Abstract
Zika virus (ZIKV) is an arbovirus from the Flaviviridae family. It is usually transmitted by mosquito bite. There have been no reports of severe symptoms caused by ZIKV infection up until the last few years. In October 2013 an outbreak was reported in French Polynesia with severe neurological complications in some affected cases. In November 2015, the Ministry of Health of Brazil attributed the increased number of neonatal microcephaly cases in northeastern Brazil to congenital ZIKV infection. The rapid spread of the virus convinced the World Health Organization to announce ZIKV infection as a “Public Health Emergency of International Concern” in February 2016. The main neuroimaging findings in congenital ZIKV infection include microcephaly which is the hallmark of the disease, other malformations of cortical development (e.g., lissencephaly, heterotopia, etc.), parenchymal calcifications, unilateral or bilateral ventriculomegaly, enlarged extra-axial CSF spaces, dysgenesis of the corpus callosum, agenesis of the cavum septum pellucidum, cerebellar and brainstem hypoplasia, and ocular abnormalities. ZIKV infection may also cause Guillain-Barré syndrome and acute disseminated encephalomyelitis in adults. Familiarity with neuroimaging findings of congenital and acquired ZIKV infection is crucial to suspect this disease in residents of endemic regions and travelers to these areas.
Similar content being viewed by others
Objective
To review neuroimaging findings of congenital and acquired Zika virus infection on ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI).
Background
Zika virus (ZIKV) is an arbovirus from the Flaviviridae family. It was first detected in a primate (rhesus macaque) in 1947 and consequently isolated from its vectors (Aedes africanus) in 1948 in Zika Valley, Uganda, Africa [1, 2]. It is typically transmitted by mosquito bite, with Aedes aegypti being the main vector nowadays, and Aedes albopictus and Aedes polynesiensis the next most important ones [3]. In addition, sexual transmission has also been reported [4].
Fever is a common presentation of ZIKV infection and other arbovirus infections such as West Nile, dengue fever, yellow fever, and Japanese encephalitis viruses [5]. No severe symptoms caused by Zika virus infection have been reported until recently. In October 2013, an outbreak of ZIKV infection was reported in French Polynesia (a country in the South Pacific Ocean) and some of the affected adult patients suffered from severe neurological and autoimmune complications [6]. In 2015, there was a Zika virus epidemic in Brazil which outspread rapidly to more than 30 nations in South America and Caribbean regions, and affected more than 2 million people [7]. In November 2015, the Ministry of Health of Brazil attributed the increased number of neonatal microcephaly cases in northeastern Brazil (particularly in Pernambuco State) to congenital ZIKV infection [8, 9]. In addition, ocular involvement has been reported in neonates with ZIKV infection, mainly affecting the macular and peri-macular areas, as well as the optic nerve [10]. Moreover, ZIKV infection has been associated with Guillain-Barré syndrome (GBS) and acute disseminated encephalomyelitis (ADEM) in affected adult cases in the endemic regions [11]. New evidence from in vitro studies suggest that ZIKV may directly infect neuronal cells [12].
The serological diagnosis of ZIKV infection is challenging because cross-reactions with other flaviviruses have been reported and also serology may be falsely negative in the early course of the disease. Real-time polymerase chain reaction (RT-PCR) is used as an accurate and rapid test for detecting virus RNA in the blood [13]. However, viremia is transient in most cases (mostly present during the first week after the beginning of presentations) and RT-PCR is likely to be negative after the viremia is resolved. By contrast, it seems that, as in other flaviviruses, ZIKV Ig-M becomes positive in the patient’s serum as the viral load starts to decrease, and will remain detectable for several months [2]. In summary, RT-PCR is the diagnostic method of choice for ZIKV infection; but in suspicious cases when RT-PCR is negative, looking for ZIKV Ig-M using the enzyme-linked immunosorbent assay (ELISA) technique may be beneficial [14].
