Abstract
The goal of the present work was to examine associations between COVID-19 pneumonia severity and pulmonary artery diameter. A total of 101 patients with COVID-19 were included in this retrospective observational study. The patients were divided into three groups based on the CT images: 41 patients with mild pneumonia, group 2 had 39 patients with moderate pneumonia, and group 3 had 21 patients with severe pneumonia. Furthermore, the diameter of the main pulmonary artery was calculated as well as ascending aorta, right and left pulmonary artery diameters. Laboratory analysis results were also compared. Analyses show an increased main pulmonary artery diameter is associated with poorer prognosis for patients with COVID-19 pneumonia. Further studies are needed into the mechanisms between severe hypoxemia, increased inflammation, and vascular resistance and higher numbers of thromboembolic events.
Zusammenfassung
Ziel der vorliegenden Arbeit war es, Zusammenhänge zwischen dem Schweregrad der COVID-19-Pneumonie und dem Durchmesser der Pulmonalarterie zu untersuchen. Insgesamt 101 Patienten mit COVID-19 wurden in diese retrospektive Beobachtungsstudie eingeschlossen. Die Patienten wurden auf Basis der Computertomographie(CT)-Bilder in 3 Gruppen eingeteilt: 41 Patienten mit leichter Pneumonie, in Gruppe 2 waren 39 Patienten mit mittelschwerer Pneumonie, und Gruppe 3 umfasste 21 Patienten mit schwerer Pneumonie. Darüber hinaus wurde der Durchmesser der Hauptpulmonalarterie sowie der aufsteigenden Aorta, der rechten und linken Pulmonalarterie berechnet. Auch die Ergebnisse der Laboranalysen wurden verglichen. Die Analysen zeigen, dass ein erhöhter Durchmesser der Hauptpulmonalarterie mit einer schlechteren Prognose für Patienten mit COVID-19-Pneumonie assoziiert ist. Weitere Studien sind erforderlich, um die Mechanismen zwischen schwerer Hypoxämie, erhöhter Entzündung und Gefäßwiderstand und höheren thromboembolischen Ereignissen zu untersuchen.
Similar content being viewed by others
Introduction
The severe acute respiratory syndrome, coronavirus 2 (SARS-CoV-2), is commonly known as the coronavirus disease 2019 (COVID-19), which is thought to be originated from Wuhan City of Hubei Province of China and it has become one of the leading causes of death worldwide [1]. Its clinical features vary from asymptomatic to acute respiratory distress syndrome and multisystem dysfunction results in catastrophic outcomes. Fever, cough, fatigue, and dyspnea are common clinical symptoms while sore throat, headache, myalgia, sputum production are less common. The reference diagnostic tool for standard confirmation of COVID-19 is the real-time polymerase chain reaction (PCR) [2]. However, computed tomography (CT) can be used instead of PCR for rapid diagnosis of COVID-19, especially in emergency services, and also to determine lung involvement [3,4,5]. Thus, CT may have a key role in the early detection and management of COVID-19 pneumonia.
The main CT findings of COVID-19 pneumonia are categorized into three stages [5]. In the first stage, lung parenchyma is normal or near normal. Stage 2 is typically characterized by an increase of ground-glass opacities (GGO) extent. The presence of consolidation, linear opacities, and crazy-paving pattern is diagnostic for stage 3 [5, 6]. It is also well known that the lung parenchymal disease severity is associated with a poorer prognosis. However, the underlying mechanism is still uncertain and is of great interest. Pulmonary artery (PA) may be one of the important affected structures due to the inflammation and hypoxia associated with pneumonia. Thus, the aim in this study is to evaluate the PA diameter in patients with COVID-19 pneumonia.
