Abstract
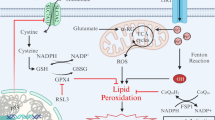
The discovery of the role of autophagy, particularly the selective form like ferritinophagy, in promoting cells to undergo ferroptosis has inspired us to investigate functional connections between diseases and cell death. Ferroptosis is a novel model of procedural cell death characterized by the accumulation of iron-dependent reactive oxygen species (ROS), mitochondrial dysfunction, and neuroinflammatory response. Based on ferroptosis, the study of ferritinophagy is particularly important. In recent years, extensive research has elucidated the role of ferroptosis and ferritinophagy in neurological diseases and anemia, suggesting their potential as therapeutic targets. Besides, the global emergence and rapid transmission of COVID-19, which is caused by SARS-CoV-2, represents a considerable risk to public health worldwide. The potential involvement of ferroptosis in the pathophysiology of brain injury associated with COVID-19 is still unclear. This review summarizes the pathophysiological changes of ferroptosis and ferritinophagy in neurological diseases, anemia, and COVID-19, and hypothesizes that ferritinophagy may be a potential mechanism of ferroptosis. Advancements in these fields will enhance our comprehension of methods to prevent and address neurological disorders, anemia, and COVID-19.








Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ACSL4:
-
acyl CoA synthase long-chain family member 4
- ACE2:
-
angiotensin-converting enzyme 2
- ATP:
-
abundant adenosine triphosphate
- AD:
-
Alzheimer’s disease
- Aβ:
-
amyloid-β
- ALS:
-
amyotrophic lateral sclerosis
- BH4:
-
tetrahydrobiopterin
- BH2:
-
dihydrobiopterin
- BBB:
-
blood-brain barrier
- CoQ10:
-
ubiquinone 10
- COVID-19:
-
coronavirus disease 2019
- CQ:
-
chloroquine
- DMT1:
-
divalent metal transporter 1
- DFP:
-
deferiprone
- FTH1:
-
ferritin heavy chain 1
- FPN:
-
ferroportin
- FSP1:
-
ferroptosis suppressor protein 1
- FTMT:
-
mitochondrial ferritin
- FALS:
-
familial ALS
- Fer-1:
-
Ferrostatin-1
- GSH:
-
glutathione
- GPX4:
-
glutathione peroxidase 4
- HSC:
-
hepatic stellate cell
- HD:
-
Huntington’s disease
- HTT:
-
Huntington
- HO-1:
-
heme-oxygenase-1
- IREBP2:
-
iron response element binding protein 2
- IS:
-
ischemic stroke
- IRE:
-
iron response element
- IRP1/2:
-
iron regulatory protein-1and -2
- IL-6:
-
inflammatory cytokines interleukin
- ICP-MS:
-
inductively coupled plasma mass spectrometry
- JNK:
-
c-Jun N-terminal kinase
- LPCAT3:
-
lysophosphatidylcholine acyltransferase 3
- LIP:
-
labile iron pool
- LA:
-
α-lipoic acid
- LAP:
-
α-Lipoic acid-plus
- MAPK8:
-
mitogen-activated protein kinase 8
- MCAO/R:
-
middle cerebral artery occlusion/reoxygenation
- MDA:
-
malondialdehyde
- MRI:
-
magnetic resonance imaging
- mHTT:
-
mutant huntingtin
- NCOA4:
-
nuclear receptor coactivator 4
- Nrf2:
-
Nuclear Factor Erythroid 2-Related Factor 2
- ND:
-
neurodegenerative diseases
- NBIA:
-
neurodegenerative with brain accumulation
- NFTs:
-
neurofibrillary tangles
- OGD/R:
-
oxygen-glucose deprivation/reoxygenation
- 8-OHdG:
-
8-Hydroxy-2-deoxyguanine
- PUFAs:
-
polyunsaturated fatty acids
- PLOOHs:
-
phospholipid hydroperoxides
- PD:
-
Parkinson’s disease
- ROS:
-
reactive oxygen species
- RSL3:
-
RAS-selective lethal 3
- STEAP3:
-
six transmembrane epithelial antigens of the prostate 3
- SARS-CoV-2:
-
syndrome coronavirus 2
- SENDA:
-
static encephalopathy of childhood with neurodegeneration in adulthood
- SPs:
-
senile plaques
- SALS:
-
sporadic ALS
- SAH:
-
subarachnoid hemorrhage
- TEM:
-
transmission electron microscopy
- TF:
-
transferrin
- TfR1:
-
transferrin receptor 1
- TBI:
-
traumatic brain injury
- TNF-α:
-
tumor necrosis factor alpha
- WDR45:
-
WD repeat domain 45
- Zip8/14:
-
Zinc-Iron regulatory protein family 8/14
References
Li Y, McGreal S, Zhao J, Huang R, Zhou Y, Zhong H, Xia M, Ding WX (2016) A cell-based quantitative high-throughput image screening identified novel autophagy modulators. Pharmacol Res 110:35–49. https://doi.org/10.1016/j.phrs.2016.05.004
Zhang L, Li J, Ma J, Chen X, Chen K, Jiang Z, Zong L, Yu S, Li X, Xu Q, Lei J, Duan W, Li W, Shan T, Ma Q, Shen X (2016) The relevance of Nrf2 pathway and autophagy in pancreatic cancer cells upon stimulation of reactive oxygen species. Oxid Med Cell Longev 2016:3897250. https://doi.org/10.1155/2016/3897250
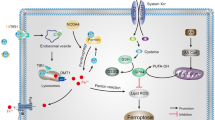
Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X (2016) Ferroptosis is an autophagic cell death process. Cell Res 26:1021–1032. https://doi.org/10.1038/cr.2016.95
Li W, Li W, Wang Y, Leng Y, Xia Z (2021) Inhibition of DNMT-1 alleviates ferroptosis through NCOA4 mediated ferritinophagy during diabetes myocardial ischemia/reperfusion injury. Cell Death Discov 7:267. https://doi.org/10.1038/s41420-021-00656-0
Walker T, Michaelides C, Ekonomou A, Geraki K, Parkes HG, Suessmilch M, Herlihy AH, Crum WR, So PW (2016) Dissociation between iron accumulation and ferritin upregulation in the aged substantia nigra: attenuation by dietary restriction. Aging (Albany NY) 8:2488–2508. https://doi.org/10.18632/aging.101069
Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, Ding H, Tan S, Chen A, Zhang F, Zheng S (2020) RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy 16:1482–1505. https://doi.org/10.1080/15548627.2019.1687985