The rapid emergence and spreading of the virus convinced the World Health Organization (WHO) to announce the ZIKV infection as a “Public Health Emergency of International Concern” on 1 February, 2016 [15]. A few reports about neuroimaging findings of ZIKV infection are available, but several questions still need to be answered. In this article, we aim to review the neuroimaging findings in fetuses, neonates, and adults with ZIKV infection. For radiologists, a detailed knowledge of the potential neuroimaging findings in patients with ZIKV infection is crucial for accurately making the diagnosis.
Methods
Protocol, data sources, and inclusion criteria
ENTREQ guidelines [16] have been followed by the authors during this study. The PubMed search engine and two other databases (i.e., Embase and Cochrane) were searched electronically among English literature for the keywords “Zika virus”, “neuroimaging”, “magnetic resonance imaging (MRI)”, “computed tomography (CT)”, “ultrasound” and related terms in articles published between January 2010 and July 2016. The reference lists of retrieved articles were also manually searched for any relevant study. There was no restriction for the study design of searched articles. The literature search was iterative, i.e., finding all available materials until reaching theoretical saturation.
Data collection and data synthesis
Study data were independently extracted by two authors of this article and disagreement was resolved by consensus. Primary screening for study selection was done by title and abstract review. Narrative synthesis and thematic analysis have been used for data synthesis.
Results
Fetal brain ultrasound
The most frequent finding on prenatal ultrasound described in the literature is microcephaly [17], i.e., head circumference (HC) 2 standard deviations (SD) below the mean for the gestational age or under the 3rd centile [23]. However, neonates with severe brain abnormalities caused by congenital ZIKV infection may have a normal HC. Microcephaly seems to depend on the timing of prenatal ZIKV infection, with infections early in pregnancy i.e., in the first and early second trimesters being mostly associated with microcephaly. Fetal microcephaly is most likely due to increased developmental neuronal apoptosis secondary to early ZIKV infection of progenitor cells [17–22]. The risk of ZIKV infection associated fetal microcephaly is estimated to be 95 (95 CI 34–191) per 10,000 pregnant women that are infected in the first trimester of pregnancy [17].
Other fetal ultrasound findings include: (1) unilateral or bilateral ventriculomegaly which may be associated with subependymal pseudocysts around the occipital horns; (2) brain parenchymal atrophy and calcifications; (3) agenesis/hypoplasia of the corpus callosum with or without interhemispheric cyst; (4) absent cavum septum pellucidum; (5) global cerebellar or vermian hypoplasia; (6) medullary and pontine hypoplasia; (7) ocular abnormalities, like microphthalmia, intraocular calcification, and cataract [17–22].
Brain parenchymal calcifications are mainly periventricular (especially in the frontal lobes) in location, but may also involve the cerebellum and basal ganglia, particularly the caudate nucleus [18].
Fetal brain MRI
Like ultrasound, fetal brain MRI reveals microcephaly in most cases. Other findings are: (1) ventriculomegaly and subependymal pseudocysts particularly near the occipital horns of lateral ventricles; (2) agenesis/hypoplasia of the corpus callosum; (3) absent cavum septum pellucidum; (4) other malformations of cortical development apart from microcephaly, such as extensive polymicrogyria, opercular dysplasia, and pachygyria; (5) abnormal cortical signal intensity suggesting cortical laminar necrosis; (6) cerebellar and vermian hypoplasia; (7) brainstem abnormalities; (8) enlarged extra-axial spaces [17, 20].