Materials and methods
Study population
A total of 101 patients with COVID-19 were included in this retrospective observational study. Patients having pneumonia from causes other than COVID-19 and patients with a history of pulmonary hypertension and pulmonary thromboembolism were excluded from the study. Baseline demographic and clinical variables were recorded from the hospital database. All blood samples (hemogram and biochemistry) were evaluated at the time of hospital admission and before hospital discharge. All subjects gave their consent for inclusion in the study. The study was approved by the local ethics committee (University of Health Sciences, Dr. Lutfi Kirdar Kartal Educational and Research Hospital, Istanbul, Turkey; 2020/514/179/21). The investigation conforms to the principles outlined in the Declaration of Helsinki.
CT imaging and evaluation
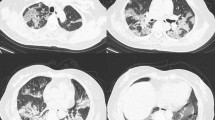
All scans were obtained using a 128 slice multidetector scanner (Philips Ingenuity, Philips Medical System, The Netherlands). The severity of pneumonia was studied in previous research and three categories were described [5, 6]. In the early stage of the disease, the pulmonary parenchyma was normal or near normal. In the stage 2 (moderate pneumonia), ground-glass opacities, typically with a peripheral and subpleural distribution, were the main CT findings. In the presence of severe pneumonia (stage 3), consolidation, linear opacities, and crazy-paving pattern were demonstrated (Fig. 1). According to this classification, our study population was divided into three groups: group 1 had 41 patients with mild pneumonia, group 2 had 39 patients with moderate pneumonia, and group 3 had 21 patients with severe pneumonia. In addition, the diameter of the main pulmonary artery was calculated in addition to the ascending aorta, right and left pulmonary artery diameters (Fig. 2). Each CT scan was displayed on a computer interface through which an observer identified the axial CT section that depicted the bifurcation of the main pulmonary artery and manually measured the diameters of the main pulmonary artery and the aorta [7]. The observer was blinded to the pulmonary arterial pressure data.
Vessel parenchyma obtained from CT scans. a In the early stage of the disease the pulmonary parenchyma was normal or near normal. b In the stage 2 (moderate pneumonia), ground glass opacities, typically with a peripheral and subpleural distribution, were the main CT findings. c In the presence of severe pneumonia (stage 3), consolidation, linear opacities and crazy-paving pattern were demonstrated. CT computed tomography
Statistical analysis
Statistical analysis was made using the computer software Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA). Pearson Χ2 analysis was performed for categorical variables and Bonferroni method was used for subgroups. Fitness to normal distribution was analyzed with the Kolmogorov-Smirnov test. Data were expressed as mean ± standard deviation (SD) for variables with normal distribution, median (25th–75th percentiles) for variables without normal distribution and n (%) for categorical variables. Kruskal–Wallis test was used for comparing quantitative variables without normal distribution while one-way analysis of variance (ANOVA) test was used for comparing the means between groups with a normal distribution. Tukey HSD and Welch tests were used for subgroups analyses with a normal distribution. Spearman analysis was used to evaluate the correlation between main PA and clinical variables. A p-value <0.05 was considered statistically significant.