Santana-Codina N, Mancias JD (2018) The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals (Basel) 11. https://doi.org/10.3390/ph11040114
Wu S, Pan R, Lu J, Wu X, Xie J, Tang H, Li X (2022) Development and verification of a prognostic ferroptosis-related gene model in triple-negative breast cancer. Front Oncol 12:896927. https://doi.org/10.3389/fonc.2022.896927
Fang X, Ardehali H, Min J, Wang F (2023) The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol 20:7–23. https://doi.org/10.1038/s41569-022-00735-4
Yoshida M, Minagawa S, Araya J, Sakamoto T, Hara H, Tsubouchi K, Hosaka Y, Ichikawa A, Saito N, Kadota T, Sato N, Kurita Y, Kobayashi K, Ito S, Utsumi H, Wakui H, Numata T, Kaneko Y, Mori S et al (2019) Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun 10:3145. https://doi.org/10.1038/s41467-019-10991-7
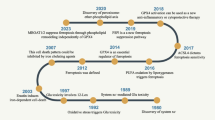
Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC (2014) Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509:105–109. https://doi.org/10.1038/nature13148
Quiles Del Rey M, Mancias JD (2019) NCOA4-Mediated ferritinophagy: a potential link to neurodegeneration. Front Neurosci 13:238. https://doi.org/10.3389/fnins.2019.00238
Nai A, Lidonnici MR, Federico G, Pettinato M, Olivari V, Carrillo F, Geninatti Crich S, Ferrari G, Camaschella C, Silvestri L, Carlomagno F (2021) NCOA4-mediated ferritinophagy in macrophages is crucial to sustain erythropoiesis in mice. Haematologica 106:795–805. https://doi.org/10.3324/haematol.2019.241232
Arosio P, Levi S (2002) Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med 33:457–463. https://doi.org/10.1016/s0891-5849(02)00842-0
Bekker-Jensen S, Rendtlew Danielsen J, Fugger K, Gromova I, Nerstedt A, Lukas C, Bartek J, Lukas J, Mailand N (2010) HERC2 coordinates ubiquitin-dependent assembly of DNA repair factors on damaged chromosomes. Nat Cell Biol 12:80–86. https://doi.org/10.1038/ncb2008
Moroishi T, Yamauchi T, Nishiyama M, Nakayama KI (2014) HERC2 targets the iron regulator FBXL5 for degradation and modulates iron metabolism. J Biol Chem 289:16430–16441. https://doi.org/10.1074/jbc.M113.541490
Mancias JD, Pontano Vaites L, Nissim S, Biancur DE, Kim AJ, Wang X, Liu Y, Goessling W, Kimmelman AC, Harper JW (2015) Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife 4. https://doi.org/10.7554/eLife.10308
Philpott CC (2020) Iron on the move: mobilizing liver iron via NCOA4. Blood 136:2604–2605. https://doi.org/10.1182/blood.2020007971
Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B (2020) Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol 36:101670. https://doi.org/10.1016/j.redox.2020.101670
Harris IS, Endress JE, Coloff JL, Selfors LM, McBrayer SK, Rosenbluth JM, Takahashi N, Dhakal S, Koduri V, Oser MG, Schauer NJ, Doherty LM, Hong AL, Kang YP, Younger ST, Doench JG, Hahn WC, Buhrlage SJ, DeNicola GM et al (2019) Deubiquitinases maintain protein homeostasis and survival of cancer cells upon glutathione depletion. Cell Metab 29:1166–1181.e6. https://doi.org/10.1016/j.cmet.2019.01.020
Li C, Sun G, Chen B, Xu L, Ye Y, He J, Bao Z, Zhao P, Miao Z, Zhao L, Hu J, You Y, Liu N, Chao H, Ji J (2021) Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol Res 174:105933. https://doi.org/10.1016/j.phrs.2021.105933
Ajoolabady A, Aslkhodapasandhokmabad H, Libby P, Tuomilehto J, Lip GYH, Penninger JM, Richardson DR, Tang D, Zhou H, Wang S, Klionsky DJ, Kroemer G, Ren J (2021) Ferritinophagy and ferroptosis in the management of metabolic diseases. Trends Endocrinol Metab 32:444–462. https://doi.org/10.1016/j.tem.2021.04.010
Zhang Z, Yao Z, Wang L, Ding H, Shao J, Chen A, Zhang F, Zheng S (2018) Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy 14:2083–2103. https://doi.org/10.1080/15548627.2018.1503146
Gryzik M, Asperti M, Denardo A, Arosio P, Poli M (2021) NCOA4-mediated ferritinophagy promotes ferroptosis induced by erastin, but not by RSL3 in HeLa cells. Biochim Biophys Acta Mol Cell Res 1868:118913. https://doi.org/10.1016/j.bbamcr.2020.118913
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, Tang D (2016) Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12:1425–1428. https://doi.org/10.1080/15548627.2016.1187366
Kuang F, Liu J, Tang D, Kang R (2020) Oxidative damage and antioxidant defense in ferroptosis. Front Cell Dev Biol 8:586578. https://doi.org/10.3389/fcell.2020.586578
Hirschhorn T, Stockwell BR (2019) The development of the concept of ferroptosis. Free Radic Biol Med 133:130–143. https://doi.org/10.1016/j.freeradbiomed.2018.09.043
Yang WS, Stockwell BR (2016) Ferroptosis: death by lipid peroxidation. Trends Cell Biol 26:165–176. https://doi.org/10.1016/j.tcb.2015.10.014
Stockwell BR, Jiang X (2020) The chemistry and biology of ferroptosis. Cell Chem Biol 27:365–375. https://doi.org/10.1016/j.chembiol.2020.03.013
Sun X, Niu X, Chen R, He W, Chen D, Kang R, Tang D (2016) Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology 64:488–500. https://doi.org/10.1002/hep.28574
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22:266–282. https://doi.org/10.1038/s41580-020-00324-8
Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E, Ye LF, Tyurina YY, Lin AJ, Shchepinov MS, Chan AY, Peguero-Pereira E, Fomich MA, Daniels JD, Bekish AV et al (2018) FINO(2) initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol 14:507–515. https://doi.org/10.1038/s41589-018-0031-6
