Abstract
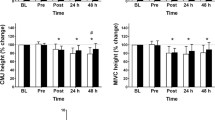
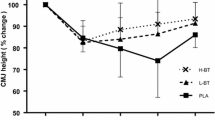
The objective of the study was to evaluate the circulating levels of chemical elements after exercise-induced muscle damage (EIMD) followed by the repeated bout effect (RBE). Seven physically active subjects (26.5 ± 4.0 years) performed two sessions of EIMD (5 sets of 20 drop jumps), the second session 14 days after the first for RBE assessment. Blood collections, countermovement jump (CMJ), squat jump (SJ), and delayed-onset muscle soreness (DOMS) were performed before (Pre), after (Post), and 24, 48, and 72 h after the exercise session. Creatine kinase (CK) was detected by biochemical analysis and the concentration of chemical elements by total reflection X-ray fluorescence (TXRF). Differences between time points and sessions were assessed with two-way ANOVA and the effect size (ES). EIMD induced a reduction in the CMJ at 24 h (P < 0.05) and an increase in DOMS at 24 h (P < 0.01) and 48 h (P < 0.01), and CK at 72 h (P < 0.05). RBE alleviated all symptoms of EIMD in the second session (P > 0.05). EIMD induced a large to very large ES for Zn reduction at 24 h (− 1.37) and 72 h (− 0.93) and Br (− 0.83) at 72 h. RBE presented large to very large ES for the increase in P at 48 h (0.92); Cl at 24 h (1.04); K at 24 h (0.91), 48 h (1.10), and 72 h (0.96); Ca at 72 h (0.92); and Fe at 24 h (0.85). RBE influenced the concentration of elements associated with fatigue (K, Ca, Cl), inflammatory response, and glucose metabolism (Zn).



Similar content being viewed by others
Data Availability
The authors declare that study data are available from the corresponding author at readers’ request.
Code Availability
Not applicable.
References
Howatson G, van Someren KA (2008) The prevention and treatment of exercise-induced muscle damage. Sports Med 38:483–503. https://doi.org/10.2165/00007256-200838060-00004
Damas F, Nosaka K, Libardi CA, Chen TC, Ugrinowitsch C (2016) Susceptibility to exercise-induced muscle damage: a cluster analysis with a large sample. Int J Sports Med 37:633–640. https://doi.org/10.1055/s-0042-100281
Owens DJ, Twist C, Cobley JN, Howatson G, Close GL (2019) Exercise-induced muscle damage: what is it, what causes it and what are the nutritional solutions? Eur J Sport Sci 19:71–85. https://doi.org/10.1080/17461391.2018.1505957
Kanda K, Sugama K, Sakuma J, Kawakami Y, Suzuki K (2014) Evaluation of serum leaking enzymes and investigation into new biomarkers for exercise-induced muscle damage. Exerc Immunol Rev 20:39–54
Clarkson PM, Hubal MJ (2002) Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 81:S52-69. https://doi.org/10.1097/00002060-200211001-00007
Koch AJ, Pereira R, Machado M (2014) The creatine kinase response to resistance exercise. J Musculoskelet Neuronal Interact 14:68–77
Carmona G, Guerrero M, Cusso R, Padulles JM, Moras G, Lloret M, Bedini JL, Cadefau JA (2015) Muscle enzyme and fiber type-specific sarcomere protein increases in serum after inertial concentric-eccentric exercise. Scand J Med Sci Sports 25:e547-557. https://doi.org/10.1111/sms.12363
Brancaccio P, Maffulli N, Limongelli FM (2007) Creatine kinase monitoring in sport medicine. Br Med Bull 81–82:209–230. https://doi.org/10.1093/bmb/ldm014
Chen TC, Huang GL, Hsieh CC, Tseng KW, Tseng WC, Chou TY, Nosaka K (2020) Comparison among three different intensities of eccentric contractions of the elbow flexors resulting in the same strength loss at one day post-exercise for changes in indirect muscle damage markers. Eur J Appl Physiol 120:267–279. https://doi.org/10.1007/s00421-019-04272-w
