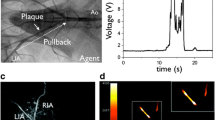
Abstract
Purpose of Review
To describe vulnerable plaque pathobiology and summarize potential targets for molecular imaging with a focus on intravascular near-infrared fluorescence (NIRF) and its translatable applications.
Recent Findings
Structural imaging alone is unable to precisely identify high-risk plaques in patients with coronary artery disease (CAD). Intravascular near-infrared fluorescence (NIRF) imaging is an emerging translational approach that can image specific in vivo molecular processes and cells that characterize vulnerable plaques. High-priority NIRF targets imaged by intravascular NIRF imaging thus far include macrophages, cathepsin protease activity, oxidized low-density lipoprotein (oxLDL), and abnormal endothelial permeability. The newest generation of NIRF catheters is multimodal in nature and combines NIRF with either IVUS or OCT, providing simultaneous co-registered morphological and pathobiological assessment of atherosclerotic plaques. While most intravascular NIRF studies have been performed in a preclinical environment, a first-in-human NIR autofluorescence-OCT trial has recently been performed. These developments suggest that clinical intravascular NIRF molecular imaging will be available within the next 3 years.
Summary
Molecular imaging is a powerful approach to enhance our understanding of atherosclerosis pathophysiology. Intravascular NIRF/OCT and NIRF/IVUS molecular imaging is nearing its use in atherosclerosis patients and will initially leverage indocyanine green (ICG) as an FDA-approved NIRF agent that reports on abnormal plaque permeability. Clinical trials are needed to assess the value of intravascular NIRF imaging using ICG as well as other novel NIRF imaging agents to better understand vulnerable plaque pathobiology, event prediction, and to enable personalized pharmacotherapy of high-risk plaques and patients.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 1 Apr 2019.
Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015;107:321–30.
Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–66.
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35.
Stone PH, Saito S, Takahashi S, Makita Y, Nakamura S, Kawasaki T, et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study. Circulation. 2012;126:172–81.
Xie Y, Mintz GS, Yang J, Doi H, Iñiguez A, Dangas GD, et al. Clinical outcome of nonculprit plaque ruptures in patients with acute coronary syndrome in the PROSPECT study. JACC Cardiovasc Imaging. 2014;7:397–405.
Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43.
Ridker PM, Everett BM, Thuren T, MacFadyen J, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74.
Chowdhury MM, Tawakol A, Jaffer FA. Molecular imaging of atherosclerosis: a clinical focus. Curr Cardiovasc Imaging Rep. 2017;10:1–11. https://doi.org/10.1007/s12410-017-9397-1.
Bourantas CV, Jaffer FA, Gijsen FJ, van Soest G, Madden SP, Courtney BK, et al. Hybrid intravascular imaging: recent advances, technical considerations, and current applications in the study of plaque pathophysiology. Eur Heart J. 2017;38:400–12.
Jaffer FA, Weissleder R. Molecular imaging in the clinical arena. JAMA. 2005;293:855–62.
Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging. 2010;3:388–97.
Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–13.
Bucerius J, Dijkgraaf I, Mottaghy FM, Schurgers LJ. Target identification for the diagnosis and intervention of vulnerable atherosclerotic plaques beyond 18F-fluorodeoxyglucose positron emission tomography imaging: promising tracers on the horizon. Eur J Nucl Med Mol Imaging. 2019;46:251–65.
Moghbel M, Al-Zaghal A, Werner TJ, Constantinescu CM, Høilund-Carlsen PF, Alavi A. The role of PET in evaluating atherosclerosis: a critical review. Semin Nucl Med. 2018;48:488–97.
Calfon MA, Rosenthal A, Mallas G, Mauskapf A, Nudelman RN, Ntziachristos V, et al. In vivo near infrared fluorescence (NIRF) intravascular molecular imaging of inflammatory plaque, a multimodal approach to imaging of atherosclerosis. J Vis Exp. 2011. https://doi.org/10.3791/2257.