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171:273–285. https://doi.org/10.1016/j.cell.2017.09.021
Liu J, Kang R, Tang D (2021) Signaling pathways and defense mechanisms of ferroptosis. Febs j. https://doi.org/10.1111/febs.16059
Gaschler MM, Stockwell BR (2017) Lipid peroxidation in cell death. Biochem Biophys Res Commun 482:419–425. https://doi.org/10.1016/j.bbrc.2016.10.086
Wang Y, Wei Z, Pan K, Li J, Chen Q (2020) The function and mechanism of ferroptosis in cancer. Apoptosis 25:786–798. https://doi.org/10.1007/s10495-020-01638-w
Bayır H, Anthonymuthu TS, Tyurina YY, Patel SJ, Amoscato AA, Lamade AM, Yang Q, Vladimirov GK, Philpott CC, Kagan VE (2020) Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chem Biol 27:387–408. https://doi.org/10.1016/j.chembiol.2020.03.014
Reichert CO, de Freitas FA, Sampaio-Silva J, Rokita-Rosa L, Barros PL, Levy D, Bydlowski SP (2020) Ferroptosis mechanisms involved in neurodegenerative diseases. Int J Mol Sci 21. https://doi.org/10.3390/ijms21228765
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S et al (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13:81–90. https://doi.org/10.1038/nchembio.2238
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA et al (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13:91–98. https://doi.org/10.1038/nchembio.2239
Konstorum A, Tesfay L, Paul BT, Torti FM, Laubenbacher RC, Torti SV (2020) Systems biology of ferroptosis: a modeling approach. J Theor Biol 493:110222. https://doi.org/10.1016/j.jtbi.2020.110222
Conrad M, Pratt DA (2019) The chemical basis of ferroptosis. Nat Chem Biol 15:1137–1147. https://doi.org/10.1038/s41589-019-0408-1
Kuhn H, Banthiya S, van Leyen K (2015) Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta 1851:308–330. https://doi.org/10.1016/j.bbalip.2014.10.002
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 113:E4966–E4975. https://doi.org/10.1073/pnas.1603244113
Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K (2008) Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke 39:2538–2543. https://doi.org/10.1161/strokeaha.108.514927
Singh NK, Rao GN (2019) Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog Lipid Res 73:28–45. https://doi.org/10.1016/j.plipres.2018.11.001
Dolma S, Lessnick SL, Hahn WC, Stockwell BR (2003) Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3:285–296. https://doi.org/10.1016/s1535-6108(03)00050-3
Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR (2014) Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3:e02523. https://doi.org/10.7554/eLife.02523
Lin CH, Lin PP, Lin CY, Lin CH, Huang CH, Huang YJ, Lane HY (2016) Decreased mRNA expression for the two subunits of system xc(-), SLC3A2 and SLC7A11, in WBC in patients with schizophrenia: evidence in support of the hypo-glutamatergic hypothesis of schizophrenia. J Psychiatr Res 72:58–63. https://doi.org/10.1016/j.jpsychires.2015.10.007
Ekoue DN, He C, Diamond AM, Bonini MG (2017) Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim Biophys Acta Bioenerg 1858:628–632. https://doi.org/10.1016/j.bbabio.2017.01.006
Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, Mehr L, Aichler M, Walch A, Lamp D, Jastroch M, Miyamoto S, Wurst W, Ursini F, Arnér ESJ et al (2018) Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172:409–422.e21. https://doi.org/10.1016/j.cell.2017.11.048
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Ursini F, Maiorino M, Gregolin C (1985) The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta 839:62–70. https://doi.org/10.1016/0304-4165(85)90182-5
Eaton JK, Furst L, Cai LL, Viswanathan VS, Schreiber SL (2020) Structure-activity relationships of GPX4 inhibitor warheads. Bioorg Med Chem Lett 30:127538. https://doi.org/10.1016/j.bmcl.2020.127538
Eaton JK, Furst L, Ruberto RA, Moosmayer D, Hilpmann A, Ryan MJ, Zimmermann K, Cai LL, Niehues M, Badock V, Kramm A, Chen S, Hillig RC, Clemons PA, Gradl S, Montagnon C, Lazarski KE, Christian S, Bajrami B et al (2020) Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat Chem Biol 16:497–506. https://doi.org/10.1038/s41589-020-0501-5
Gsaller F, Hortschansky P, Beattie SR, Klammer V, Tuppatsch K, Lechner BE, Rietzschel N, Werner ER, Vogan AA, Chung D, Mühlenhoff U, Kato M, Cramer RA, Brakhage AA, Haas H (2014) The Janus transcription factor HapX controls fungal adaptation to both iron starvation and iron excess. Embo j 33:2261–2276. https://doi.org/10.15252/embj.201489468
Frazer DM, Anderson GJ (2014) The regulation of iron transport. Biofactors 40:206–214. https://doi.org/10.1002/biof.1148
Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y (2016) Regulators of Iron Homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci 41:274–286. https://doi.org/10.1016/j.tibs.2015.11.012
Ganz T (2013) Systemic iron homeostasis. Physiol Rev 93:1721–1741. https://doi.org/10.1152/physrev.00008.2013
Zhang J, Li X, Olmedo M, Holdorf AD, Shang Y, Artal-Sanz M, Yilmaz LS, Walhout AJM (2019) A delicate balance between bacterial iron and reactive oxygen species supports optimal C. elegans development. Cell Host Microbe 26:400–411.e3. https://doi.org/10.1016/j.chom.2019.07.010
Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, Jiang X (2019) Role of Mitochondria in Ferroptosis. Mol Cell 73:354–363.e3. https://doi.org/10.1016/j.molcel.2018.10.042