Chen TC, Liu HW, Russell A, Barthel BL, Tseng KW, Huang MJ, Chou TY, Nosaka K (2020) Large increases in plasma fast skeletal muscle troponin I after whole-body eccentric exercises. J Sci Med Sport 23:776–781. https://doi.org/10.1016/j.jsams.2020.01.011
Coratella G, Chemello A, Schena F (2016) Muscle damage and repeated bout effect induced by enhanced eccentric squats. J Sports Med Phys Fitness 56:1540–1546
Goodall S, Thomas K, Barwood M, Keane K, Gonzalez JT, St Clair Gibson A, Howatson G (2017) Neuromuscular changes and the rapid adaptation following a bout of damaging eccentric exercise. Acta Physiol (Oxf) 220:486–500. https://doi.org/10.1111/apha.12844
Howatson G, Van Someren K, Hortobagyi T (2007) Repeated bout effect after maximal eccentric exercise. Int J Sports Med 28:557–563. https://doi.org/10.1055/s-2007-964866
Hyldahl RD, Chen TC, Nosaka K (2017) Mechanisms and mediators of the skeletal muscle repeated bout effect. Exerc Sport Sci Rev 45:24–33. https://doi.org/10.1249/JES.0000000000000095
Chen TC, Yang TJ, Huang MJ, Wang HS, Tseng KW, Chen HL, Nosaka K (2019) Damage and the repeated bout effect of arm, leg, and trunk muscles induced by eccentric resistance exercises. Scand J Med Sci Sports 29:725–735. https://doi.org/10.1111/sms.13388
Milias GA, Nomikos T, Fragopoulou E, Athanasopoulos S, Antonopoulou S (2006) Effects of baseline serum levels of Se on markers of eccentric exercise-induced muscle injury. BioFactors 26:161–170. https://doi.org/10.1002/biof.5520260301
Malliaropoulos N, Tsitas K, Porfiriadou A, Papalada A, Ames PR, Del Buono A, Lippi G, Maffulli N (2013) Blood phosphorus and magnesium levels in 130 elite track and field athletes. Asian J Sports Med 4:49–53. https://doi.org/10.5812/asjsm.34531
Karakukcu C, Polat Y, Torun YA, Pac AK (2013) The effects of acute and regular exercise on calcium, phosphorus and trace elements in young amateur boxers. Clin Lab 59:557–562. https://doi.org/10.7754/clin.lab.2012.120505
Soria M, Anson M, Escanero JF (2016) Correlation analysis of exercise-induced changes in plasma trace element and hormone levels during incremental exercise in well-trained athletes. Biol Trace Elem Res 170:55–64. https://doi.org/10.1007/s12011-015-0466-5
Speich M, Pineau A, Ballereau F (2001) Minerals, trace elements and related biological variables in athletes and during physical activity. Clin Chim Acta 312:1–11. https://doi.org/10.1016/s0009-8981(01)00598-8
Padoin S, de Freitas VH, Cleto DAM, Zeffa AC, Nakamura FY, Andrello AC, de Paula RS (2020) Effects of futsal demands on serum and salivary levels of trace elements and minerals detected by total reflection X-ray fluorescence. Biol Trace Elem Res 193:73–80. https://doi.org/10.1007/s12011-019-01697-4
Jablan J, Inic S, Stosnach H, Hadziabdic MO, Vujic L, Domijan AM (2017) Level of minerals and trace elements in the urine of the participants of mountain ultra-marathon race. J Trace Elem Med Biol 41:54–59. https://doi.org/10.1016/j.jtemb.2017.02.004
Maynar M, Munoz D, Alves J, Barrientos G, Grijota FJ, Robles MC, Llerena F (2018) Influence of an acute exercise until exhaustion on serum and urinary concentrations of molybdenum, selenium, and zinc in athletes. Biol Trace Elem Res 186:361–369. https://doi.org/10.1007/s12011-018-1327-9
Otag A, Hazar M, Otag I, Gurkan AC, Okan I (2014) Responses of trace elements to aerobic maximal exercise in elite sportsmen. Glob J Health Sci 6:90–96. https://doi.org/10.5539/gjhs.v6n3p90
Maynar M, Llerena F, Grijota FJ, Alves J, Robles MC, Bartolome I, Munoz D (2017) Serum concentration of several trace metals and physical training. J Int Soc Sports Nutr 14:e19. https://doi.org/10.1186/s12970-017-0178-7