• Yoo H, Kim JW, Shishkov M, et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat Med. 2011;17:1680–4 This study validated the first use of a hybrid OCT-NIRF microstructural-molecular catheter system to visualize fibrin in cadaveric human coronary arteries with implanted stents and in vivo plaque inflammation in rabbit iliac artery.
• Abran M, Stähli BE, Merlet N, Mihalache-Avram T, Mecteau M, Rhéaume E, et al. Validating a bimodal intravascular ultrasound (IVUS) and near-infrared fluorescence (NIRF) catheter for atherosclerotic plaque detection in rabbits. Biomed Opt Express. 2015;6:3989–99 This study validated the use of bimodal IVUS-NIRF catheter and ICG in rabbit aortas and right iliac arteries to detect atherosclerotic plaque areas.
• Bozhko D, Osborn EA, Rosenthal A, et al. Quantitative intravascular biological fluorescence-ultrasound imaging of coronary and peripheral arteries in vivo. Eur Heart J Cardiovasc Imaging. 2017;18:1253–61 This study detailed the first in vivo IVUS and NIRF molecular-structural imaging system, accounting for distance correction using IVUS data. Corrected NIRF-IVUS was used to image plaque structure and inflammation in swine peripheral arteries with angioplasty-induced vascular injury, coronary artery stents with fibrin deposition, and of rabbit aorta atheroma.
• Ughi GJ, Verjans J, Fard AM, Wang H, Osborn E, Hara T, et al. Dual modality intravascular optical coherence tomography (OCT) and near-infrared fluorescence (NIRF) imaging: a fully automated algorithm for the distance-calibration of NIRF signal intensity for quantitative molecular imaging. Int J Card Imaging. 2015;31:259–68 This study validated the use of automatic distance-corrected algorithm for NIRF images using OCT-NIRF hybrid catheter in atherosclerotic rabbit aortas, compared to manual segmentation.
•• Ughi GJ, Wang H, Gerbaud E, Gardecki JA, Fard AM, Hamidi E, et al. Clinical Characterization of Coronary Atherosclerosis With Dual-Modality OCT and Near-Infrared Autofluorescence Imaging. JACC Cardiovasc Imaging. 2016;9:1304–14 First-in-human intravascular NIR fluorescence study using NIRAF-OCT catheter to detect plaque NIR autofluorescence 633 nm-based signal in non-culprit lesions associated with fibroatheroma, plaque rupture and in-stent restenosis.
Celeng C, de Keizer B, Merkely B, de Jong P, Leiner T, Takx RAP. PET molecular targets and near-infrared fluorescence imaging of atherosclerosis. Curr Cardiol Rep. 2018;20:11.
Bode MF, Jaffer FA. IVUS and OCT: current state-of-the-art in intravascular coronary imaging. Curr Cardiovasc Imaging Rep. 2019;12:29.
Swamy PM, Mamas MA, Bharadwaj AS. Role of near-infrared spectroscopy (NIRS) in intracoronary imaging. Curr Cardiovasc Imaging Rep. 2019;12:34.
Calfon MA, Rosenthal A, Mallas G, Mauskapf A (2011) In vivo near infrared fluorescence (NIRF) intravascular molecular imaging of inflammatory plaque, a multimodal approach to imaging of atherosclerosis. JoVE (Journal of.
•• van Dam GM, Themelis G, Crane LMA, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med. 2011;17:1315–9 This study was a landmark fluorescence molecular imaging study in patients. Folate-fluorescein isothiocyanate (FITC) is a NIRF molecular imaging agent that binds to FR-α receptor on epithelial ovarian cancer. Folate-FITC-based molecular imaging enabled greater detection of tumors by cancer surgeons using widefield fluorescence imaging.
Harlaar NJ, Koller M, de Jongh SJ, van Leeuwen B, Hemmer PH, Kruijff S, et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: a single-centre feasibility study. Lancet Gastroenterol Hepatol. 2016;1:283–90.
Hong G, Antaris AL, Dai H. Near-infrared fluorophores for biomedical imaging. Nat Biomed Eng. 2017;1:0010.