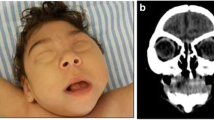
Neonatal brain CT scan (Fig. 1)
Microcephaly is seen in nearly all affected neonates. Other described findings include: (1) intraparenchymal calcifications which have been reported in almost all infants with congenital ZIKV infection. Calcifications are mainly located at the corticomedullary junction within the frontal and parietal lobes. Other locations include basal ganglia and thalami in a decreasing order. Calcifications are predominantly punctate in shape and band-like in distribution; (2) ventriculomegaly is seen in nearly all affected neonates and is severe in about half of them. Ventriculomegaly may involve the whole ventricular system, but in about 40 % of patients it occurs only in the lateral ventricles with predominant enlargement of the trigones and posterior horns; (3) malformations of cortical development, such as hypogyration, is seen in almost all infants with congenital ZIKV infection. It is severe (i.e., agyria) in about 80 % of cases. Other malformations described in the literature include polymicrogyria, heterotopia, and schizencephaly; (4) cerebellar and brainstem hypoplasia may also be noted in approximately 75 % and 10 % of cases, respectively; (5) white-matter hypodensity is seen in almost all neonates and is diffuse in near 90 % of them; no further characterization of white matter changes is possible on CT, but MRI studies suggest that white matter changes seen on CT are due to dysmyelination or delayed myelination; (6) chronic encephalomalacia is reported in one case in the territory of the middle cerebral artery (MCA) which is most likely caused by sequelae of prenatal ischemic stroke in early intrauterine life; (7) skull molding with a pointed occiput and overriding of bones mainly in the frontal and occipital regions is another described feature in head CT scans of affected neonates [24–27].
Axial brain CT scan findings in congenital microcephaly due to intrauterine Zika virus infection in four different infants. Multiple isolated (a, b, f) and band-like (c–e) calcifications at the corticomedullary junction or periventricular white matter are noted. In addition, diffuse hypogyration (a–f), ventriculomegaly (a–f), and cerebellar hypoplasia (g–h) are seen
Neonatal brain MRI
Craniofacial disproportion and microcephaly (HC < 32 cm) are seen in almost all brain MRI studies [25–27].
Other findings that are seen in almost all cases include: (1) brain atrophy and reduced brain cortical thickness; (2) enlarged subarachnoid spaces; (3) lissencephaly; (4) ventriculomegaly which is non-hypertensive and secondary to brain atrophy; (5) agenesis/hypoplasia of the corpus callosum; (6) coarse calcifications that are most commonly seen in subcortical-cortical transition and the basal ganglia [25–27].
Less common findings include: (1) a large choroid plexus; (2) intraventricular septations; (3) periventricular calcifications; (4) cerebellar and brainstem hypoplasia; (5) schizencephaly; (6) gray matter heterotopia [8, 25–27].
ZIKV-related Guillain-Barré syndrome
Brain and spine MRI findings in ZIKV-related GBS are similar to those in patients with GBS due to other etiologies and include: (1) post-contrast enhancement of cranial nerves, such as trigeminal and facial nerves; (2) post-contrast enhancement of the conus medullaris and cauda equina nerve roots with more prominent and common enhancement of the ventral roots compared to the dorsal ones; (3) T2-hyperintensity and contrast-enhancement of the lumbar spinal ganglia bilaterally. The findings are secondary to autoimmune inflammation and demyelination, along with blood-neuronal barrier breakdown [11, 28]. So far, no significant imaging difference between ZIKV-related GBS and GBS in other settings has been reported.
ZIKV-related acute disseminated encephalomyelitis
Neuroimaging findings in ZIKV-related ADEM do not differ from those caused by other etiologies, and no specific imaging finding for ZIKV-related ADEM distinguishing it from other causes of ADEM has been reported yet. Neuroimaging findings of ADEM typically include multiple, asymmetrically distributed, and poorly marginated lesions involving the white matter and deep gray matter nuclei that are hyperintense on T2-weighted and fluid-attenuated inversion recovery (FLAIR) MR images. Open-ring post-contrast enhancement is common, but complete-ring or punctate enhancement as well as lack of enhancement are also possible. In the acute phase, peripheral restricted diffusion is typically seen in contrast to central restriction in brain abscesses [29, 30].