Nishiie-Yano R, Hirayama S, Tamura M, Kanemochi T, Ueno T, Hirayama A, Hori A, Ai T, Hirose N, Miida T (2020) Hemolysis is responsible for elevation of serum iron concentration after regular exercises in judo athletes. Biol Trace Elem Res 197:63–69. https://doi.org/10.1007/s12011-019-01981-3
Peeling P, Sim M, Badenhorst CE, Dawson B, Govus AD, Abbiss CR, Swinkels DW, Trinder D (2014) Iron status and the acute post-exercise hepcidin response in athletes. PLoS ONE 9:e93002. https://doi.org/10.1371/journal.pone.0093002
Huang Z, Rose AH, Hoffmann PR (2012) The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16:705–743. https://doi.org/10.1089/ars.2011.4145
Li L, Yang X (2018) The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxid Med Cell Longev 2018:e7580707. https://doi.org/10.1155/2018/7580707
Olechnowicz J, Tinkov A, Skalny A, Suliburska J (2018) Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci 68:19–31. https://doi.org/10.1007/s12576-017-0571-7
Winter WE, Bazydlo LA, Harris NS (2014) The molecular biology of human iron metabolism. Lab Med 45:92–102. https://doi.org/10.1309/lmf28s2gimxnwhmm
Alshammari E, Shafi S, Nurmi-Lawton J, Taylor A, Lanham-New S, Ferns G (2010) Altered antioxidant and trace-element status in adolescent female gymnasts. Int J Sport Nutr Exerc Metab 20:291–298. https://doi.org/10.1123/ijsnem.20.4.291
Tremblay MS, Warburton DE, Janssen I, Paterson DH, Latimer AE, Rhodes RE, Kho ME, Hicks A, Leblanc AG, Zehr L, Murumets K, Duggan M (2011) New Canadian physical activity guidelines. Appl Physiol Nutr Metab 36(36–46):47–58. https://doi.org/10.1139/H11-009
Howatson G, Goodall S, van Someren KA (2009) The influence of cold water immersions on adaptation following a single bout of damaging exercise. Eur J Appl Physiol 105:615–621. https://doi.org/10.1007/s00421-008-0941-1
Davies G, Riemann BL, Manske R (2015) Current concepts of plyometric exercise. Int J Sports Phys Ther 10:760–786
Kamandulis S, Skurvydas A, Snieckus A, Masiulis N, Aagaard P, Dargeviciute G, Brazaitis M (2011) Monitoring markers of muscle damage during a 3 week periodized drop-jump exercise programme. J Sports Sci 29:345–353. https://doi.org/10.1080/02640414.2010.530676
Paulsen G, Mikkelsen UR, Raastad T, Peake JM (2012) Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev 18:42–97
Bosco C, Luhtanen P, Komi PV (1983) A simple method for measurement of mechanical power in jumping. Eur J Appl Physiol Occup Physiol 50:273–282. https://doi.org/10.1007/BF00422166
Cook DB, O’Connor PJ, Oliver SE, Lee Y (1998) Sex differences in naturally occurring leg muscle pain and exertion during maximal cycle ergometry. Int J Neurosci 95:183–202. https://doi.org/10.3109/00207459809003340
Espinoza-Quinones FR, Modenes AN, Dos Santos J, Obregon PL, de Pauli AR (2018) Insights on limits of detection, precision and accuracy in TXRF analysis of trace and major elements in environmental solid suspensions. Appl Radiat Isot 137:80–90. https://doi.org/10.1016/j.apradiso.2018.03.016
Rodriguez-Saldana V, Fobil J, Basu N (2020) Lead (Pb) exposure assessment in dried blood spots using Total Reflection X-Ray Fluorescence (TXRF). Environ Res 10:110444. https://doi.org/10.1016/j.envres.2020.110444
Schober P, Bossers SM, Schwarte LA (2018) Statistical significance versus clinical importance of observed effect sizes: what do P values and confidence intervals really represent? Anesth Analg 126:1068–1072. https://doi.org/10.1213/ANE.0000000000002798
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc 82:591–605. https://doi.org/10.1111/j.1469-185X.2007.00027.x
Wang G (2016) Chloride flux in phagocytes. Immunol Rev 273:219–231. https://doi.org/10.1111/imr.12438