Results
Baseline demographic and clinical variables of the whole study group were demonstrated in Table 1. There were no significant differences in age, gender, history of diabetes mellitus, hypertension, pulmonary disease, coronary artery disease, and congestive heart failure, usage of azitromycine, chloroquine, favipiravir, high-molecular-weight kininogen, and ritonavir & lopinavir combination, PCR positivity, duration of hospitalization, saturation of O2, glucose, urea, creatinine, sodium, potassium, alanine aminotransferase (ALT), monocyte, mean cell volume (MCV), mean platelet volume (MPV), thrombocyte, D‑dimer, ferritin, procalcitonin, chloride, body temperature, creatine phosphokinase (CPK) levels, aortic diameter, left and right pulmonary artery diameters and ratio of main PA to ascending aorta. Oseltamivir usage was higher in group 2 than group 1 (36 [92.3%], 24 [60.0%], 17 [81.0%]; groups 1, 2, and 3 respectively; p = 0.003). Antibiotic usage was higher in group 2 and 3 (27 [67.5%], 36 [92.3%], 21 [100%]; groups 1, 2, and 3 respectively; p < 0.001). The ratio of noninvasive mechanical ventilation usage was higher in group 3 compared to group 1 (6 [14.6%], 12 [30.8%], 13 [61.9%]; group 1, 2, and 3 respectively; p = 0.001). Calcium (8.81 [8.60–9.18], 8.50 [8.10–8.70], 8.75 [8.15–9.00]; groups 1, 2, and 3 respectively; p = 0.004) and albumin (3.89 ± 0.48, 3.49 ± 0.37, 3.57 ± 0.44; groups 1, 2, and 3 respectively; p = 0.008) levels were lower in group 2 than group 1. Aspartate aminotransferase (AST; 23 [19–30], 28.5 [23–35], 30 [23–42]; group 1, 2, and 3 respectively; p = 0.009), C‑reactive protein (CRP; 7.33 [4.32–28.0], 27.35 [7.0–52.0], 96.0 [19.0–119.0]; groups 1, 2, and 3 respectively; p < 0.001) and lactate dehydrogenase (LDH; 196 [172.5–242], 264 [223–345], 265 [224–408]; groups 1, 2, and 3 respectively; p = 0.001) levels were higher in group 2 and 3 than group 1. Leukocyte level (4.5 [3.85–6.65], 5.15 [3.9–7.5], 7.05 [4.4–9.3]; groups 1, 2, and 3 respectively; p = 0.039) and neutrophil to lymphocyte ratio (NLR; 2.31 [1.62–4.0], 3.22 [2.18–5.14], 4.26 [2.01–13.02]; groups 1, 2, and 3 respectively; p = 0.045) were higher in group 3 compared to group 1. Hemoglobin level (13.46 ± 1.67, 13.58 ± 1.41, 12.54 ± 1.24; groups 1, 2, and 3 respectively; p = 0.033) was lower in group 3 than group 2. The main PA diameter was higher (26.11 ± 3.72, 26.65 ± 2.95, 28.59 ± 3.63; groups 1, 2, and 3 respectively; p = 0.027) in patients with severe pneumonia compared to patients with mild pneumonia (Fig. 3).
Spearman correlation analysis revealed that there was a negative correlation between main PA diameter saturation O2 at the hospitalization (H; r = −0.296, p = 0.004) and discharge from the hospital (D; r = −0.277, p = 0.011). Additionally, there was a positive correlation between PA diameter and CRP-H (r = 0.310, p = 0.003), CRP-D (r = 0.258, p = 0.025), body temperature‑H (r = 0.302, p = 0.015), neutrophil‑D (r = 0.222, p = 0.047), and NLR‑H (r = 0.230, p = 0.026) (Table 2; Fig. 4).
Correlation between main pulmonary artery diameter and clinical and laboratory parameters; CRP-H (a), CRP-D (b), body temperature-H (c), saturation O2-H (d), saturation O2-D (e) and NLR-H (f). CRP C-reactive protein, D discharge from hospital, H hospitalization, NLR neutrophil to lymphocyte ratio, PAD pulmonary artery diameter
Discussion
To the best of our knowledge this is the first time that an association of COVID-19 pneumonia severity and PA diameter was demonstrated. In addition, the main PA diameter was found to be correlated with saturation O2, body temperature, and inflammatory markers such as CRP and NLR.