Discussion and data synthesis
Many prenatal viral infections such as TORCH infections may interfere with various processes of brain development like neuronal migration, cortical organization, and myelination, and hence result in various brain injuries and congenital brain anomalies. The earlier the mother is infected, the more severe the abnormalities will be because the main process of organogenesis takes place during the first trimester and early second trimester.
The brain damage may be a consequence of either direct viral invasion and apoptosis of fetal neuronal tissue, or an inflammatory response due to inflammatory mediators released from the placenta in the infected pregnant woman [31]; however, Mlakar et al. [32] have isolated ZIKV from the brain of an aborted fetus affected by Zika infection which proves the neurotropism of this virus and favors the first pathomechanism against the second one.
The neuroimaging findings described in the literature suggest that ZIKV may disrupt various stages of the normal cortical development because of abnormal cell proliferation/apoptosis (e.g., microcephaly), abnormal neuronal migration (e.g., lissencephaly, heterotopia), or abnormal post-migrational development (also known as abnormal cortical organization) (e.g., polymicrogyria, cortical dysplasia, and schizencephaly). Recent experimental studies also support a disruptive pathomechanism. In experimental models ZIKV has been shown to target human brain cells, reducing their viability and growth [33–35]. These results suggest that Zika virus abrogates neurogenesis during human brain development. In addition, Zika virus infection causes a downregulation of genes involved in cell cycle pathways, dysregulation of cell proliferation, and upregulation of genes involved in apoptotic pathways resulting in cell death [34].
Intracranial calcifications are a common finding in TORCH infections. It is considered to be a part of the healing phase. Morphology, distribution, and location of calcifications may differ between patients affected by different viral congenital infections. In congenital ZIKV infection, intracranial calcifications are typically punctate in form, are located at the cerebral gray-white matter junction, and have band-like distribution; however, calcifications may be also seen in the basal ganglia, periventricular white matter, and cerebellum.
The distinctive finding described in congenital Zika is the corpus callosum dysgenesis indicating that the insult has occurred at about 8–12 weeks of gestation. The normal development of the corpus callosum begins from the genu (anterior) and progresses posteriorly; however, the rostrum appears as the last part. Insult at any stage of development will result in different outcomes, ranging from total agenesis to hypoplasia, due to encephalomalacia in the corpus callosum white matter bundles, also known as the bundles of Probst [36].
The ventriculomegaly and enlarged extra-axial CSF spaces are best explained in the context of brain volume reduction with an ex-vacuo mechanism.
In adults, ZIKV may cause neuronal damage and demyelination with inflammatory mediators. Two described conditions that may complicate Zika virus infection in adults are Guillain-Barré syndrome (GBS) and acute disseminated encephalomyelitis (ADEM). Since they are usually of autoimmune pathogenesis, they may develop secondary to ZIKV cross-reactivity with human neuronal antigens theoretically.
Conclusion
We reviewed neuroimaging findings of congenital and acquired Zika virus infection on ultrasound, CT scan, and MRI.
Fetal ZIKV infection causes severe central nervous system (CNS) developmental abnormalities. The neuroimaging findings in congenital Zika infection are not pathognomonic; but in combination with the patient history (especially residence or history of travel in endemic areas) may be suggestive of ZIKV infection.
In addition, ZIKV may cause neurological complications in adults. Familiarity with the neuroimaging findings of these potential conditions is important for making the correct diagnosis in the infected patients.
References
Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev. 2016;29(3):487–524.
Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–63.
Singh RK, Dhama K, Malik YS, Ramakrishnan MA, Karthik K, Tiwari R, Saurabh S, Sachan S, Joshi SK. Zika virus: emergence, evolution, pathology, diagnosis, and control: current global scenario and future perspectives—a comprehensive review. Vet Q. 2016;36(3):150–75.
Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21(2):359–61.
Shuaib W, Stanazai H, Abazid AG, Mattar AA. Re-emergence of zika virus: a review on pathogenesis, clinical manifestations, diagnosis, treatment, and prevention. Am J Med. 2016; 129(8):879.e7–879.
Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44(7):302–7.
Anderson KB, Thomas SJ, Endy TP. The emergence of zika virus: a narrative review. Ann Intern Med. 2016;165(3):175–83.
Chibueze EC, Tirado V, da Silva Lopes K, Balogun OO, Takemoto Y, Swa T, et al. Zika virus infection in pregnancy: a systematic review of disease course and complications. Bull World Health Organ. E-pub: 2016.
Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, et al. Brazilian Medical Genetics Society—Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62.
de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, Belfort R, Jr. Ocular findings in infants with microcephaly associated with presumed zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol. 2016.
Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F. Zika virus infection complicated by Guillain-Barré syndrome — case report, French Polynesia, December 2013. Euro Surveill. 2014;19:20720.
Simões E, Silva AC, Moreira JM, Romanelli RM, Teixeira AL. Zika virus challenges for neuropsychiatry. Neuropsychiatr Dis Treat. 2016;12:1747–60.
Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J. 2013;22(10):311.
Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65:122–127.
Gulland A. Zika virus is a global public health emergency, declares WHO. BMJ. 2016;352:i657-i657.
Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181.
Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–32.
Oliveira Melo AS, Malinger G, Ximenes R, et al. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47:6–7.
Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report. N Engl J Med. doi: 10.1056/NEJMoa1602412.
Guillemette-Artur P, Besnard M, Eyrolle-Guignot D, et al. Prenatal brain MRI of fetuses with Zika virus infection. Pediatr Radiol. 2016;46:1032.
Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jääskeläinen AJ, Smura T, Rosenberg A, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374(22):2142–51.
Franca GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 live births with complete investigation. Lancet. 2016; Epub June 29.
Tarrant A, Garel C, Germanaud D, et al. Microcephaly: a radiological review. Pediatr Radiol. 2009;39:772–80.
Hazin AN, Poretti A, Cruz DDCS, et al. Computed tomographic findings in microcephaly associated with Zika virus. N Engl J Med. 2016;374:2193–5.
De Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. The BMJ. 2016; 353:i1901.
Cavalheiro S, Lopez A, Serra S, Da Cunha A, da Costa MD, Moron A, Lederman HM. Microcephaly and Zika virus: neonatal neuroradiological aspects. Childs Nerv Syst. 2016;32(6):1057–60.
Baptista T, Quaghebeur G, Alarcon A. Neuroimaging findings of babies with microcephaly and presumed congenital Zika virus infection. The BMJ. 2016;353:i2194.
Fontes CA, Dos Santos AA, Marchiori E. Magnetic resonance imaging findings in Guillain-Barré syndrome caused by Zika virus infection. Neuroradiology. Published online April 11, 2016.
Hynson JL, Kornberg AJ, Coleman LT, et al. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56(10):1308–12.
Young NP, Weinshenker BG, Lucchinetti CF. Acute disseminated encephalomyelitis: current understanding and controversies. Semin Neurol. 2008;28(1):84–94.
Adibi JJ, Marques ET Jr, Cartus A, Beigi RH. Teratogenic effects of the Zika virus and the role of the placenta. Lancet. 2016;387(10027):1587–90.
Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, et al. Zika virus associated with microcephaly. N Engl J Med. 374:951–958.
Garcez PP, Loiola EC, Madeiro da Costa R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816–8.
Tang H, Hammack C, Ogden SC, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587–90.
Qian X, Nguyen HN, Song MM, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–54.
Kornienko VN, Pronin IN. Diagnostic neuroradiology. New York: Springer; 2009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
About this article
Cite this article
Zare Mehrjardi, M., Keshavarz, E., Poretti, A. et al. Neuroimaging findings of Zika virus infection: a review article. Jpn J Radiol 34, 765–770 (2016). https://doi.org/10.1007/s11604-016-0588-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-016-0588-5