Abstract
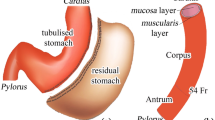
Obesity is one of the main health concerns worldwide. Bariatric Surgery (BS) is the gold standard treatment for severe obesity. Nevertheless, unsatisfactory weight loss and complications can occur. The efficacy of BS is mainly defined on experiential bases; therefore, a more rational approach is required. The here reported activities aim to show the strength of experimental and computational biomechanics in evaluating stomach functionality depending on bariatric procedure. The experimental activities consisted in insufflation tests on samples of swine stomach to assess the pressure-volume behaviour both in pre- and post-surgical configurations. The investigation pertained to two main bariatric procedures: adjustable gastric banding (AGB) and laparoscopic sleeve gastrectomy (LSG). Subsequently, a computational model of the stomach was exploited to validate and to integrate results from experimental activities, as well as to broad the investigation to a wider scenario of surgical procedures and techniques. Furthermore, the computational approach allowed analysing stress and strain fields within stomach tissues because of food ingestion. Such fields elicit mechanical stimulation of gastric receptors, contributing to release satiety signals. Pressure-volume curves assessed stomach capacity and stiffness according to the surgical procedure. Both AGB and LSG proved to reduce stomach capacity and to increase stiffness, with markedly greater effect for LSG. At an internal pressure of 5 kPa, outcomes showed that in pre-surgical configuration the inflated volume was about 1000 mL, after AGB the inflated volume was slightly lower, while after LSG it fell significantly, reaching 100 mL. Computational modelling techniques showed the influence of bariatric intervention on mechanical stimulation of gastric receptors due to food ingestion. AGB markedly enhanced the mechanical stimulation within the fundus region, while LSG significantly reduced stress and strain intensities. Further computational investigations revealed the potentialities of hybrid endoscopic procedures to induce both reduction of stomach capacity and enhancement of gastric receptors mechanical stimulation. In conclusion, biomechanics proved to be useful for the investigation of BS effects. Future exploitations of the biomechanical methods may largely improve BS reliability, efficacy and penetration rate.








Similar content being viewed by others
References
ABAQUS. ABAQUS Documentation. 2018
Abbas, M., and L. Khaitan. Primary endoluminal bariatric procedures. Tech. Gastrointest. Endosc. 20:194–200, 2018.
Angrisani, L., A. Santonicola, P. Iovino, G. Formisano, H. Buchwald, and N. Scopinaro. Bariatric Surgery Worldwide 2013. Obes. Surg. 25:1822–1832, 2015.
Angrisani, L., A. Santonicola, P. Iovino, A. Vitiello, K. Higa, J. Himpens, H. Buchwald, and N. Scopinaro. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes. Surg. 28:3783–3794, 2018.
Angrisani, L., A. Santonicola, P. Iovino, A. Vitiello, N. Zundel, H. Buchwald, and N. Scopinaro. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes. Surg. 27:1–11, 2017.
Arterburn, D. E., and A. P. Courcoulas. Bariatric surgery for obesity and metabolic conditions in adults. BMJ 349:1–11, 2014.
Berthoud, H., and T. L. Powley. Vagal Merent Innervation of the Rat Fundic Stomach : Morphological Characterization of the Gastric Tension Receptor. J Comp Neural 19:276, 1992.
Berthoud, H. R., P. a Lynn, and L. a Blackshaw. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280:R1371–R1381, 2001.
Buchwald, H., and S. E. Williams. Bariatric surgery worldwide 2003. Obes. Surg. 14:1157–1164, 2004.
Carniel, E. L., A. Frigo, C. G. Fontanella, G. M. De Benedictis, A. Rubini, L. Barp, G. Pluchino, B. Sabbadini, and L. Polese. A biomechanical approach to the analysis of methods and procedures of bariatric surgery. J. Biomech. 56:32–41, 2017.
Carniel, E. L., A. Rubini, A. Frigo, and A. N. Natali. Analysis of the biomechanical behaviour of gastrointestinal regions adopting an experimental and computational approach. Comput. Methods Programs Biomed. 113:338–345, 2014.