Tang LJ, Zhou YJ, Xiong XM, Li NS, Zhang JJ, Luo XJ, Peng J (2021) Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med 162:339–352. https://doi.org/10.1016/j.freeradbiomed.2020.10.307
Gammella E, Recalcati S, Rybinska I, Buratti P, Cairo G (2015) Iron-induced damage in cardiomyopathy: oxidative-dependent and independent mechanisms. Oxid Med Cell Longev 2015:230182. https://doi.org/10.1155/2015/230182
Lv H, Shang P (2018) The significance, trafficking and determination of labile iron in cytosol, mitochondria and lysosomes. Metallomics 10:899–916. https://doi.org/10.1039/c8mt00048d
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D (2016) Ferroptosis: process and function. Cell Death Differ 23:369–379. https://doi.org/10.1038/cdd.2015.158
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H et al (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16:1180–1191. https://doi.org/10.1038/ncb3064
Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575:688–692. https://doi.org/10.1038/s41586-019-1705-2
Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, Mourão A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A et al (2019) FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575:693–698. https://doi.org/10.1038/s41586-019-1707-0
Elguindy MM, Nakamaru-Ogiso E (2015) Apoptosis-inducing factor (AIF) and its family member protein, AMID, are rotenone-sensitive NADH: ubiquinone oxidoreductases (NDH-2). J Biol Chem 290:20815–20826. https://doi.org/10.1074/jbc.M115.641498
Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X, Anastasov N, Kössl J, Brandner S, Daniels JD, Schmitt-Kopplin P, Hauck SM, Stockwell BR, Hadian K, Schick JA (2020) GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci 6:41–53. https://doi.org/10.1021/acscentsci.9b01063
Dai E, Meng L, Kang R, Wang X, Tang D (2020) ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem Biophys Res Commun 522:415–421. https://doi.org/10.1016/j.bbrc.2019.11.110
Dai E, Zhang W, Cong D, Kang R, Wang J, Tang D (2020) AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem Biophys Res Commun 523:966–971. https://doi.org/10.1016/j.bbrc.2020.01.066
Chen X, Yu C, Kang R, Kroemer G, Tang D (2021) Cellular degradation systems in ferroptosis. Cell Death Differ 28:1135–1148. https://doi.org/10.1038/s41418-020-00728-1
Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, Qin C, Sun F, Shi Z, Zhu Y, Jiang S, Lu L (2020) Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 30:343–355. https://doi.org/10.1038/s41422-020-0305-x
Yesudhas D, Srivastava A, Gromiha MM (2021) COVID-19 outbreak: history, mechanism, transmission, structural studies and therapeutics. Infection 49:199–213. https://doi.org/10.1007/s15010-020-01516-2
Arbour N, Day R, Newcombe J, Talbot PJ (2000) Neuroinvasion by human respiratory coronaviruses. J Virol 74:8913–8921. https://doi.org/10.1128/jvi.74.19.8913-8921.2000
Abobaker A, Raba AA (2021) Does COVID-19 affect male fertility? World J Urol 39:975–976. https://doi.org/10.1007/s00345-020-03208-w
Divani AA, Andalib S, Biller J, Di Napoli M, Moghimi N, Rubinos CA, Nobleza CO, Sylaja PN, Toledano M, Lattanzi S, McCullough LD, Cruz-Flores S, Torbey M, Azarpazhooh MR (2020) Central nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep 20:60. https://doi.org/10.1007/s11910-020-01079-7
Beaud V, Crottaz-Herbette S, Dunet V, Vaucher J, Bernard-Valnet R, Du Pasquier R, Bart PA, Clarke S (2021) Pattern of cognitive deficits in severe COVID-19. J Neurol Neurosurg Psychiatry 92:567–568. https://doi.org/10.1136/jnnp-2020-325173
Habib HM, Ibrahim S, Zaim A, Ibrahim WH (2021) The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother 136:111228. https://doi.org/10.1016/j.biopha.2021.111228
Moreira AC, Mesquita G, Gomes MS (2020) Ferritin: an inflammatory player keeping iron at the core of pathogen-host interactions. Microorganisms 8. https://doi.org/10.3390/microorganisms8040589
Cassat JE, Skaar EP (2013) Iron in infection and immunity. Cell Host Microbe 13:509–519. https://doi.org/10.1016/j.chom.2013.04.010
Colafrancesco S, Alessandri C, Conti F, Priori R (2020) COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev 19:102573. https://doi.org/10.1016/j.autrev.2020.102573
Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, Sougrat R, Morgenstern A, Galy B, Hentze MW, Lazaro FJ, Rouault TA, Meyron-Holtz EG (2010) Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 116:1574–1584. https://doi.org/10.1182/blood-2009-11-253815
Jia F, Liu H, Kang S (2021) NCOA4-mediated ferritinophagy: a vicious culprit in COVID-19 pathogenesis? Front Mol Biosci 8:761793. https://doi.org/10.3389/fmolb.2021.761793
Khan R, Naseem T, Hussain MJ, Hussain MA, Malik SS (2020) Possible potential outcomes from COVID-19 complications on testes: lesson from SARS infection. J Coll Physicians Surg Pak 30:118–120. https://doi.org/10.29271/jcpsp.2020.supp2.118
Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, Liu S, Yang JK (2020) Gender Differences in patients with COVID-19: focus on severity and mortality. Front Public Health 8:152. https://doi.org/10.3389/fpubh.2020.00152
Santambrogio P, Biasiotto G, Sanvito F, Olivieri S, Arosio P, Levi S (2007) Mitochondrial ferritin expression in adult mouse tissues. J Histochem Cytochem 55:1129–1137. https://doi.org/10.1369/jhc.7A7273.2007