Sevick-Muraca EM, Rasmussen JC. Molecular imaging with optics: primer and case for near-infrared fluorescence techniques in personalized medicine. J Biomed Opt. 2008;13:041303.
Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV, et al. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118:1802–9.
•• Jaffer FA, Calfon MA, Rosenthal A, Mallas G, Razansky RN, Mauskapf A, et al. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol. 2011;57:2516–26 Landmark study that used the first two-dimensional rotational NIRF intravascular catheter with automated pullback allowing in vivo molecular imaging of stented rabbit aortas.
Lee S, Lee MW, Cho HS, Song JW, Nam HS, Oh DJ, et al. Fully integrated high-speed intravascular optical coherence tomography/near-infrared fluorescence structural/molecular imaging in vivo using a clinically available near-infrared fluorescence-emitting indocyanine green to detect inflamed lipid-rich atheromata in coronary-sized vessels. Circ Cardiovasc Interv. 2014;7:560–9.
Kim JB, Park K, Ryu J, et al. Intravascular optical imaging of high-risk plaques in vivo by targeting macrophage mannose receptors. Sci Rep. 2016;6:22608.
• Kim S, Lee MW, Kim TS, et al. Intracoronary dual-modal optical coherence tomography-near-infrared fluorescence structural-molecular imaging with a clinical dose of indocyanine green for the assessment of high-risk plaques and stent-associated inflammation in a beating coronary artery. Eur Heart J. 2016;37:2833–44 Injecting clinical dose of ICG along with using OCT-NIRF hybrid catheter was shown to accurately assess plaque inflammation and DES-related inflammation in in vivo swine coronary arteries.
Maehara A, Matsumura M, Ali ZA, Mintz GS, Stone GW. IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. JACC Cardiovasc Imaging. 2017;10:1487–503.
Dixon AJ, Hossack JA. Intravascular near-infrared fluorescence catheter with ultrasound guidance and blood attenuation correction. J Biomed Opt. 2013;18:56009.
Bertrand M-J, Abran M, Maafi F, et al. In vivo near-infrared fluorescence imaging of atherosclerosis using local delivery of novel targeted molecular probes. Sci Rep. 2019;9:2670.
Li Y, Jing J, Qu Y, Miao Y, Zhang B, Ma T, et al. Fully integrated optical coherence tomography, ultrasound, and indocyanine green-based fluorescence tri-modality system for intravascular imaging. Biomed Opt Express. 2017;8:1036–44.
Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824:68–88.
Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–83.
Lutgens E, Lutgens SPM, Faber BCG, Heeneman S, Gijbels MM, de Winther MP, et al. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107.
Rodgers KJ, Watkins DJ, Miller AL, Chan PY, Karanam S, Brissette WH, et al. Destabilizing role of cathepsin S in murine atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26:851–6.
Jaffer FA, Weissleder R. Seeing within: molecular imaging of the cardiovascular system. Circ Res. 2004;94:433–45.
Newby AC. Metalloproteinase production from macrophages—a perfect storm leading to atherosclerotic plaque rupture and myocardial infarction. Exp Physiol. 2016;101:1327–37.
• Calfon Press MA, Mallas G, Rosenthal A, et al. Everolimus-eluting stents stabilize plaque inflammation in vivo: assessment by intravascular fluorescence molecular imaging. Eur Heart J Cardiovasc Imaging. 2017;18:510–8 This in vivo NIRF molecular imaging study demonstrated that everolimus-eluting stents can suppress plaque macrophages and, hence, inflammation.
Abstract 656: In Vivo Plaque Inflammation and Endothelial Permeability Independently Predict Atherosclerosis Progression: A Serial Multimodality Imaging Study | Arteriosclerosis, Thrombosis, and Vascular Biology. Arterioscler. Thromb. Vasc. Biol.
Chang S-H, Johns M, Boyle JJ, McConnell E, Kirkham PA, Bicknell C, et al. Model IgG monoclonal autoantibody-anti-idiotype pair for dissecting the humoral immune response to oxidized low density lipoprotein. Hybridoma. 2012;31:87–98.
