Abstract
Objectives
To describe micafungin pharmacokinetic (PK) alterations of sepsis induced in piglets and to determine whether the porcine septic model is able to predict the PK of micafungin in septic patients at the plasma and peritoneal sites.
Methods
From healthy (n = 8) and septic piglet group (n = 16), total micafungin concentrations were subject to a population PK analysis using Monolix®. Data from 16 septic humans patients from others studies was used to compare micafungin PK between septic piglets and septic patients.
Results
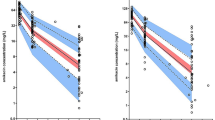
Sepsis induced in piglets slightly alters the total clearance and the volume of distribution, while inter-compartment clearance is increased (from 3.88 to 5.74 L/h) as well as the penetration into peritoneal cavity (from 61 to 90%). In septic human patients, PK parameters are similar except for the Vd, which is corrected by an allometric factor based on the body weight of each species. Micafungin penetration into peritoneal cavity of humans is lower than in septic piglets (40 versus 90%).
Conclusions
The sepsis induced in the porcine model alters the PK of micafungin comparable to that in humans. In addition, micafungin PK is similar between these two species at the plasma level taking into account the allometric relationship of the body weight of these species on the central volume of distribution. The porcine septic plasma model would be able to predict the micafungin PK in the septic patients. However, further studies on peritoneal penetration are necessary to characterize this inter-species difference.




Similar content being viewed by others
References
Angus DC, van der Poll T. Severe Sepsis and Septic Shock. N Engl J Med. 29 août 2013;369(9):840–51.
Guillon A, Preau S, Aboab J, Azabou E, Jung B, Silva S, et al. Preclinical septic shock research: why we need an animal ICU. Ann Intensive Care déc. 2019;9(1):66.
Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol mars. 2012;49(2):344–56.
Mair KH, Sedlak C, Käser T, Pasternak A, Levast B, Gerner W, et al. The porcine innate immune system: an update. Dev Comp Immunol août. 2014;45(2):321–43.
Balls M, Goldberg AM, Fentem JH, Broadhead CL, Burch RL, Festing MFW, et al. The Three Rs: The Way Forward. ATLA. 1995;23:838–66.
Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol janv. 2012;20(1):50–7.
Sinnollareddy M, Peake SL, Roberts MS, Lipman J, Roberts JA. Using pharmacokinetics and pharmacodynamics to optimise dosing of antifungal agents in critically ill patients: a systematic review. Int J Antimicrob Agents janv. 2012;39(1):1–10.
Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient: Crit Care Med. mars 2009;37(3):840‑51.
Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study*. Crit Care Med févr. 2006;34(2):344–53.
Vincent J-L. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA. 2 déc 2009;302(21):2323.
Pappas PG, Rotstein CMF, Betts RF, Nucci M, Talwar D, Waele JJD, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive Candidiasis. Clin Infect Dis. 2007;45(7):883–93.
Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJC, Baron EJ, et al. Diagnosis and management of complicated intra‐abdominal infection in adults and children: guidelines by the surgical infection society and the infectious diseases society of America. Clin Infect Dis. 15 janv 2010;50(2):133‑64.
Wasmann RE, Muilwijk EW, Burger DM, Verweij PE, Knibbe CA, Brüggemann RJ. Clinical pharmacokinetics and pharmacodynamics of micafungin. Clin Pharmacokinet mars. 2018;57(3):267–86.
Aboab J, Sebille V, Jourdain M, Mangalaboyi J, Gharbi M, Mansart A, et al. Effects of esmolol on systemic and pulmonary hemodynamics and on oxygenation in pigs with hypodynamic endotoxin shock. Intensive Care Med août. 2011;37(8):1344–51.
Food and Drug Administration. Guidance for industry: bioanalytical method validation. 2018;Rockville: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM).
Chan PLS, Jacqmin P, Lavielle M, McFadyen L, Weatherley B. The use of the SAEM algorithm in MONOLIX software for estimation of population pharmacokinetic-pharmacodynamic-viral dynamics parameters of maraviroc in asymptomatic HIV subjects. J Pharmacokinet Pharmacodyn févr. 2011;38(1):41–61.
Garbez N, Mbatchi LC, Maseda E, Luque S, Grau S, Wallis SC, et al. A loading micafungin dose in critically Ill patients undergoing continuous veno-venous hemofiltration or continuous veno-venous hemodiafiltration: a population pharmacokinetic analysis. Ther Drug Monit [Internet]. 30 juill 2021 [cité 6 août 2021]; Disponible sur: https://journals.lww.com/drug-monitoring/Abstract/9000/A_Loading_Micafungin_Dose_in_Critically_Ill.98654.aspx
Garbez N, Mbatchi L, Wallis SC, Muller L, Lipman J, Roberts JA, et al. Prospective cohort study of micafungin population pharmacokinetic analysis in plasma and peritoneal fluid in septic patients with intra-abdominal infections. Antimicrob Agents Chemother [Internet]. 17 juin 2021 [cité 6 août 2021];65(7). Disponible sur: https://journals.asm.org/doi/https://doi.org/10.1128/AAC.02307-20
West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 4 avr 1997;276(5309):122‑6.
Gumbo T, Hiemenz J, Ma L, Keirns JJ, N. Buell D, Drusano GL. Population pharmacokinetics of micafungin in adult patients. Diagn Microbiol Infect Dis. mars 2008;60(3):329‑31.
Grau S, Luque S, Campillo N, Samsó E, Rodríguez U, García-Bernedo CA, et al. Plasma and peritoneal fluid population pharmacokinetics of micafungin in post-surgical patients with severe peritonitis. J Antimicrob Chemother. 2015;70(10):2854–61.
Martial LC, ter Heine R, Schouten JA, Hunfeld NG, van Leeuwen HJ, Verweij PE, et al. Population pharmacokinetic model and pharmacokinetic target attainment of micafungin in intensive care unit patients. Clin Pharmacokinet. 2017;56(10):1197–206.
Gastine S, Lanckohr C, Blessou M, Horn D, Fobker M, Bause D, et al. Pharmacokinetics of micafungin in critically Ill patients. Sci Rep déc. 2019;9(1):17741.
Schelstraete W, Clerck LD, Govaert E, Millecam J, Devreese M, Deforce D, et al. Characterization of porcine hepatic and intestinal drug metabolizing CYP450: comparison with human orthologues from a quantitative, activity and selectivity perspective. Sci Rep déc. 2019;9(1):9233.
Hall C, Lueshen E, Mošat’ A, Linninger AA. Interspecies scaling in pharmacokinetics: a novel whole-body physiologically based modeling framework to discover drug biodistribution mechanisms in vivo. J Pharm Sci. mars 2012;101(3):1221‑41.
Funding
This study was supported by an academic funding from the Nimes University Hospital to conduct this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Jeffrey Lipman received honoraria from MSD and Pfizer and received Institution support from MSD. Claire Roger received consultancy fees from MSD, Pfizer, and Fresenius Medical Care.
Nicolas Garbez, Litaty Mbatchi, Guillaume Louart, Steven C. Wallis, Laurent Muller, Jason A. Roberts, Jean-Yves Lefrant have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garbez, N., Mbatchi, L.C., Louart, G. et al. Micafungin Population PK Analysis in Healthy and Septic Pigs: Can the Septic Porcine Model Predict the Micafungin PK in Septic Patients?. Pharm Res 38, 1863–1871 (2021). https://doi.org/10.1007/s11095-021-03137-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03137-2