Campanella A, Rovelli E, Santambrogio P, Cozzi A, Taroni F, Levi S (2009) Mitochondrial ferritin limits oxidative damage regulating mitochondrial iron availability: hypothesis for a protective role in Friedreich ataxia. Hum Mol Genet 18:1–11. https://doi.org/10.1093/hmg/ddn308
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13:1045–1060. https://doi.org/10.1016/s1474-4422(14)70117-6
Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR (2004) Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5:863–873. https://doi.org/10.1038/nrn1537
Angelova DM, Brown DR (2015) Iron, aging, and neurodegeneration. Metals 5:2070–2092. https://doi.org/10.3390/met5042070
Biasiotto G, Di Lorenzo D, Archetti S, Zanella I (2016) Iron and neurodegeneration: is ferritinophagy the link? Mol Neurobiol 53:5542–5574. https://doi.org/10.1007/s12035-015-9473-y
Ullrich SR, González C, Poehlein A, Tischler JS, Daniel R, Schlömann M, Holmes DS, Mühling M (2016) Gene loss and horizontal gene transfer contributed to the genome evolution of the extreme acidophile “Ferrovum”. Front Microbiol 7:797. https://doi.org/10.3389/fmicb.2016.00797
Anastassova N, Aluani D, Hristova-Avakumova N, Tzankova V, Kondeva-Burdina M, Rangelov M, Todorova N, Yancheva D (2022) Study on the neuroprotective, radical-scavenging and MAO-B inhibiting properties of new benzimidazole arylhydrazones as potential multi-target drugs for the treatment of parkinson’s disease. Antioxidants (Basel) 11. https://doi.org/10.3390/antiox11050884
Sandström KO, Baltzersen OB, Marsman A, Lemvigh CK, Boer VO, Bojesen KB, Nielsen M, Lundell H, Sulaiman DK, Sørensen ME, Fagerlund B, Lahti AC, Syeda WT, Pantelis C, Petersen ET, Glenthøj BY, Siebner HR, Ebdrup BH (2022) Add-on memantine to dopamine antagonism to improve negative symptoms at first psychosis- the AMEND trial protocol. Front Psychiatry 13:889572. https://doi.org/10.3389/fpsyt.2022.889572
Ito S, Yamanaka Y, Ojika M, Wakamatsu K (2016) The metabolic fate of ortho-Quinones derived from catecholamine metabolites. Int J Mol Sci 17. https://doi.org/10.3390/ijms17020164
Saitsu H, Nishimura T, Muramatsu K, Kodera H, Kumada S, Sugai K, Kasai-Yoshida E, Sawaura N, Nishida H, Hoshino A, Ryujin F, Yoshioka S, Nishiyama K, Kondo Y, Tsurusaki Y, Nakashima M, Miyake N, Arakawa H, Kato M et al (2013) De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet 45:445–449e1. https://doi.org/10.1038/ng.2562
Seibler P, Burbulla LF, Dulovic M, Zittel S, Heine J, Schmidt T, Rudolph F, Westenberger A, Rakovic A, Münchau A, Krainc D, Klein C (2018) Iron overload is accompanied by mitochondrial and lysosomal dysfunction in WDR45 mutant cells. Brain 141:3052–3064. https://doi.org/10.1093/brain/awy230
Lan B, Ge JW, Cheng SW, Zheng XL, Liao J, He C, Rao ZQ, Wang GZ (2020) Extract of Naotaifang, a compound Chinese herbal medicine, protects neuron ferroptosis induced by acute cerebral ischemia in rats. J Integr Med 18:344–350. https://doi.org/10.1016/j.joim.2020.01.008
Au A, Griffiths LR, Irene L, Kooi CW, Wei LK (2017) The impact of APOA5, APOB, APOC3 and ABCA1 gene polymorphisms on ischemic stroke: evidence from a meta-analysis. Atherosclerosis 265:60–70. https://doi.org/10.1016/j.atherosclerosis.2017.08.003
Brouns R, De Deyn PP (2009) The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 111:483–495. https://doi.org/10.1016/j.clineuro.2009.04.001
Dávalos A, Castillo J, Marrugat J, Fernandez-Real JM, Armengou A, Cacabelos P, Rama R (2000) Body iron stores and early neurologic deterioration in acute cerebral infarction. Neurology 54:1568–1574. https://doi.org/10.1212/wnl.54.8.1568
Patt A, Horesh IR, Berger EM, Harken AH, Repine JE (1990) Iron depletion or chelation reduces ischemia/reperfusion-induced edema in gerbil brains. J Pediatr Surg 25:224–227. https://doi.org/10.1016/0022-3468(90)90407-z
Hanson LR, Roeytenberg A, Martinez PM, Coppes VG, Sweet DC, Rao RJ, Marti DL, Hoekman JD, Matthews RB, Frey WH 2nd, Panter SS (2009) Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther 330:679–686. https://doi.org/10.1124/jpet.108.149807
Zhang Y, Lu X, Tai B, Li W, Li T (2021) Ferroptosis and its multifaceted roles in cerebral stroke. Front Cell Neurosci 15:615372. https://doi.org/10.3389/fncel.2021.615372
Speer RE, Karuppagounder SS, Basso M, Sleiman SF, Kumar A, Brand D, Smirnova N, Gazaryan I, Khim SJ, Ratan RR (2013) Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by “antioxidant” metal chelators: from ferroptosis to stroke. Free Radic Biol Med 62:26–36. https://doi.org/10.1016/j.freeradbiomed.2013.01.026
Chang CF, Cho S, Wang J (2014) (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol 1:258–271. https://doi.org/10.1002/acn3.54
Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, Liuyang ZY, Roisman L, Zhang ST, Ayton S, Wang Q, Crouch PJ, Ganio K, Wang XC, Pei L, Adlard PA, Lu YM, Cappai R, Wang JZ et al (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22:1520–1530. https://doi.org/10.1038/mp.2017.171
Long JM, Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179:312–339. https://doi.org/10.1016/j.cell.2019.09.001
Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, György B, Breakefield XO, Tanzi RE, Moir RD (2018) Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 99:56–63.e3. https://doi.org/10.1016/j.neuron.2018.06.030
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189. https://doi.org/10.1101/cshperspect.a006189
