm, TMP at 3.8 ppm, DMT at 4.45 and 8.6 ppm and EC at 5.1 ppm. Figure 10f shows the exact same peaks for NMC811/AG TF = 70 °C electrolyte. These NMR results are in agreement with the GC-MS results in Fig. 9, and unambiguously confirm the presence of TMP, EC, and DMT after formation at high temperature.
31P NMR spectra of extracted electrolytes
Figure 11 shows the 31P NMR spectra of the electrolytes measured in Figs. 9 and 10. Figure 11a and 11b show PO3F2− in pristine electrolyte around -20 ppm, which results from reaction between LiPF6 and small amounts of residual water commonly found in alkyl carbonate electrolytes. 35,36 There are no other significant peaks besides PF6 − around -145 ppm. The addition of DMT in Fig. 11b does not change the spectrum, since DMT does not contain any phosphorus.
Figure 11. 31P NMR spectra of (a) pristine DMC + 1.5 M LiPF6 electrolyte, (b) the same electrolyte with 0.1 M DMT and DMC electrolyte extracted from (c, d) LFP/AG and (e, f) NMC 811/AG pouch cells after formation at TF = 25 or 70 °C. All cells were stored for 1 week at 25 °C before electrolyte extraction to allow for electrolyte equilibration.
Download figure:
Standard image High-resolution imageFigure 11c and 11e show only PF6 − peaks in electrolytes after formation at TF = 25 °C, whereas Figs. 11d and 11f show an additional peak at 0 ppm for TMP in electrolytes after formation at TF = 70 °C. This is in agreement with the GC-MS and 1H NMR results in Figs. 9 and 10, respectively. EC and DMT do not show up in 31P NMR since they do not contain any phosphorus. Interestingly, there is no PO3F2− in electrolytes after formation. This could mean that residual water is consumed by other reactions during formation (see Discussion section). 35
19F NMR spectra of extracted electrolytes
Figure 12 shows the 19F NMR spectra of the electrolytes measured in Figs. 9, 10 and 11.
Figure 12. 19F NMR spectra of (a) pristine DMC + 1.5 M LiPF6 electrolyte, (b) the same electrolyte with 0.1 M DMT and DMC electrolyte extracted from (c, d) LFP/AG and (e, f) NMC 811/AG pouch cells after formation at TF = 25 or 70 °C. All cells were stored for 1 week at 25 °C before electrolyte extraction to allow for electrolyte equilibration.
Download figure:
Standard image High-resolution imageThe pristine electrolytes in Figs. 12a and 12b show signs of water contamination since they contain PO3F2− around -85 ppm, PO2F2 − around −90 ppm and HF at −157 ppm. 35,36 This agrees with the PO3F2− signals in Figs. 11a and 11b. No other significant peaks are seen besides PF6 − at approximately -75 ppm. Electrolytes after formation at TF = 25 °C show no water contamination (see Figs. 12c and 12e), and electrolytes after formation at TF = 70 °C show only minor PO3F2− signals (see Figs. 12d and 12f), indicating consumption of residual water during formation. 35 TMP, EC and DMT do not show up in 19F NMR since they do not contain any fluorine.
GC-MS, 1H, 19F, and 31P NMR experiments (see Figs. 9–12) leave DMT as the only potential redox shuttle molecule that could be detected in the extracted electrolytes. Most redox shuttle molecules known in literature feature an aromatic ring in their chemical structure, which makes DMT a reasonable candidate. 37–39 In the following, DMT is added to pristine electrolyte to test redox shuttle behavior in Al/Li coin cells.
Visual observations during preparation of coin cells with DMT-containing electrolyte
Figure 13 shows a coin cell can with lithium metal foil at the bottom after addition of 0.1 ml electrolyte consisting of DMC, 1.5 M LiPF6 and 0.1 M DMT. The soaked separator was placed on the lithium foil and then removed with tweezers. DMT is a white powder and after dissolving it in pristine electrolyte, the electrolyte remains completely transparent. It is only after contact with lithium metal in the coin cell can that it turns dark red. However, this color change is slow; most likely because the lithium metal surface easily passivates in carbonate electrolyte. Firmly pressing a coin cell spacer on the lithium metal foil accelerates the color change significantly, and within seconds the dark red color is observed. Due to its low chemical potential, lithium metal is highly reducing and the dark red color likely comes from DMT in its reduced form. Note that pristine electrolyte without DMT does not show this color change. Further note that many redox active compounds change color depending on their oxidation state. 40,41
Figure 13. Picture of coin cell can with lithium metal foil and electrolyte consisting of 1.5 M LiPF6 in DMC + 0.1 M DMT. The soaked separator was placed on the lithium foil and then removed with tweezers.
