Abstract
Purpose
To perform a large-scale gene profiling of the liver in a mouse model of fatty liver induced by high carbohydrate (sucrose) diet (HCD) to gain a deeper insight into potential mechanisms of diet-induced hepatic steatosis.
Methods
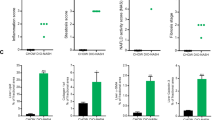
C57BL/6 male mice were fed either a purified, control diet or a HCD for 16 weeks. HCD feeding led to marked liver steatosis without inflammation or necrosis. The expression of 42,500 genes/sequences was assessed.
Results
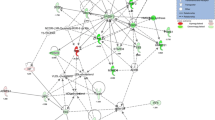
A number of genes (471) underwent significant expression changes in HCD- as compared to standard diet-fed mice (n = 5/group; P < 0.01). Of these genes, 211 were down- and 260 up-regulated. The latter group includes 20 genes encoding enzymes involved in carbohydrate conversion to fat. The genes that underwent expression changes perform a large variety of molecular functions, and the vast majority of these have never been tested before in non-alcoholic fatty liver of nutritional origin. They reveal novel aspects of the disease and allow identification of candidate genes that may underlie the initiation of hepatic steatosis and progression to non-alcoholic steatohepatitis.
Conclusions
HCD-fed laboratory animals provide a model of early non-alcoholic fatty liver disease resembling the disease in humans. The genome wide gene profiling of the liver reveals the complexity of the disease, unravels novel aspects of HCD-induced hepatic steatosis, and helps elucidate its nature and mechanisms.




Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine-2-oxoglutarate amino transferase (EC 2.6.1.2)
- HCD:
-
High-carbohydrate diet
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- TBARS:
-
Thiobarbituric acid reactive substances
References
McClain CJ, Mokshagundam PL, Barve SS, Song Z, Hill DB, Chen T, Deaciuc I. Mechanisms of non-alcoholic steatohepatitis. Alcohol 2004;34:67–79.
Den Boer M, Voshol PG, Kuipers F, Havekes LM, Romijn JA. Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Artherioscl Thromb Vasc Biol 2004;24:644–9.
Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis 2001;21:89–104.
Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, et al. Model of non-alcoholic steatohepatitis. Am J Clin Nutr 2004;79:502–9.
Nanji AA. Animal models of nonalcoholic fatty liver disease and steatohepatitis. Clin Liver Dis 2004;8:559–74.
Portincasa P, Grattagliano I, Palmieri VO, Palasciano G. Nonalcoholic steatohepatitis: recent advances from experimental models to clinical management. Clin Biochem 2005;38:203–17.
Deng QG, She H, Cheng JH, French SW, Koop DR, Xion S, et al. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology 2005;42:905–14.
Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol 2003;39:978–83.
Friedman HA, Nylund B. Intestinal fat digestion, absorption and transport. Am J Clin Nutr 1980;33:1108–39.
Foufelle F, Girard J, Ferre P. Regulation of lipogenic enzyme expression by glucose in liver and adipose tissue: a review of the potential cellular and molecular mechanisms. Adv Enzyme Regul 1996;36:199–226.
Flatt JP. Conversion of carbohydrate to fat in the adipose tissue: an energy-yielding and, therefore, self-limiting process. J Lip Res 1970;11:131–43.
Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab 2005;2:5. doi:10.1186/1743-7075-2-5.
Diraison F, Yankah V, Letexier D, Dusserre E, Jones P, Beylot M. Differences in the regulation of adipose tissue and liver lipogenesis by carbohydrates in humans. J Lipid Res 2003;44:846–53.
Hudgins L, Hellerstein M, Seidman C, Seidman C, Neese R, Diakun J, et al. Human fatty acid synthesis is stimulated by eucaloric low fat, high carbohydrate diet. J Clin Invest 1996;97:2081–91.
Schwartz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low carbohydrate and low-fat, high carbohydrate isoenergetic diets. Am J Clin Nutr 2003;77:43–50.
Quintanilha AT, Packer L, Davies JM, Racanelli TM, Davies KJ. Membrane effects of vitamin E deficiency: bioenergetic and surface charge density studies of skeletal muscle and liver mitochondria. Ann NY Acad Sci 1982;393:32–47.