Concors, S. J., B. L. Ecker, R. Maduka, A. Furukawa, S. E. Raper, D. D. Dempsey, N. N. Williams, and K. R. Dumon. Complications and Surveillance After Bariatric Surgery. Curr. Treat. Options Neurol. 18:1–12, 2016.
Dobbs, R., C. Sawers, F. Thompson, J. Manyika, J. Woetzel, P. Child, S. McKenna, and A. Spatharou. Overcoming obesity: an initial economic analysis. Discussion paper. McKinsey Glob. Inst. 1–71, 2014.
Fayad, L., A. Adam, M. Schweitzer, L. J. Cheskin, T. Ajayi, M. Dunlap, D. S. Badurdeen, C. Hill, N. Paranji, S. Alezari, A. N. Kalloo, M. A. Khashab, and V. Kumbhari. Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: a case-matched study. Gastrointest. Endosc. 89:782–788, 2018.
Foletto, M., and R. Rosenthal. The Globesity Challenge to General Surgery: A Guide to Strategy and Techniques. Berlin: Springer, 2014.
Fontanella, C. G., C. Salmaso, I. Toniolo, N. de Cesare, A. Rubini, G. M. De Benedictis, and E. L. Carniel. Computational Models for the Mechanical Investigation of Stomach Tissues and Structure. Ann. Biomed. Eng. 47:1237–1249, 2019.
Fox, E. A., R. J. Phillips, F. A. Martinson, E. A. Baronowsky, and T. L. Powley. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: Morphology and topography. J. Comp. Neurol. 428:558–576, 2000.
Frattini, F., G. Borroni, M. Lavazza, X. Liu, H. Y. Kim, R. Liu, A. Anuwong, S. Rausei, and G. Dionigi. New endoscopic procedures for diabetes mellitus type 2 and obesity treatment. Gland Surg. 5:458–464, 2016.
Fung, Y. C. Biomechanics - Mechanical properties of living tissues. Berlin: Springer Science + Business Media, 1993.
Gloy, V. L., M. Briel, D. L. Bhatt, S. R. Kashyap, P. R. Schauer, G. Mingrone, H. C. Bucher, and A. J. Nordmann. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 347:f5934–f5934, 2013.
Guide, A. P. Obesity. Cham: Bariatric and Metabolic Surgery. Springer, 2016.
Harrold, J. A., T. M. Dovey, J. E. Blundell, and J. C. G. Halford. CNS regulation of appetite. Neuropharmacology 63:3–17, 2012.
Hayes, K., and G. Eid. Laparoscopic Sleeve Gastrectomy: Surgical Technique and Perioperative Care. Surg. Clin. North Am. 96:763–771, 2016.
Holtmann, G., J. Gschossmann, J. Neufang-Hüber, G. Gerken, and N. J. Talley. Differences in gastric mechanosensory function after repeated ramp distensions in non-consulters with dyspepsia and healthy controls. Gut 47:332–336, 2000.
Holtmann, G., and N. J. Talley. The stomach-brain axis. Best Pract. Res. Clin. Gastroenterol. 28:967–979, 2014.
Kararli, T. Review article comparison. Biopharm. Drug Dispos. 16:351–380, 1995.
Kararli, T. T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 16:351–380, 1995.
Kueper, M. A., K. M. Kramer, A. Kirschniak, A. Königsrainer, R. Pointner, and F. A. Granderath. Laparoscopic sleeve gastrectomy: Standardized technique of a potential stand-alone bariatric procedure in morbidly obese patients. World J. Surg. 32:1462–1465, 2008.
Kurian, M., M. Kroh, B. Chand, D. Mikami, K. Reavis, and L. Khaitan. SAGES review of endoscopic and minimally invasive bariatric interventions: a review of endoscopic and non-surgical bariatric interventions. Surg. Endosc. 32:4063–4067, 2018.
Lee, W. J., and Y. H. Lin. Single-Anastomosis Gastric Bypass (SAGB): Appraisal of Clinical Evidence. Obes. Surg. 24:1749–1756, 2014.