Rogers JT, Randall JD, Cahill CM, Eder PS, Huang X, Gunshin H, Leiter L, McPhee J, Sarang SS, Utsuki T, Greig NH, Lahiri DK, Tanzi RE, Bush AI, Giordano T, Gullans SR (2002) An iron-responsive element type II in the 5′-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem 277:45518–45528. https://doi.org/10.1074/jbc.M207435200
Nuñez MT, Chana-Cuevas P (2018) New perspectives in iron chelation therapy for the treatment of neurodegenerative diseases. Pharmaceuticals (Basel) 11. https://doi.org/10.3390/ph11040109
Hambright WS, Fonseca RS, Chen L, Na R, Ran Q (2017) Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12:8–17. https://doi.org/10.1016/j.redox.2017.01.021
Ersche KD, Acosta-Cabronero J, Jones PS, Ziauddeen H, van Swelm RP, Laarakkers CM, Raha-Chowdhury R, Williams GB (2017) Disrupted iron regulation in the brain and periphery in cocaine addiction. Transl Psychiatry 7:e1040. https://doi.org/10.1038/tp.2016.271
Belaidi AA, Masaldan S, Southon A, Kalinowski P, Acevedo K, Appukuttan AT, Portbury S, Lei P, Agarwal P, Leurgans SE, Schneider J, Conrad M, Bush AI, Ayton S (2022) Apolipoprotein E potently inhibits ferroptosis by blocking ferritinophagy. Mol Psychiatry. https://doi.org/10.1038/s41380-022-01568-w
Mahoney-Sanchez L, Belaidi AA, Bush AI, Ayton S (2016) The complex role of apolipoprotein E in Alzheimer’s disease: an overview and update. J Mol Neurosci 60:325–335. https://doi.org/10.1007/s12031-016-0839-z
van Bergen JM, Li X, Hua J, Schreiner SJ, Steininger SC, Quevenco FC, Wyss M, Gietl AF, Treyer V, Leh SE, Buck F, Nitsch RM, Pruessmann KP, van Zijl PC, Hock C, Unschuld PG (2016) Colocalization of cerebral iron with amyloid beta in mild cognitive impairment. Sci Rep 6:35514. https://doi.org/10.1038/srep35514
Ayton S, Faux NG, Bush AI (2017) Association of cerebrospinal fluid ferritin level with preclinical cognitive decline in APOE-ε4 carriers. JAMA Neurol 74:122–125. https://doi.org/10.1001/jamaneurol.2016.4406
Song B, Cha Y, Ko S, Jeon J, Lee N, Seo H, Park KJ, Lee IH, Lopes C, Feitosa M, Luna MJ, Jung JH, Kim J, Hwang D, Cohen BM, Teicher MH, Leblanc P, Carter BS, Kordower JH et al (2020) Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J Clin Invest 130:904–920. https://doi.org/10.1172/jci130767
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909. https://doi.org/10.1016/s0896-6273(03)00568-3
Stefanis L (2012) α-Synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med 2:a009399. https://doi.org/10.1101/cshperspect.a009399
Liu MZ, Kong N, Zhang GY, Xu Q, Xu Y, Ke P, Liu C (2022) The critical role of ferritinophagy in human disease. Front Pharmacol 13:933732. https://doi.org/10.3389/fphar.2022.933732
Faucheux BA, Martin ME, Beaumont C, Hunot S, Hauw JJ, Agid Y, Hirsch EC (2002) Lack of up-regulation of ferritin is associated with sustained iron regulatory protein-1 binding activity in the substantia nigra of patients with Parkinson’s disease. J Neurochem 83:320–330. https://doi.org/10.1046/j.1471-4159.2002.01118.x
Baksi S, Singh N (2017) α-Synuclein impairs ferritinophagy in the retinal pigment epithelium: implications for retinal iron dyshomeostasis in Parkinson’s disease. Sci Rep 7:12843. https://doi.org/10.1038/s41598-017-12862-x
Rhodes SL, Ritz B (2008) Genetics of iron regulation and the possible role of iron in Parkinson’s disease. Neurobiol Dis 32:183–195. https://doi.org/10.1016/j.nbd.2008.07.001
Ammal Kaidery N, Ahuja M, Thomas B (2019) Crosstalk between Nrf2 signaling and mitochondrial function in Parkinson’s disease. Mol Cell Neurosci 101:103413. https://doi.org/10.1016/j.mcn.2019.103413
Sun Y, He L, Wang T, Hua W, Qin H, Wang J, Wang L, Gu W, Li T, Li N, Liu X, Chen F, Tang L (2020) Activation of p62-Keap1-Nrf2 pathway protects 6-hydroxydopamine-induced ferroptosis in dopaminergic cells. Mol Neurobiol 57:4628–4641. https://doi.org/10.1007/s12035-020-02049-3
Shi L, Huang C, Luo Q, Xia Y, Liu W, Zeng W, Cheng A, Shi R, Zhengli C (2020) Clioquinol improves motor and non-motor deficits in MPTP-induced monkey model of Parkinson’s disease through AKT/mTOR pathway. Aging (Albany NY) 12:9515–9533. https://doi.org/10.18632/aging.103225
Wyant KJ, Ridder AJ, Dayalu P (2017) Huntington’s disease-update on treatments. Curr Neurol Neurosci Rep 17:33. https://doi.org/10.1007/s11910-017-0739-9
Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA, Scahill RI, Wetzel R, Wild EJ, Tabrizi SJ (2015) Huntington disease. Nat Rev Dis Primers 1:15005. https://doi.org/10.1038/nrdp.2015.5
Jimenez-Sanchez M, Licitra F, Underwood BR, Rubinsztein DC (2017) Huntington’s disease: mechanisms of pathogenesis and therapeutic strategies. Cold Spring Harb Perspect Med 7. https://doi.org/10.1101/cshperspect.a024240
McColgan P, Tabrizi SJ (2018) Huntington’s disease: a clinical review. Eur J Neurol 25:24–34. https://doi.org/10.1111/ene.13413
Rosas HD, Chen YI, Doros G, Salat DH, Chen NK, Kwong KK, Bush A, Fox J, Hersch SM (2012) Alterations in brain transition metals in Huntington disease: an evolving and intricate story. Arch Neurol 69:887–893. https://doi.org/10.1001/archneurol.2011.2945
Chen CM, Wu YR, Cheng ML, Liu JL, Lee YM, Lee PW, Soong BW, Chiu DT (2007) Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington’s disease patients. Biochem Biophys Res Commun 359:335–340. https://doi.org/10.1016/j.bbrc.2007.05.093
Magtanong L, Dixon SJ (2018) Ferroptosis and brain injury. Dev Neurosci 40:382–395. https://doi.org/10.1159/000496922