Download figure:
Standard image High-resolution imageThe fact that DMT changes color upon reduction, is a strong indication that it is indeed the redox shuttle molecule present in discolored electrolytes extracted from LFP/AG and NMC811/AG pouch cells (see Fig. 5). Electrolytes with a specific dark red discoloration show significantly higher shuttling currents (see Fig. 7). The electrolytes without red discoloration did not show redox shuttle behavior (see Figs. 5 and 7).
CVs of DMT-containing electrolyte
Figure 14a shows CVs of DMC electrolytes extracted from LFP/AG (see Fig. 14a-1) and NMC811/AG (see Fig. 14a-2) pouch cells after formation at TF = 70 °C. Note that these CVs were already presented in Figs. 7a-4 and 7b-4 and are now shown as reference. Figure 14b shows CVs of pristine DMC electrolytes with 0.1 M (see Figs. 14b-1) and 0.05 M DMT (see Figs. 14b-2). All electrolytes in Fig. 14 show clear shuttling currents with a maximum current around 6 μA. This highly suggests that DMT is the redox shuttle molecule.
Figure 14. (a) CVs of 1.5 M LiPF6 DMC electrolytes extracted from (a-1) LFP/AG and (a-2) NMC811/AG pouch cells after formation at TF = 70 °C. Pouch cells were stored for 1 week at 25 °C before electrolyte extraction to allow for electrolyte equilibration. (b) CVs of pristine electrolytes consisting of 1.5 M LiPF6 in DMC with (b-1) 0.1 M or (b-2) 0.05 M DMT. All CVs were recorded in Al/Li coin cells at 0.1 mV s−1 and 25 °C.
Download figure:
Standard image High-resolution imageThe concentration of 0.1 M DMT in the coin cells of Figs. 14b-1, is the same as the concentration used for the GC-MS control injection in Fig. 9a. In the GC-MS spectra, the DMT peak area for pristine DMC electrolyte with 0.1 M DMT is an order of magnitude higher than the DMT peak area of extracted electrolytes (compare Fig. 9a to Figs. 9b and 9c). This approximate order of magnitude difference is also visible in the 1H NMR spectra with and without DMT addition (compare Fig. 10b to Figs. 10d and 10f). However, the shuttling currents are almost identical for electrolyte with DMT addition and electrolyte extracted from pouch cells. Furthermore, reducing the DMT concentration to 0.05 M does not reduce the shuttling current in coin cells (see Fig. 14b-2). This suggests that both concentrations are well above the saturation concentration, where current flow is limited by other factors, e.g., the charge transfer kinetics at the electrode interfaces. Note that the selected DMT concentrations of 50 and 100 mM in Fig. 14b are significantly higher than the Fc concentrations of 0.88, 4.4 and 8.8 mM in Fig. 6, for which a clear concentration dependent shuttling current has been observed. In future work, we will probe lower DMT concentrations and determine if there is a correlation to the shuttling current.
Discussion
TMP generation in LiPF6-containing electrolytes
The GC-MS and NMR spectra in Figs. 9–11 prove the formation of TMP in LFP/AG and NMC811/AG cells with DMC electrolyte at TF = 70 °C. Figure 15 shows the proposed formation mechanism of TMP. It can form in electrolytes that contain LiPF6, a linear alkyl carbonate (here DMC) and water. As shown by Weber et al., the reaction is also possible with ethyl methyl carbonate (EMC) or diethyl carbonate (DEC). 30 Weber et al. demonstrated that TMP formation can be a purely chemical reaction in the liquid electrolyte phase at elevated temperatures of 95 °C; electrochemical reactions, e.g., charge transfer at the interfaces are not needed. 30 However, it is also possible to form TMP in lithium-ion cells charged to high voltages, even at moderate temperatures of 20 °C. 31
Figure 15. Proposed formation mechanism of TMP at high temperatures using LiPF6, H2O and DMC. Adapted and modified from Weber et al. 30,31
Download figure:
Standard image High-resolution imageThe formation mechanism starts with the thermal decomposition of LiPF6 into LiF and PF5 at elevated temperature. 42 The subsequent reaction of PF5 with small amounts of residual water in the electrolyte creates HF and POF3. 43 POF3 then undergoes a nucleophilic reaction with DMC releasing fluoromethane (CH3F), followed by a decarboxylation reaction releasing CO2. The latter two steps repeat two more times to form the final TMP product (see Fig. 15).