Zorbas YG, Naexu KA, Federenko YF (1992) Blood serum biochemical changes in physically conditioned and unconditioned subjects during bed rest and chronic hyperhydration. Clin Exp Pharmacol Physiol 19:137–145. https://doi.org/10.1111/j.1440-1681.1992.tb00432.x
McKenna MJ, Heigenhauser GJ, McKelvie RS, MacDougall JD, Jones NL (1997) Sprint training enhances ionic regulation during intense exercise in men. J Physiol 501(Pt3):687–702. https://doi.org/10.1111/j.1469-7793.1997.687bm.x
McKenna MJ, Bangsbo J (1985) Renaud JM (2008) Muscle K+, Na+, and Cl disturbances and Na+-K+ pump inactivation: implications for fatigue. J Appl Physiol 104:288–295. https://doi.org/10.1152/japplphysiol.01037.2007
Cairns SP, Lindinger MI (2008) Do multiple ionic interactions contribute to skeletal muscle fatigue? J Physiol 586:4039–4054. https://doi.org/10.1113/jphysiol.2008.155424
McKelvie RS, Lindinger MI, Heigenhauser GJ, Sutton JR, Jones NL (1989) Renal responses to exercise-induced lactic acidosis. Am J Physiol 257:R102-108. https://doi.org/10.1152/ajpregu.1989.257.1.R102
Shushakov V, Stubbe C, Peuckert A, Endeward V, Maassen N (2007) The relationships between plasma potassium, muscle excitability and fatigue during voluntary exercise in humans. Exp Physiol 92:705–715. https://doi.org/10.1113/expphysiol.2006.036384
Nielsen JJ, Mohr M, Klarskov C, Kristensen M, Krustrup P, Juel C, Bangsbo J (2004) Effects of high-intensity intermittent training on potassium kinetics and performance in human skeletal muscle. J Physiol 554:857–870. https://doi.org/10.1113/jphysiol.2003.050658
Wilson JR, Kapoor SC (1985) Krishna GG (1994) Contribution of potassium to exercise-induced vasodilation in humans. J Appl Physiol 77:2552–2557. https://doi.org/10.1152/jappl.1994.77.6.2552
Lindinger MI (1995) Potassium regulation during exercise and recovery in humans: implications for skeletal and cardiac muscle. J Mol Cell Cardiol 27:1011–1022. https://doi.org/10.1016/0022-2828(95)90070-5
Wang W, Soltero L, Zhang P, Huang XR, Lan HY, Adrogue HJ (2007) Renal inflammation is modulated by potassium in chronic kidney disease: possible role of Smad7. Am J Physiol Renal Physiol 293:F1123-1130. https://doi.org/10.1152/ajprenal.00104.2007
Tabbaa A, Shaker M, Lopez R, Hoshemand K, Nobili V, Alkhouri N (2015) Low serum potassium levels associated with disease severity in children with nonalcoholic fatty liver disease. Pediatr Gastroenterol Hepatol Nutr 18:168–174. https://doi.org/10.5223/pghn.2015.18.3.168
Staniszewski K, Lygre H, Berge T, Rosen A (2019) Serum analysis in patients with temporomandibular disorders: a controlled cross-sectional study in Norway. Pain Res Manag 2019:1360725. https://doi.org/10.1155/2019/1360725
Tsantoulas C (2015) Emerging potassium channel targets for the treatment of pain. Curr Opin Support Palliat Care 9:147–154. https://doi.org/10.1097/SPC.0000000000000131
Cheng AJ, Place N, Westerblad H (2018) Molecular basis for exercise-induced fatigue: the importance of strictly controlled cellular Ca(2+) handling. Cold Spring Harb Perspect Med 8:849. https://doi.org/10.1101/cshperspect.a029710
Beaton LJ, Tarnopolsky MA, Phillips SM (2002) Contraction-induced muscle damage in humans following calcium channel blocker administration. J Physiol 544:849–859. https://doi.org/10.1113/jphysiol.2002.022350
Buratti P, Gammella E, Rybinska I, Cairo G, Recalcati S (2015) Recent advances in iron metabolism: relevance for health, exercise, and performance. Med Sci Sports Exerc 47:1596–1604. https://doi.org/10.1249/MSS.0000000000000593
Hinton PS (2014) Iron and the endurance athlete. Appl Physiol Nutr Metab 39:1012–1018. https://doi.org/10.1139/apnm-2014-0147