Coronaviruses are enveloped positive-sense RNA viruses with spike-like projections on their surface membrane [8]. The clinical spectrum of the COVID-19 varies from asymptomatic to acute respiratory tract disease and multiorgan dysfunction. Fever, cough, fatigue, and dyspnea are the most common symptoms of the disease. Sore throat, headache, myalgia, breathlessness, rhinorrhea, chest pain, hemoptysis, conjunctival congestion, diarrhea, nausea, and vomiting are also less common symptoms [2]. These symptoms are similar to other respiratory infections. However, the progression of the disease to pneumonia can be fast and can result in multiorgan dysfunction and death. The reference diagnostic tool for standard confirmation of COVID-19 is real-time PCR [3]. However, CT had a broader usage to detect pneumonia linked to COVID-19. In a study by Ai et al., based on 1014 patients revealed a 97% sensitivity of chest CT for the diagnosis of COVID-19 [5]. The presence of ground-glass opacities is accepted as the main CT finding of COVID-19 pneumonia, typically with a peripheral and subpleural distribution in the intermediate grade of the disease. Consolidation, linear opacities, and crazy-paving pattern generally occur in severe COVID-19 pneumonia [6]. In addition, the severity of the disease is correlated with the severity of pneumonia and adverse clinical outcomes. In the early stage of the disease, near-normal pulmonary compliance was observed without severe hypoxemia. Contrary to this, in the presence of severe pneumonia decreased pulmonary compliance results in severe hypoxemia and acute respiratory distress syndrome. We hypothesized that prolonged hypoxemia linked to severe pneumonia could result in higher pulmonary vascular resistance and also increased PA diameter. Supporting this, in our study population, patients with severe CT findings of COVID-19 pneumonia had higher main PA diameter as well as increased hypoxemia was found to be correlated with higher PA diameter. This may be one of the most important reasons for poorer outcomes in COVID-19 patients with severe pneumonia.
Increased inflammatory status is accompanied by the parallel prolongation in the severity of the disease. Lymphopenia, thrombocytopenia, and leukopenia are the most common laboratory findings in COVID-19 patients, while patients with severe disease had higher neutrophil counts [2, 8]. In addition, many patients had elevated CRP, erythrocyte sedimentation rate, serum ferritin, D-dimer, LDH, CPK, prothrombin time, ALT, and AST levels [9,10,11]. Progression is also associated with increased inflammatory cytokines levels such as interleukin (IL)-6, IL2, IL7, IL10, tumor necrosis factor (TNF)-α, interferon‑γ inducible protein (IP)-10, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP) 1‑α, and granulocyte-colony stimulating factor (G-CSF) [8]. These biomarkers were related to the severity of the disease and increased mortality rates [12]. In our study, we also found that the COVID-19 pneumonia severity is related to higher CRP, AST, LDH, leukocyte, neutrophil, and NLR levels. This increased inflammatory status may result in a decrease in lung capacity and increased PA pressure. Furthermore, increased liver parameters in patients from the sever pneumonia group as possible evidence of higher pulmonary hypertension. Thus, it could be one of the possible etiological reasons for increased PA diameter in patients with severe COVID-19 pneumonia in our study. Supporting these, we revealed the correlation between the PA diameter and CRP level and NLR.
The incidence of acute pulmonary embolism in patients with COVID-19 undergoing pulmonary CT angiography was reported 23–30% in different series [13, 14]. Thrombotic complications are related to increased morbidity and mortality in patients with COVID-19 [15] and it is well known that the incidence rate of acute pulmonary embolism is higher in patients with the severe pulmonary disease even if the prophylactic anticoagulation is performed. Thus, patients having severe pneumonia had a higher incidence of pulmonary embolism in contrast to patients with early stage of pulmonary disease. Although the incidence of pulmonary embolism was not demonstrated in our study, this well-known relationship could be one of the reasons for increased PA diameter in patients with severe COVID-19 pneumonia compared to patients with normal CT findings of pulmonary parenchyma.
COVID-19 is a multisystem disease that also affects the heart. The underlying mechanism of cardiac injury is still controversial. The possible mechanisms are as follows: increased cardiac stress due to respiratory failure and hypoxemia, the direct myocardial infection of SARS-CoV‑2, the indirect effect of the increased inflammatory status or a combination of these. Regardless of the mechanism, myocardial damage, and heart failure contributed to 40% of deaths in COVID-19 patients [16]. In the presence of myocardial damage and heart failure, increased PA pressure may result in increased PA diameter. Based on an analysis of the present data, we revealed that the severity of COVID-19 pneumonia was associated with the higher PA diameter. However, large-scaled studies are needed for future investigations to demonstrate the certain underlying reasons for this relationship and its clinical importance.
