Abstract
Background and Objectives
Cotadutide is a balanced dual glucagon-like peptide-1/glucagon receptor agonist under development for the treatment of nonalcoholic steatohepatitis and chronic kidney disease with type 2 diabetes. The objectives of the analysis were to characterize the population pharmacokinetics of cotadutide following daily subcutaneous injection in subjects with type 2 diabetes and to evaluate the effect of demographic and clinical variables of interest on cotadutide pharmacokinetics.
Methods
This study analyzed 8834 plasma concentrations of cotadutide from 759 subjects with type 2 diabetes who received daily subcutaneous doses from 20 to 600 μg from six clinical studies. The impact of covariates on cotadutide pharmacokinetics was quantified, and body weight effect on cotadutide exposure was further evaluated using a simulation approach. The model performance was evaluated through prediction-corrected visual predictive checks.
Results
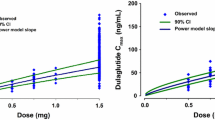
A one-compartment model with first-order absorption and elimination described cotadutide pharmacokinetic data well. The mean values for cotadutide apparent clearance, apparent distribution volume, absorption rate constant, and half-life were 1.04 L/h (interindividual variability [IIV]: 26.5%), 18.7 L (IIV: 28.7%), 0.343 h−1 (IIV: 38.6%), and 12.9 h, respectively. Higher body weight, lower albumin, and higher alanine aminotransferase were associated with an increase in cotadutide clearance, while an increase in anti-drug antibody titers was associated with a decrease in cotadutide clearance. These statistically significant effects were not considered clinically significant and did not warrant dose adjustment. Effects of other tested baseline covariates (age, sex, body mass index, hemoglobin A1c, renal function, duration of diabetes) were not found to statistically significantly affect cotadutide pharmacokinetics.
Conclusions
Cotadutide pharmacokinetics was adequately described by a one-compartment linear model with first-order absorption and elimination. Body weight-based dosing is not necessary for cotadutide based on the simulation using the final population pharmacokinetic modeling. This model will be used to evaluate exposure–response relationships for efficacy and safety in different indications that are being studied for cotadutide.








Similar content being viewed by others
References
Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269–73. https://doi.org/10.5001/omj.2012.68.
Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean MEJ, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36:843–54. https://doi.org/10.1038/ijo.2011.158.
Lynch AM, Pathak N, Pathak V, O’Harte FP, Flatt PR, Irwin N, et al. A novel DPP IV-resistant C-terminally extended glucagon analogue exhibits weight-lowering and diabetes-protective effects in high-fat-fed mice mediated through glucagon and GLP-1 receptor activation. Diabetologia. 2014;57(9):1927–36. https://doi.org/10.1007/s00125-014-3296-7.
Habegger KM, Stemmer K, Cheng C, Müller TD, Heppner KM, Ottaway N, et al. Fibroblast growth factor 21 mediates specific glucagon actions. Diabetes. 2013;62(5):1453–63. https://doi.org/10.2337/db12-1116.
Wynne K, Park A, Small C, Meeran K, Ghatei M, Frost G, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes. 2006;30(12):1729–36. https://doi.org/10.1038/sj.ijo.0803344.
Cegla J, Troke RC, Jones B, Tharakan G, Kenkre J, McCullough KA, et al. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63(11):3711–20. https://doi.org/10.2337/db14-0242.
Schjoldager BT, Baldissera FG, Mortensen PE, Holst JJ, Christiansen J. Oxyntomodulin: a potential hormone from the distal gut: pharmacokinetics and effects on gastric acid and insulin secretion in man. Eur J Clin Investig. 1988;18(5):499–503. https://doi.org/10.1111/j.1365-2362.1988.tb01046.x.
Pocai A. Action and therapeutic potential of oxyntomodulin. Mol Metab. 2014;3(3):241–51. https://doi.org/10.1016/j.molmet.2013.12.001.
Henderson SJ, Konkar A, Hornigold DC, Trevaskis JL, Jackson R, Fritsch Fredin M, et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diab Obes Metab. 2016;18:1176–90. https://doi.org/10.1111/dom.12735.
Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–9. https://doi.org/10.1021/jm9909645.
Ambery PD, Klammt S, Posch MG, Petrone M, Pu W, Rondinone C, et al. MEDI0382, a GLP-1/glucagon receptor dual agonist, meets safety and tolerability endpoints in a single-dose, healthy-subject, randomized, phase 1 study. Br J Clin Pharmacol. 2018;84:2325–35. https://doi.org/10.1111/bcp.13688.
Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391(10140):2607–18. https://doi.org/10.1016/S0140-6736(18)30726-8.
Parker VER, Robertson D, Wang T, Hornigold DC, Petrone M, Cooper AT, et al. Efficacy, safety, and mechanistic insights of cotadutide, a dual receptor glucagon-like peptide-1 and glucagon agonist. J Clin Endocrinol Metab. 2020;105(3):803–20. https://doi.org/10.1210/clinem/dgz047.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria 2014. http://www.R-project.org/. Accessed 19 Mar 2021.
Bauer RJ. NONMEM tutorial part II: estimation methods and advanced examples. CPT Pharmacomet Syst Pharmacol. 2019;8(8):538–56. https://doi.org/10.1002/psp4.12422.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
Jacobsen LV, Flint A, Olsen AK, Ingwersen SH. Liraglutide in type 2 diabetes mellitus: clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2016;55(6):657–72. https://doi.org/10.1007/s40262-015-0343-6.