Bauer P, Zeissler S, Walscheid R, Frech T, Hillebrecht A (2018) Acute effects of high-intensity exercise on hematological and iron metabolic parameters in elite male and female dragon boating athletes. Phys Sportsmed 46:335–341. https://doi.org/10.1080/00913847.2018.1482187
Goto K, Kasai N, Kojima C, Ishibashi A (2018) Postexercise serum hepcidin response to repeated sprint exercise under normoxic and hypoxic conditions. Appl Physiol Nutr Metab 43:221–226. https://doi.org/10.1139/apnm-2017-0418
McClung JP, Martini S, Murphy NE, Montain SJ, Margolis LM, Thrane I, Spitz MG, Blatny JM, Young AJ, Gundersen Y, Pasiakos SM (2013) Effects of a 7-day military training exercise on inflammatory biomarkers, serum hepcidin, and iron status. Nutr J 12:141. https://doi.org/10.1186/1475-2891-12-141
Goto K, Kojima C, Kasai N, Sumi D, Hayashi N, Hwang H (2020) Resistance exercise causes greater serum hepcidin elevation than endurance (cycling) exercise. PLoS ONE 15:e0228766. https://doi.org/10.1371/journal.pone.0228766
Myers SA, Nield A, Chew GS, Myers MA (2013) The zinc transporter, Slc39a7 (Zip7) is implicated in glycaemic control in skeletal muscle cells. PLoS ONE 8:e79316. https://doi.org/10.1371/journal.pone.0079316
Hernandez-Camacho JD, Vicente-Garcia C, Parsons DS, Navas-Enamorado I (2020) Zinc at the crossroads of exercise and proteostasis. Redox Biol 35:e101529. https://doi.org/10.1016/j.redox.2020.101529
Jackson MJ, Jones DA, Edwards RH (1982) Tissue zinc levels as an index of body zinc status. Clin Physiol 2:333–343. https://doi.org/10.1111/j.1475-097x.1982.tb00038.x
Soria M, Gonzalez-Haro C, Anson M, Lopez-Colon JL, Escanero JF (2015) Plasma levels of trace elements and exercise induced stress hormones in well-trained athletes. J Trace Elem Med Biol 31:113–119. https://doi.org/10.1016/j.jtemb.2015.04.004
Granell J (2014) Zinc and copper changes in serum and urine after aerobic endurance and muscular strength exercise. J Sports Med Phys Fitness 54:232–237
Volpe SL, Lowe NM, Woodhouse LR, King JC (2007) Effect of maximal exercise on the short-term kinetics of zinc metabolism in sedentary men. Br J Sports Med 41:156–161. https://doi.org/10.1136/bjsm.2006.030346
Acknowledgements
The authors would like to thank the Edge academy (Londrina-PR) for allowing the use of their facilities for evaluation and data collection.
Funding
The study had financial support from Coordination for the Improvement of Higher Education Personnel (CAPES-Brazil) for the Master’s scholarship to SP (grant n. 1798841/2018).
Author information
Authors and Affiliations
Contributions
S.S. Dias: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing the original draft.
M.G. Weber: methodology, investigation, data curation, writing the original draft.
S. Padoin: conceptualization, methodology, investigation, resources.
A.C. Andrello: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing the original draft.
E.I. Jussiani: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing the original draft.
S.de Paula Ramos: supervision, project administration, resources, writing the original data.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Ethics Committee for Studies Involving Human Beings at the State University of Londrina (protocol No. 2,650,252).
Consent to Participate
The participants signed an informed consent form before the study started.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dias, S.S., Weber, M.G., Padoin, S. et al. Circulating Concentration of Chemical Elements During Exercise-Induced Muscle Damage and the Repeated Bout Effect. Biol Trace Elem Res 200, 1060–1070 (2022). https://doi.org/10.1007/s12011-021-02737-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02737-8