While the formation of TMP at the expense of LiPF6 and DMC is in principle an unwanted parasitic reaction, it should be noted that this reaction removes residual water from the electrolyte. Thermally stable salts like LiFSI that do not react with water cannot remove water from battery electrolytes in the same way. Logan et al. showed that cells with hygroscopic, high surface area LFP contain considerable amounts of residual water even after drying at 120 °C. 1 This is consistent with the fact that electrolyte from LFP/AG cells shows more TMP than electrolyte from NMC811/AG cells (see Figs. 9 and 11). Logan et al. also showed that LFP/AG cells with LiFSI operating at 40 °C have higher charge endpoint capacity slippage in UHPC and more heat flow in microcalorimetry than their LiPF6 counterparts, despite better capacity retention. 5 This could only be explained by a reversible shuttle reaction. 5 If redox shuttle generation involved residual water, it could be more severe for LiFSI cells. The role of residual water on redox shuttle generation will be discussion in the following.
Proposed formation mechanism of DMT and EC by depolymerization of PET
The GC-MS and NMR spectra in Figs. 9 and 10 prove the formation of DMT and EC in LFP/AG and NMC811/AG cells with DMC electrolyte at TF = 70 °C. Figure 16 shows a proposed mechanism that can explain the formation of both DMT and EC. The mechanism begins with the formation of methanol (CH3OH), a typical contaminant in lithium-ion battery electrolytes that either stems from the synthesis of DMC (typically, EC + methanol → DMC + ethylene glycol, followed by distillation to remove residual alcohols to a level of ∼50 ppm) or the hydrolysis of DMC with residual water (see Fig. 16a-1). 35,44 As shown by Heider et al. the hydrolysis reaction is strongly temperature activated; in their case electrolytes were stored at 70 °C for several days. 35 DMC can also react electrochemically at the non-passivated negative electrode during formation and form LiOMe upon reduction (see Fig. 16a-2). 16,26 It is important to emphasize that DMC reduction would be strongly supressed in the presence of effective SEI-forming electrolyte additives like VC, as shown by Petibon et al. and Strehle et al. 16,28 Thus, LiOMe is only formed in the absence of SEI-building additives.
Figure 16. Proposed formation mechanism of DMT and EC from polyethylene terephthalate (PET) and DMC. The reaction is catalyzed by LiOMe and involves ethylene glycol (EG) and methanol. (a-1) Methanol is formed from DMC and water; (a-2) LiOMe is formed by reduction of DMC; (b) DMT and EG are formed by the reaction of PET and methanol catalyzed by LiOMe; (c) EG reacts with DMC to form methanol and EC. Combining reactions (b) and (c) gives the overall reaction (d). The length of the arrows indicates in which direction the reaction equilibrium lies. Adapted and modified from Tanaka et al. 45
Download figure:
Standard image High-resolution imageMethanol then further reacts with polyethylene terephthalate (PET) to form DMT and ethylene glycol (EG, see Fig. 16b). Tanaka et al. showed that this reaction is efficiently catalyzed by LiOMe. 45 Starting from 100 mg PET, 1.5 ml DMC, 0.2 ml MeOH and 5 mol% LiOMe, the authors achieved a yield of 90% DMT and EC after only 3 h at 28 °C. 45 Without LiOMe the reaction does not proceed and there is no measurable yield even after 5 h at 65 °C. 45 This shows that LiOMe is an essential catalyst for the formation of the DMT redox shuttle molecule from PET. In the presence of VC, LiOMe formation is supressed, there will be no generation of DMT and accordingly also the reversible self-discharge will be minimal. The latter has been experimentally observed by Logan et al. and Buechele et al. 1,2 We will discuss the origin of PET in these cells at the end of this section.