Carlsson Petri KC, Ingwersen SH, Flint A, Zacho J, Overgaard RV. Semaglutide s.c. once-weekly in type 2 diabetes: a population pharmacokinetic analysis. Diabetes Ther. 2018;9(4):1533–47. https://doi.org/10.1007/s13300-018-0458-5.
Rini C, Roberts BC, Morel D, Klug R, Selvage B, Pettis RJ. Evaluating the impact of human factors and pen needle design on insulin pen injection. J Diabetes Sci Technol. 2019;13(3):533–45. https://doi.org/10.1177/1932296819836987.
Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18(4):317–32. https://doi.org/10.1111/dom.12596.
Endocrinologic and Metabolic Drug Advisory Committee. Semaglutide s.c. OW NDA 209637 briefing document. Treatment to improve glycemic control in adults with type 2 diabetes mellitus. 2017. https://www.fda.gov/media/108311/download. Accessed 19 Mar 2021.
Fineman MS, Mace KF, Diamant M, Darsow T, Cirincione BB, Booker Porter TK, et al. Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes Metab. 2012;14(6):546–54. https://doi.org/10.1111/j.1463-1326.2012.01561.x.
European Medicines Agency. Assessment report of Lyxumia. 2012. https://www.ema.europa.eu/en/documents/assessment-report/lyxumia-epar-public-assessment-report_en.pdf. Accessed 19 Mar 2021.
US FDA Center for Drug Evaluation and Research. Application number 022200Orig1s000. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022200Orig1s000ClinPharmR.pdf. Accessed 19 Mar 2021.
US FDA Center for Drug Evaluation and Research, application number 208471Orig1s000. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208471Orig1s000OtherR.pdf. Accessed 19 Mar 2021.
Baekdal TA, Thomsen M, Kupčová V, Hansen CW, Anderson TW. Pharmacokinetics, safety, and tolerability of oral semaglutide in subjects with hepatic impairment. J Clin Pharmacol. 2018;58(10):1314–23. https://doi.org/10.1002/jcph.1131.
Jensen L, Kupcova V, Arold G, Pettersson J, Hjerpsted JB. Pharmacokinetics and tolerability of semaglutide in people with hepatic impairment. Diabetes Obes Metab. 2018;20(4):998–1005. https://doi.org/10.1111/dom.13186.
Flint A, Nazzal K, Jagielski P, Hindsberger C, Zdravkovic M. Influence of hepatic impairment on pharmacokinetics of the human GLP-1 analogue, liraglutide. Br J Clin Pharmacol. 2010;70(6):807–14. https://doi.org/10.1111/j.1365-2125.2010.03762.x.
Scheen AJ. Pharmacokinetics in patients with chronic liver disease and hepatic safety of incretin-based therapies for the management of type 2 diabetes mellitus. Clin Pharmacokinet. 2014;53(9):773–85. https://doi.org/10.1007/s40262-014-0157-y.
Casteele NV, Mould DR, Coarse J, Hasan I, Gils A, Feagan B, Sandborn WJ. Accounting for pharmacokinetic variability of certolizumab pegol in patients with Crohn’s disease. Clin Pharmacokinet. 2017;56(12):1513–23. https://doi.org/10.1007/s40262-017-0535-3.
Xu C, Su Y, Paccaly A, Kanamaluru V. Population pharmacokinetics of sarilumab in patients with rheumatoid arthritis. Clin Pharmacokinet. 2019;58(11):1455–67. https://doi.org/10.1007/s40262-019-00765-1.
Acknowledgments
This study was funded by AstraZeneca. The authors thank Lars Hansen, M.D., Ph.D., (Early Clinical Development, AstraZeneca) for his review and valued medical field support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by AstraZeneca.
Conflict of interest
Rosalinda H. Arends is an employee of AstraZeneca and owns stock in AstraZeneca. Ye Guan, Neang Ly, and Jing Li were employees of AstraZeneca. Neang Ly and Jing Li owned stock in AstraZeneca at the time the study was conducted.
Ethics approval
All studies were conducted in accordance with the principles in the Declaration of Helsinki. Clinical protocols were reviewed and approved for each site by institutional review boards.
Consent to participate
All subjects enrolled provided written informed consent prior to participation in the studies.
Consent for publication
Not applicable.
Code availability
Not applicable.
Availability of data and material
No data have been shared.
Authors’ contributions
YG and JL wrote the manuscript. YG, NL, and JL designed and conducted the pharmacometric analysis. YG, NL, JL, and RA contributed to data acquisition and interpretation. All authors revised the manuscript and accepted its form for submission. This work focuses on population pharmacokinetic modeling using pooled data from six clinical trials, which involves multiple principal investigators across different countries. As the principal investigators of the clinical trials did not participate in the modeling analysis work, they are not included as authors on this paper. However, Lars Hansen, MD, PhD, (Early Clinical Development, AstraZeneca) oversees the clinical aspects for the cotadutide program including the current analysis.
Additional information
Ye Guan, Neang Ly, Jing Li were former employees of AstraZeneca.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guan, Y., Ly, N., Li, J. et al. Population Pharmacokinetics of Cotadutide in Subjects with Type 2 Diabetes. Clin Pharmacokinet 61, 833–845 (2022). https://doi.org/10.1007/s40262-021-01094-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-021-01094-y