Background
Adipose tissue (AT) metabolism is altered in obese subjects, and the reestablishment of energy homeostasis requires the identification and regulation of genes with altered patterns. The aim of this study was to compare mRNA expression of PPARβ/δ and PPARγ1-3 in morbidly obese and nonobese patients. The expression pattern of these receptors in various abdominal adipose tissues, subcutaneous (SAT), retroperitoneal (RAT) and visceral (VAT), was also evaluated.
Methods
The AT depots were obtained by surgery. Total RNAs were extracted using TRIzol. PPARs reverse transcripts were determined by quantitative polymerase chain reaction (qRT-PCR).
Results
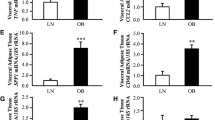
The amounts of PPARβ/δ mRNA in different depots of morbidly obese AT showed a significant decrease in VAT (P < 0.05). In the non-obese group, the level of PPARβ/δ was higher in SAT (P < 0.05), but PPARγ1-3 was not differentially expressed in obese and non-obese depots. When comparing obese and non-obese, the results revealed a decrease in PPARβ/δ expression in SAT (P = 0.058) and VAT (P = 0.094) of the morbidly obese. PPARγ1-3 mRNA expression was increased significantly in SAT (P = 0.022) and decreased in RAT (P = 0.034) in morbidly obese subjects. PPARβ/δ expression in SAT and VAT correlated negatively with hip size and insulin serum respectively. PPARγ1-3 expression in RAT correlated negatively with waist and hip circumference and in VAT correlated positively with waist size.
Conclusions
The present study demonstrates that PPARβ/δ and PPARγ1-3 mRNAs are quantitatively different in AT of morbidly obese individuals compared to non-obese, and that PPARβ/δ mRNA levels are characteristic for each AT depot.
Similar content being viewed by others
References
Campos MG, Cañete RR, Gil A. Adiponectin. The missing link in insulin resistance and obesity. Clin Nutr 2004; 23: 963–74.
Adami GF, Camerini GG, Ravera NS et al. Metabolic syndrome in severely obese patients. Obes Surg 2001; 11: 543–5.
Deitel M. Overweight and obesity worldwide now estimated to involve 1.7 billion people (Editorial). Obes Surg 2003; 13: 329–30.
Lee WJ, Wang W. Bariatric surgery: Asia-Pacific perspective. Obes Surg 2005; 15: 751–7.
Misra A, Vikram NK. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition 2003; 19: 457–66.
Vohl MC, Sladek R, Robitaille J et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res 2004; 12: 1217–22.
Takahashi S, Tanaka T, Kodama T et al. Peroxisome proliferator-activated receptor δ (PPARδ). A novel target site for drug discovery in metabolic syndrome. Pharmacol Res 2006; 53: 501–7.
Tanaka T, Yamamoto J, Iwasaki S et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. PNAS 2003; 100: 15924–9.
Grimaldi PA. The roles of PPARs in adipocyte differentiation. Prog Lipid Res 2001; 40: 269–81.
Kota BP, Huang THW, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacol Res 2005; 51: 85–94.
Meirhaeghe A, Amouyel P. Impact of genetic variation of PPAR γ in humans. Mol Genet Metab 2004; 83: 93–102.
Panunti B, Fonseca V. Effects of PPAR gamma agonists on cardiovascular function in obese non-diabetic patients. Vasc Pharmacol 2006; 45: 29–35.
Mehrabi MR, Haslmayer P, Humpeler S et al. Quantitative analysis of peroxisome proliferator-activated receptor gamma (PPARγ) expression in arteries and hearts of patients with ischaemic or dilated cardiomyopathy. Eur J Heart Fail 2003; 5: 733–9.
Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med 2004; 10: 1–7.
Wang YX, Lee CH, Tiep S et al. Peroxisome proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 2003; 113: 159–70.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 2001; 25: 402–8.
Rattarasarn C. Physiological and pathophysiological regulation of regional adipose tissue in the development of insulin resistance and type 2 diabetes. Acta Physiol 2006; 186: 87–101.
Ferré P. The biology of peroxisome proliferator-activated receptors: Relationship with lipid metabolism and insulin sensitivity. Diabetes 2004; 53: S43–S50.
Montague CT, Prins JB, Sanders L et al. Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes 1998; 47: 1384–91.
Barish GD, Narkar VA, Evans RM. PPARδ: a dagger in the heart of the metabolic syndrome. J Clin Invest 2006; 116: 590–7.
Krempler F, Breban D, Oberkofler H et al. Leptin, peroxisome proliferator-activated receptor-γ, and CAAAT/enhancer binding protein-α mRNA expression in adipose tissue of humans and their relation to cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2000; 20: 443–9.
Auboeuf D, Riuesset J, Fajas J et al. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: No alterations in adipose tissue of obese and NIDDM patients. Diabetes 1997; 46: 1319–27.
Lefebre AM, Laville M, Vega N et al. Depot-specific differences in adipose tissue gene expression in lean and obese subjects. Diabetes 1998; 47: 98–103.
Yanase T, Yashiro T, Takitani K et al. Differential expression of PPAR γ1 and γ2 isoforms in human adipose tissue. Biochem Biophys Res Commun 1997; 233: 320–4.
Bouchard C, Despres J-P, Mauriege P. Genetic and nongenetic determinants of regional fat distribution. Endocr Rev 1993; 14: 72–93.
Vidal-Puig AJ, Considine RV, Jimenez-Liñan M et al. Peroxisome proliferator-activated receptor gene expression in human tissues: effects of obesity. Weight loss and regulation by insulin and glucocorticoids. J Clin Invest 1997; 99: 2416–22.
Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med 2003; 115: 42S–48S.
Pelton PD, Zhou L, Demarest KT et al. PPARδ activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem Biophys Res Commun 1999; 261:456–8.
Albrektsen T, Frederiksen KS, Holmes WE et al. Novel genes regulated by the insulin sensitizer rosiglitazone during adipocyte differentiation. Diabetes 2002; 51: 1042–51.
Gross BS, Fruchart JC, Staels B. Peroxisome Proliferator-Activated Receptorβ/δ: A novel target for the reduction of atherosclerosis. Drug Discovery Today: Therapeutical Strategies 2005; 2: 237–43.
Baranova A, Collantes R, Gowder SJ et al. Obesityrelated differential gene expression in the visceral adipose tissue. Obes Surg 2005; 15: 758–65.
Lee YH, Nair S, Rousseau E et al. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia 2005; 48: 1776–83.
Von Eyben FE, Kroustrup JP, Larsen JF et al. Comparison of gene expression in intra-abdominal and subcutaneous fat: a study of men with morbid obesity and nonobese men using microarray and proteomics. Ann N Y Acad Sci 2004; 1030: 508–36.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bortolotto, J.W., Margis, R., Ferreira, Â.C.B. et al. Adipose Tissue Distribution and Quantification of PPARβ/δ and PPARγ1-3 mRNAs: Discordant Gene Expression in Subcutaneous, Retroperitoneal and Visceral Adipose Tissue of Morbidly Obese Patients. OBES SURG 17, 934–940 (2007). https://doi.org/10.1007/s11695-007-9172-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-007-9172-5