The GC-MS and NMR spectra in Figs. 9 and 10 do not show any methanol or EG. Since methanol is consumed in the depolymerization of PET (see Fig. 16b), its absence is easily explained. As shown by Tanaka et al. EG is also consumed since it reacts with DMC to form EC and methanol (see Fig. 16c). 45 The reverse reaction is supressed due to the stable ring structure of EC. Note that the as-formed methanol is consumed again by PET depolymerization (see Fig. 16b), which also shifts the reaction equilibrium towards the EC side (see Fig. 16b). 45
Figure 16d shows the proposed net reaction that forms EC and the redox shuttle molecule DMT in our lithium-ion cells at elevated formation temperature. Note that high temperature is needed because the hydrolysis of DMC is temperature activated (see Fig. 16a-1), whereas charge transfer reactions during formation are needed to generate LiOMe (see Fig. 16a-2).
Water is a necessary starting reactant for the mechanism presented in Fig. 16. Cells with more water would likely generate more methanol and thus also more DMT shuttle molecule. Buechele et al. found that LFP/AG cells, which are notorious for containing relatively large amounts of residual water, 1 show more reversible self-discharge than NMC811/AG cells when the cells do not contain electrolyte additives. 2 This is consistent with the proposed mechanism for redox shuttle generation.
Source of PET in the lithium-ion cells used in this study
A remaining question is where PET comes from in our lithium-ion cells. Figure 17a shows ATR-FTIR spectra of inactive cell components extracted from the battery cells used in this study as well as a reference spectrum for pure PET film. Figure 17b shows photographs of the cathode of an unwound jelly roll (left) with green tapes, commonly used to secure the ends of the jelly roll after winding. Figure 17b also shows the separator (middle) extracted from the cell and pieces of the pouch bag (right) into which the jelly roll was placed. The pouch bag is a multiplayer foil that contains several polymer layers (matt outside) and an aluminum layer that blocks gas diffusion (shiny inside). 46 Comparing the ATR-FTIR spectra of these cell components to the PET reference spectrum (green line in Fig. 17a), it becomes obvious that the outside of the pouch bag, as well as both tapes show the characteristic absorption bands of PET at 720, 1080, 1250, and 1720 cm−1 wavenumbers. While the outside of the pouch bag is not in contact with electrolyte, the two tapes are in direct contact with the electrolyte. Hence, the tapes are the source of PET in our lithium-ion cells, which undergoes depolymerization in the presence of methanol and catalytic amounts of lithium methoxide to form the DMT redox shuttle.
Figure 17. (a) ATR-FTIR spectra of (b) inactive cell components extracted from the cells used in this study. The green spectrum is reference data for a pure PET film.
Download figure:
Standard image High-resolution imageIn other battery cells, PET may be contained in other cell components, e.g., the separators. 47–51 Future work will identify the exact reaction conditions and reactant concentrations needed for DMT generation. The performance of PET-containing cells will be compared to PET-free cells with respect to self-discharge during storage and inefficiencies during ultra-high precision coulometry.
Conclusions
This study showed the in situ generation of a redox shuttle molecule in LFP/AG and NMC811/AG pouch cells after formation at elevated temperature with common alkyl carbonate electrolyte. Several electrolyte additives were investigated for their ability to supress the shuttle generation. The effectiveness of the additives to form a passivating SEI layer on the negative electrode was characterized by the additive reduction onset potential, first cycle inefficiency and gas evolution during formation at temperatures between 25 and 70 °C. After formation, electrolyte was extracted from pouch cells for visual inspection and quantification of redox shuttle activity in coin cells by cyclic voltammetry. It was found that electrolytes show dark red discoloration in the absence of effective SEI-forming additives and that these electrolytes show the highest shuttling currents. The redox shuttle molecule was identified as DMT by GC-MS and NMR. Addition of DMT to pristine electrolyte generates intense, dark red discoloration when DMT is present in its reduced form and shuttling currents of similar magnitude as in extracted electrolytes. According to the proposed formation mechanism, DMT stems from the LiOMe catalyzed reaction of methanol with PET. The former can be formed by hydrolysis of DMC with residual water, the latter is found in the tape used to secure the ends of the jelly roll after winding.
Acknowledgments
This work was funded under the auspices of the NSERC/Tesla Canada Alliance Grant program. ERL and AE thank NSERC and The Nova Scotia Graduate Scholarship program for scholarship support.


