•• Khamis RY, Woollard KJ, Hyde GD, et al. Near infrared fluorescence (NIRF) molecular imaging of oxidized LDL with an autoantibody in experimental atherosclerosis. Sci Rep. 2016;6:21785 The group synthesized LO1-750, a NIRF agent that binds to oxidized LDL and demonstrated the ability to image oxidized LDL and thus oxidative stress using preclinical intravascular NIRF imaging.
• Stein-Merlob AF, Hara T, McCarthy JR, Mauskapf A, Hamilton JA, Ntziachristos V, et al. Atheroma susceptible to thrombosis exhibit impaired endothelial permeability in vivo as assessed by nanoparticle-based fluorescence molecular imaging. Circ Cardiovasc Imaging. 2017. https://doi.org/10.1161/CIRCIMAGING.116.005813 CLIO-CyAm7 nanoparticle was shown to deposit in plaque cells of areas of impaired endothelial barrier and thrombosed plaques, as detected by standalone intravascular NIRF imaging.
Ciesienski KL, Caravan P. Molecular MRI of thrombosis. Curr Cardiovasc Imaging Rep. 2010;4:77–84.
Hara T, Bhayana B, Thompson B, Kessinger CW, Khatri A, McCarthy JR, et al. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC Cardiovasc Imaging. 2012;5:607–15.
• Hara T, Ughi GJ, McCarthy JR, Erdem SS, Mauskapf A, Lyon SC, et al. Intravascular fibrin molecular imaging improves the detection of unhealed stents assessed by optical coherence tomography in vivo. Eur Heart J. 2017;38:447–55 Intravascular NIRF-OCT coupled to FTP11-Cy7, a fibrin specific NIRF agent, was demonstrated to detect fibrin in unhealed stents that otherwise was not detectable by standalone OCT imaging. These findings may help better understand stent healing and the development of new stents or scaffolds.
•• Vinegoni C, Botnaru I, Aikawa E, Calfon MA, Iwamoto Y, Folco EJ, et al. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci Transl Med. 2011;3:84ra45 An important study that showed that FDA-approved ICG could serve as a targeted NIRF vulnerable plaque imaging agent. ICG accumulated in lipid-rich macrophages and was detectable by intravascular NIRF in atherosclerotic rabbit model.
•• Verjans JW, Osborn EA, Ughi GJ, et al. Targeted near-infrared fluorescence imaging of atherosclerosis: clinical and intracoronary evaluation of indocyanine green. JACC Cardiovasc Imaging. 2016;9:1087–95 First targeted atherosclerosis NIRF human trial during which ICG was injected into patients undergoing carotid endartectomy. NIRF-OCT intravascular imaging was used in resected carotid plaques and demonstrated that ICG accumulated in areas of disrupted fibrous cap, neovascularization and intraplaque hemorrhage.
Wang H, Gardecki JA, Ughi GJ, Jacques PV, Hamidi E, Tearney GJ. Ex vivo catheter-based imaging of coronary atherosclerosis using multimodality OCT and NIRAF excited at 633 nm. Biomed Opt Express. 2015;6:1363–75.
Htun NM, Chen YC, Lim B, et al. Near-infrared autofluorescence induced by intraplaque hemorrhage and heme degradation as marker for high-risk atherosclerotic plaques. Nat Commun. 2017;8:75.
Funding
This work was supported by NIH 1R01HL137913 (F.A.J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Khraishah has no conflict of interest to disclose. Dr. Jaffer has received sponsored research grants from Canon and Siemens; he is a consultant for Boston Scientific, Abbott Vascular, Siemens, Philips, and Acrostak. Massachusetts General Hospital has a patent licensing arrangement with Canon, and Dr. Jaffer has the right to receive royalties.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Khraishah, H., Jaffer, F.A. Intravascular Molecular Imaging to Detect High-Risk Vulnerable Plaques: Current Knowledge and Future Perspectives. Curr Cardiovasc Imaging Rep 13, 8 (2020). https://doi.org/10.1007/s12410-020-9527-z
Published:
DOI: https://doi.org/10.1007/s12410-020-9527-z