Thus, COVID-19 pneumonia and its clinical findings are still important. In patients with moderate to severe pneumonia, hypoxemia can occur due to impaired pulmonary compliance. The radiological findings of COVID-19 pneumonia are important due to its impact on prognosis. In a retrospective study by Yuan et al., severe CT findings were found to be related to increased mortality in COVID-19 patients [6]. Furthermore, increased inflammation and higher thromboembolic events such as pulmonary embolism are related with poorer prognosis in patients with COVID-19 [12, 15]. Thus, increased PA diameter, as a result of the mechanisms mentioned above, can be a rapid and easy diagnostic tool to predict high-risk patients and be a guide for early and effective treatment decisions.
Study limitations
The small sample size of the study was the main limitation. We found a relationship between PA diameter and the COVID-19 pneumonia severity during active disease. However, we did not have any knowledge about the diameter of the PA before the COVID-19 and also we did not follow up the diameter of PA after the disease had been treated. Lack of data about the incidence of pulmonary embolism that can affect the PA diameter was another limitation. Furthermore, we did not know the cardiac status of the patient. Lack of data about the electrocardiographic and echocardiographic imaging was a limitation and our results did not have cardiac biomarkers such as troponin levels as a predictor of cardiac injury. Finally, the COVID-19 patients were not clinically followed up. Thus, we do not know the clinical impact of the PA diameter in this population.
Conclusion
It is known that the severity of the COVID-19 pneumonia is related to the poorer prognosis. In our study, we demonstrated that it was associated with the increased main PA diameter. Severe hypoxemia, increased inflammation, and vascular resistance and higher thromboembolic events may be the underlying mechanisms of this.
References
World Health Organization (2020) Coronavirus disease 2019 (COVID-19): situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed 6 Aug 2020
Corman VM, Landt O, Kaiser M et al (2020) Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25:2000045
Fang Y, Zhang H, Xie J et al (2020) Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology 296:115–117
Ai T, Yang Z, Hou H et al (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 296:32–40
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (2020) Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 215:87–93
Yuan M, Yin W, Tao Z et al (2020) Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS ONE 15:e230548
Corson N, Armato SG, Labby ZE et al (2014) CT-based pulmonary artery measurements for the assessment of pulmonary hypertension. Acad Radiol 21:523–530
Chen N, Zhou M, Dong X et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513
Xu XW, Wu XX, Jiang XG et al (2020) Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: retrospective case series. BMJ 368:606
Zhang JJ, Dong X, Cao YY et al (2020) Clinical characteristics of 140 patients infected by SARSCoV‑2 in Wuhan, China. Allergy 75:1730–1741
Fu L, Wang B, Yuan T et al (2020) Clinical characteristics of coronavirus disease 2019 (COVID-19) in China. A systematic review and meta-analysis. J Infect 80:656–665
Zhou F, Yu T, Du R et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062
Grillet F, Behr J, Calame P et al (2020) Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology 296(3):E186–E188
Leonard-Lorant I, Delabranche X, Severac F et al (2020) Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D‑dimer levels. Radiology 296(3):E189–E191
Bikdeli B, Madhavan MV, Jimenez D et al (2020) COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 75:2950–2973
Ruan Q, Yang K, Wang W et al (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46:846–848
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Yildiz,S. Yadigar,B.Ş. Yildiz, N.B. Aladag, O. Keskin, R.S. Ozer, C. Topel andS. Kahraman declare that they have no competing interests.
All subjects gave their consent for inclusion in the study. The study was approved by the local ethics committee (University of Health Sciences, Dr. Lutfi Kirdar Kartal Educational and Research Hospital, Istanbul, Turkey; 2020/514/179/21). The investigation conforms to the principles outlined in the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Yildiz, M., Yadigar, S., Yildiz, B.Ş. et al. Evaluation of the relationship between COVID-19 pneumonia severity and pulmonary artery diameter measurement. Herz 46, 56–62 (2021). https://doi.org/10.1007/s00059-020-05014-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-020-05014-x