Mathus-Vliegen, E. M. H., and J. Dargent. Current endoscopic/laparoscopic bariatric procedures. In: Bariatric Therapy. Cham: Springer, 2018, pp. 85–176.
McGrice, M., and K. Don-Paul. Interventions to improve long-term weight loss in patients following bariatric surgery: challenges and solutions. Diabetes Metab. Syndr. Obes. Targets Ther. 8:263–274, 2015.
Moran, T. H. Neural and hormonal controls of food intake and satiety. In: Physiology of the gastrointestinal tract, edited by K. Barrett, F. K. Ghishan, J. L. Merchant, H. M. Said, J. W. Wood, and L. R. Joh. Amsterdam: Elsevier, 2006, pp. 877–894.
Natali, A. N., E. L. Carniel, A. Frigo, P. G. Pavan, S. Todros, P. Pachera, C. G. Fontanella, A. Rubini, L. Cavicchioli, Y. Avital, and G. M. De Benedictis. Experimental investigation of the biomechanics of urethral tissues and structures. Exp. Physiol. 101:641–656, 2016.
Neff, K. J., T. Olbers, and C. W. LeRoux. Bariatric surgery: the challenges with candidate selection, individualizing treatment and clinical outcomes. BMC Med. 11:8, 2013.
Nguyen, N. T., and J. E. Varela. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat. Rev. Gastroenterol. Hepatol. 14:160–169, 2017.
Omalu, B. I., D. G. Ives, A. M. Buhari, J. L. Lindner, P. R. Schauer, C. H. Wecht, and L. H. Kuller. Obesity, mortality, and bariatric surgery death rates. JAMA 298:2406–2408, 2007.
Pories, W. J. Bariatric surgery: risks and rewards. J. Clin. Endocrinol. Metab. 93:s89–s96, 2008.
Powley, T. L., J. M. Gilbert, E. A. Baronowsky, C. N. Billingsley, F. N. Martin, and R. J. Phillips. Vagal sensory innervation of the gastric sling muscle and antral wall: Implications for gastro-esophageal reflux disease? Neurogastroenterol. Motil. 24:526–537, 2012.
Powley, T. L., C. N. Hudson, J. L. Mcadams, E. A. Baronowsky, and R. J. Phillips. Vagal intramuscular arrays: the specialized mechanoreceptor arbors that innervate the smooth muscle layers of the stomach examined in the rat. J. Comp. Neurol. 524:713–737, 2016.
Pucci, A., and R. L. Batterham. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. J. Endocrinol. Invest. 42:117–128, 2019.
Puzziferri, N., T. B. Roshek, H. G. Mayo, R. Gallagher, S. H. Belle, and E. H. Livingston. Long-term follow-up after bariatric surgery: a systematic review. JAMA 312:934–942, 2014.
Schroeder, R., T. D. Harrison, and S. L. McGraw. Treatment of adult obesity with bariatric surgery. Am. Fam. Physician 93:31–37, 2016.
Swindle, M. M., A. Makin, A. J. Herron, F. J. Clubb, and K. S. Frazier. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 49:344–356, 2012.
The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377:13–27, 2017.
Tremmel, M., U. G. Gerdtham, P. M. Nilsson, and S. Saha. Economic burden of obesity: a systematic literature review. Int. J. Environ. Res. Public Health 14:1–18, 2017.
Wang, F. B., and T. L. Powley. Topographic inventories of vagal afferents in gastrointestinal muscle. J. Comp. Neurol. 421:302–324, 2000.
Wang, G. J., D. Tomasi, W. Backus, R. Wang, F. Telang, A. Geliebter, J. Korner, A. Bauman, J. S. Fowler, P. K. Thanos, and N. D. Volkow. Gastric distention activates satiety circuitry in the human brain. Neuroimage 39:1824–1831, 2008.
Welbourn, R., M. Hollyman, R. Kinsman, J. Dixon, R. Liem, J. Ottosson, A. Ramos, V. Våge, S. Al-Sabah, W. Brown, R. Cohen, P. Walton, and J. Himpens. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO global registry report 2018. Obes. Surg. 29:782–795, 2019.