Wyttenbach A, Sauvageot O, Carmichael J, Diaz-Latoud C, Arrigo AP, Rubinsztein DC (2002) Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet 11:1137–1151. https://doi.org/10.1093/hmg/11.9.1137
Perera ND, Sheean RK, Lau CL, Shin YS, Beart PM, Horne MK, Turner BJ (2018) Rilmenidine promotes MTOR-independent autophagy in the mutant SOD1 mouse model of amyotrophic lateral sclerosis without slowing disease progression. Autophagy 14:534–551. https://doi.org/10.1080/15548627.2017.1385674
Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, van den Berg LH (2017) Amyotrophic lateral sclerosis. Nat Rev Dis Primers 3:17071. https://doi.org/10.1038/nrdp.2017.71
Vahsen BF, Gray E, Thompson AG, Ansorge O, Anthony DC, Cowley SA, Talbot K, Turner MR (2021) Non-neuronal cells in amyotrophic lateral sclerosis - from pathogenesis to biomarkers. Nat Rev Neurol 17:333–348. https://doi.org/10.1038/s41582-021-00487-8
Xiao Y, Karam C, Yi J, Zhang L, Li X, Yoon D, Wang H, Dhakal K, Ramlow P, Yu T, Mo Z, Ma J, Zhou J (2018) ROS-related mitochondrial dysfunction in skeletal muscle of an ALS mouse model during the disease progression. Pharmacol Res 138:25–36. https://doi.org/10.1016/j.phrs.2018.09.008
Wang T, Tomas D, Perera ND, Cuic B, Luikinga S, Viden A, Barton SK, McLean CA, Samson AL, Southon A, Bush AI, Murphy JM, Turner BJ (2022) Ferroptosis mediates selective motor neuron death in amyotrophic lateral sclerosis. Cell Death Differ 29:1187–1198. https://doi.org/10.1038/s41418-021-00910-z
Da Cruz S, Bui A, Saberi S, Lee SK, Stauffer J, McAlonis-Downes M, Schulte D, Pizzo DP, Parone PA, Cleveland DW, Ravits J (2017) Misfolded SOD1 is not a primary component of sporadic ALS. Acta Neuropathol 134:97–111. https://doi.org/10.1007/s00401-017-1688-8
Ignjatović A, Stević Z, Lavrnić D, Nikolić-Kokić A, Blagojević D, Spasić M, Spasojević I (2012) Inappropriately chelated iron in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler 13:357–362. https://doi.org/10.3109/17482968.2012.665929
Kwan JY, Jeong SY, Van Gelderen P, Deng HX, Quezado MM, Danielian LE, Butman JA, Chen L, Bayat E, Russell J, Siddique T, Duyn JH, Rouault TA, Floeter MK (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7:e35241. https://doi.org/10.1371/journal.pone.0035241
Hemerková P, Vališ M (2021) Role of oxidative stress in the pathogenesis of amyotrophic lateral sclerosis: antioxidant metalloenzymes and therapeutic strategies. Biomolecules 11. https://doi.org/10.3390/biom11030437
Moreau C, Danel V, Devedjian JC, Grolez G, Timmerman K, Laloux C, Petrault M, Gouel F, Jonneaux A, Dutheil M, Lachaud C, Lopes R, Kuchcinski G, Auger F, Kyheng M, Duhamel A, Pérez T, Pradat PF, Blasco H et al (2018) Could conservative iron chelation lead to neuroprotection in amyotrophic lateral sclerosis? Antioxid Redox Signal 29:742–748. https://doi.org/10.1089/ars.2017.7493
Evans RC, Chen L, Na R, Yoo K, Ran Q (2022) The Gpx4NIKO mouse is a versatile model for testing interventions targeting ferroptotic cell death of spinal motor neurons. Neurotox Res 40:373–383. https://doi.org/10.1007/s12640-021-00469-0
Lee JY, He Y, Sagher O, Keep R, Hua Y, Xi G (2009) Activated autophagy pathway in experimental subarachnoid hemorrhage. Brain Res 1287:126–135. https://doi.org/10.1016/j.brainres.2009.06.028
Hao S, Song C, Shang L, Yu J, Qiao T, Li K (2016) Phosphorylation of Akt by SC79 prevents iron accumulation and ameliorates early brain injury in a model of experimental subarachnoid hemorrhage. Molecules 21:325. https://doi.org/10.3390/molecules21030325
Wang Y, Gao A, Xu X, Dang B, You W, Li H, Yu Z, Chen G (2015) The neuroprotection of lysosomotropic agents in experimental subarachnoid hemorrhage probably involving the apoptosis pathway triggering by cathepsins via chelating intralysosomal iron. Mol Neurobiol 52:64–77. https://doi.org/10.1007/s12035-014-8846-y
Yan H, Hao S, Sun X, Zhang D, Gao X, Yu Z, Li K, Hang CH (2015) Blockage of mitochondrial calcium uniporter prevents iron accumulation in a model of experimental subarachnoid hemorrhage. Biochem Biophys Res Commun 456:835–840. https://doi.org/10.1016/j.bbrc.2014.12.073
Li Y, Liu Y, Wu P, Tian Y, Liu B, Wang J, Bihl J, Shi H (2021) Inhibition of ferroptosis alleviates early brain injury after subarachnoid hemorrhage in vitro and in vivo via reduction of lipid peroxidation. Cell Mol Neurobiol 41:263–278. https://doi.org/10.1007/s10571-020-00850-1
Jiang JY, Gao GY, Feng JF, Mao Q, Chen LG, Yang XF, Liu JF, Wang YH, Qiu BH, Huang XJ (2019) Traumatic brain injury in China. Lancet Neurol 18:286–295. https://doi.org/10.1016/s1474-4422(18)30469-1
Pavlovic D, Pekic S, Stojanovic M, Popovic V (2019) Traumatic brain injury: neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 22:270–282. https://doi.org/10.1007/s11102-019-00957-9
Xie BS, Wang YQ, Lin Y, Mao Q, Feng JF, Gao GY, Jiang JY (2019) Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci Ther 25:465–475. https://doi.org/10.1111/cns.13069
Dheen ST, Kaur C, Ling EA (2007) Microglial activation and its implications in the brain diseases. Curr Med Chem 14:1189–1197. https://doi.org/10.2174/092986707780597961
Chodobski A, Zink BJ, Szmydynger-Chodobska J (2011) Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2:492–516. https://doi.org/10.1007/s12975-011-0125-x