Woods, S. C. Gastrointestinal satiety signals I: an overview of gastrointestinal signals that influence food intake. Am. J. Physiol. Gastrointest. Liver Physiol. 286:7–13, 2004.
World Health Organization. Obesity and overweight. Geneva: WHO, 2018.
Zagorodnyuk, V. P., and S. J. Brookes. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J. Neurosci. 20:6249–6255, 2000.
Zagorodnyuk, V. P., B. N. Chen, and S. J. H. Brookes. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol 534:255–268, 2001.
Zhao, J., D. Liao, P. Chen, P. Kunwald, and H. Gregersen. Stomach stress and strain depend on location, direction and the layered structure. J. Biomech. 41:3441–3447, 2008.
Ziegler, A., L. Gonzalez, and A. Blikslager. Large animal models: the key to translational discovery in digestive disease research. CMGH 2:716–724, 2016.
Disclosure
Claudia Salmaso, Ilaria Toniolo, Pietro Da Roit, Chiara Giulia Fontanella, Alice Albanese, Luca Polese, Cesare Stefanini, Mirto Foletto and Emanuele Luigi Carniel have no conflicts of interest.
Funding
This study has been supported by University of Padova, BIRD 2018, Project No. BIRD183013, titled SMARTBAR: SMART Tools for the effectiveness assessment and the optimization of BARiatric surgery.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Umberto Morbiducci oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
An anisotropic visco-hyperelastic formulation was exploited to characterize the mechanical behavior of stomach tissues, as fully reported by Fontanella et al.16 The model was based on the following relationship between the first Piola-Kirchhoff stress tensor P, the right Cauchy-Green strain tensor (c) and viscous variables qi:
where \( W^{0} \) is an hyperelastic potential that specifies the instantaneous response of the tissue, while F is the deformation gradient. The evolution of viscous variables qi was specified by standard differential equations:
relaxation time \( \tau^{i} \) evaluates the time the ith viscous process requires to develop. Relative stiffness parameter \( \gamma^{i} \) specifies the contribution of the ith viscous process to the stress drop the material undergoes because of relaxation phenomena.
The fiber-reinforced configuration of both connective stratum and muscularis externa suggested to define the strain energy function by means of contributions from an isotropic ground matrix, as \( W_{m}^{0} \), and from fibers, as \( W_{f}^{0} \):
where \( I_{1} \) and \( I_{3} \) are the first and the third invariants of the right Cauchy-Green strain tensor, \( {\mathbf{a}}_{0} \) and \( {\mathbf{b}}_{0} \) define the orientation of collagen (within the connective stratum) or muscular (within the muscularis externa) fibers, while \( I_{4} \) and \( I_{6} \) are structural invariants that specify the square of tissue stretch along directions \( {\mathbf{a}}_{0} \) and \( {\mathbf{b}}_{0} \), respectively. The term p is a Lagrange multiplier that ensures the incompressibility constraint. Constitutive parameter \( C_{1} \) specifies the tissue initial shear stiffness, while parameter \( \alpha_{1} \) regulates the non-linearity of the shear response. Parameters \( C_{4} \) and \( C_{6} \) are constants that define the fibers initial stiffness, while \( \alpha_{4} \) and \( \alpha_{6} \) depend on fibers stiffening with stretch.
Appendix 2
Figure 9 shows rough experimental points that were obtained from the experimentations on the ten swine stomachs (six AGB and four LSG). Each curve was composed of the data at the relaxation points (the pressure values at the end of the rest period of 600 s). Although samples were taken from similar animals, morphometry and stiffness were necessarily slightly different, leading to a high rate of scatter, which is typical in biological tissues and structures mechanics.
Rights and permissions
About this article
Cite this article
Salmaso, C., Toniolo, I., Fontanella, C.G. et al. Computational Tools for the Reliability Assessment and the Engineering Design of Procedures and Devices in Bariatric Surgery. Ann Biomed Eng 48, 2466–2483 (2020). https://doi.org/10.1007/s10439-020-02542-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02542-9