Fischer TD, Hylin MJ, Zhao J, Moore AN, Waxham MN, Dash PK (2016) Altered mitochondrial dynamics and TBI pathophysiology. Front Syst Neurosci 10:29. https://doi.org/10.3389/fnsys.2016.00029
Anthonymuthu TS, Kenny EM, Lamade AM, Kagan VE, Bayır H (2018) Oxidized phospholipid signaling in traumatic brain injury. Free Radic Biol Med 124:493–503. https://doi.org/10.1016/j.freeradbiomed.2018.06.031
Morera-Fumero AL, Abreu-Gonzalez P (2013) Role of melatonin in schizophrenia. Int J Mol Sci 14:9037–9050. https://doi.org/10.3390/ijms14059037
Cao B, Yan L, Ma J, Jin M, Park C, Nozari Y, Kazmierczak OP, Zuckerman H, Lee Y, Pan Z, Brietzke E, McIntyre RS, Lui LMW, Li N, Wang J (2019) Comparison of serum essential trace metals between patients with schizophrenia and healthy controls. J Trace Elem Med Biol 51:79–85. https://doi.org/10.1016/j.jtemb.2018.10.009
Wirshing DA, Bartzokis G, Pierre JM, Wirshing WC, Sun A, Tishler TA, Marder SR (1998) Tardive dyskinesia and serum iron indices. Biol Psychiatry 44:493–498. https://doi.org/10.1016/s0006-3223(97)00453-8
Sussulini A, Erbolato HM, Pessôa GS, Arruda MAZ, Steiner J, Martins-de-Souza D (2018) Elemental fingerprinting of schizophrenia patient blood plasma before and after treatment with antipsychotics. Eur Arch Psychiatry Clin Neurosci 268:565–570. https://doi.org/10.1007/s00406-017-0836-4
Keleş Altun İ, Atagün M, Erdoğan A, Oymak Yenilmez D, Yusifova A, Şenat A, Erel Ö (2021) Serum hepcidin / ferroportin levels in bipolar disorder and schizophrenia. J Trace Elem Med Biol 68:126843. https://doi.org/10.1016/j.jtemb.2021.126843
Wang DM, Chen DC, Wang L, Zhang XY (2021) Sex differences in the association between symptoms and superoxide dismutase in patients with never-treated first-episode schizophrenia. World J Biol Psychiatry 22:325–334. https://doi.org/10.1080/15622975.2020.1805510
Ikawa T, Sato M, Oh-Hashi K, Furuta K, Hirata Y (2021) Oxindole-curcumin hybrid compound enhances the transcription of γ-glutamylcysteine ligase. Eur J Pharmacol 896:173898. https://doi.org/10.1016/j.ejphar.2021.173898
Cruz BF, de Campos-Carli SM, de Oliveira AM, de Brito CB, Garcia ZM, do Nascimento Arifa RD, de Souza DDG, Teixeira AL, Salgado JV (2021) Investigating potential associations between neurocognition/social cognition and oxidative stress in schizophrenia. Psychiatry Res 298:113832. https://doi.org/10.1016/j.psychres.2021.113832
Krieg L, Milstein O, Krebs P, Xia Y, Beutler B, Du X (2011) Mutation of the gastric hydrogen-potassium ATPase alpha subunit causes iron-deficiency anemia in mice. Blood 118:6418–6425. https://doi.org/10.1182/blood-2011-04-350082
Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388:482–488. https://doi.org/10.1038/41343
Haschka D, Hoffmann A, Weiss G (2021) Iron in immune cell function and host defense. Semin Cell Dev Biol 115:27–36. https://doi.org/10.1016/j.semcdb.2020.12.005
Weber GJ, Choe SE, Dooley KA, Paffett-Lugassy NN, Zhou Y, Zon LI (2005) Mutant-specific gene programs in the zebrafish. Blood 106:521–530. https://doi.org/10.1182/blood-2004-11-4541
An X, Schulz VP, Li J, Wu K, Liu J, Xue F, Hu J, Mohandas N, Gallagher PG (2014) Global transcriptome analyses of human and murine terminal erythroid differentiation. Blood 123:3466–3477. https://doi.org/10.1182/blood-2014-01-548305
Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, Cantwell J, Luu C, Cornella-Taracido I, Harrington E, Fekkes P, Lei H, Fang Q, Digan ME, Burdick D et al (2014) Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol 16:1069–1079. https://doi.org/10.1038/ncb3053
Gao X, Lee HY, Li W, Platt RJ, Barrasa MI, Ma Q, Elmes RR, Rosenfeld MG, Lodish HF (2017) Thyroid hormone receptor beta and NCOA4 regulate terminal erythrocyte differentiation. Proc Natl Acad Sci U S A 114:10107–10112. https://doi.org/10.1073/pnas.1711058114
Bellelli R, Federico G, Matte A, Colecchia D, Iolascon A, Chiariello M, Santoro M, De Franceschi L, Carlomagno F (2016) NCOA4 deficiency impairs systemic iron homeostasis. Cell Rep 14:411–421. https://doi.org/10.1016/j.celrep.2015.12.065
Truman-Rosentsvit M, Berenbaum D, Spektor L, Cohen LA, Belizowsky-Moshe S, Lifshitz L, Ma J, Li W, Kesselman E, Abutbul-Ionita I, Danino D, Gutierrez L, Li H, Li K, Lou H, Regoni M, Poli M, Glaser F, Rouault TA, Meyron-Holtz EG (2018) Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood 131:342–352. https://doi.org/10.1182/blood-2017-02-768580
Funding
This work was supported by the National Natural Science Foundation of China (No. 82374326), and the Zhejiang Provincial Science and Technology Innovation Leading Talent Project of “Ten Thousand Talents Plan” (2019).
Author information
Authors and Affiliations
Contributions
The contribution of each author in this manuscript is as follows: Yu He contributes to the conception. Siqi Li writes the initial draft. Ping Huang and Feifan Lai modify the manuscript. Ting Zhang, Jiaqi Guan, Haitong Wan, and Yu He review and edit the manuscript. All authors read and approved the final manuscript. The corresponding authors attest that all listed authors meet the authorship criteria.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
Not applicable
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Huang, P., Lai, F. et al. Mechanisms of Ferritinophagy and Ferroptosis in Diseases. Mol Neurobiol 61, 1605–1626 (2024). https://doi.org/10.1007/s12035-023-03640-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03640-0