Deaciuc IV, Peng X, D’Souza NB, Shedlofsky SI, Burikhanov R. Microarray gene analysis of the liver in a rat model of chronic, voluntary alcohol uptake. Alcohol 2004;32:113–27.
Järveläinen HA, Fang C, Ingelman-Sundberg M, Lindros KO. Effect of chronic administration of endotoxin and ethanol on rat liver pathology and proinflammatory and antiinflammatory cytokines. Hepatology 1999;29:1602–14.
Bulera SJ, Eddy SM, Ferguson E, Jatkoe TA, Reindel JF, Bleavins MR, et al. RNA expression in the early characterization of hepatotoxicants in Wistar rats by high-density DNA microarrays. Hepatology 2002;33:1239–58.
Deaciuc IV, Doherty DE, Burikhanov R, Lee EY, Stromberg AJ, Peng X, et al. Large-scale gene profiling of the liver in a mouse model of chronic, intragastric ethanol infusion. J Hepatol 2004;40:219–27.
Spencer AF, Lowenstein JM. The supply of precursors for the synthesis of fatty acids. J Biol Chem 1962;237:3640–8.
Wise EM, Ball EG. Malic enzyme and lipogenesis. Proc Natl Acad Sci USA 1964;52:1255–63.
Müller-Wieland D, Kotzka J. SREBP-1: gene regulatory key to syndrome X? Ann NY Acad Sci 2002;967:19–27.
Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 2004;86:839–48.
Tan NS, Michalik L, Desvregne B, Wahli W. Multiple expression control mechanisms of peroxisome proliferator-activated receptors and their target genes. J Steroid Biochem Mol Biol 2005;93:99–105.
Dela Pena A, Leclerq I, Field J, George J, Jones B, Farrell G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepetitis. Gastroenterology 2005;129:1663–74.
Louthan MV, Barve S, McClain CJ, Joshi-Barve S. Decreased serum adiponectin: an early event in pediatric non-alcoholic fatty liver disease. J Pediatr 2005;147:835–8.
Ballestar E, Wolffe AP. Methyl-CpG-binding proteins. Targeting specific gene repression. Eur J Biochem 2001;268:1–6.
El-Osta A, Wolffe AP. DNA methylation and histone deacetylation in the control of gene expression: basic biochemistry to human development and disease. Gene Expr 2000;9:63–75.
Mehlman MA. Dangerous and cancer-causing properties of products and chemicals in the oil refining and petrochemical industry. VIII. Health effects of motor fuels: carcinogenicity of gasoline—scientific update. Environ Res 1992;59(1):238–49.
Cave M, Deaciuc IV, Mendez C, Song Z, Joshi-Barve S, Barve S, et al. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem 2007;18:184–95.
Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. Can Med Assoc J 2005;172:899–905.
Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004;114:147–52.
Browning JD, Szczepaniak LS, Dobbins R, Nurember P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–95.
Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med 2004;10:1–7.
Hamaguchi M, Kojima T, Takeda N, Taniguchi H, Fujii K, Omatsu T, et al. The metabolic syndrome as a predictor of non-alcoholic fatty liver disease. Ann Intern Med 2005;143:722–8.
Villafuerte BC, Phillips LS, Rane MJ, Zhao W. Insulin-response element-binding protein 1. A novel Akt substrate involved in transcriptional action of insulin. J Biol Chem 2004;279:36650–9.
Villafuerte BC, Kaytor EN. An insulin-response element-binding protein that ameliorates hyperglycemia in diabetes. J Biol Chem 2005;280:20010–20.
Foufelle F, Ferre P. Regulation of carbohydrate metabolism by insulin: role of transcription factor SREBP-1c in the hepatic transcriptional effects of the hormone. J Soc Biol 2001;195:243–8.
Griffin MJ, Sul HS. Insulin regulation of fatty acid synthase gene transcription: roles of USF and SREBP-1c. Life 2004;56:595–600.
Acknowledgements
The work reported in this study was supported by National Institutes of Health grants (to I.V.D., Z.S., S.S.B., T.B.K., A.V.S., and C.J.M.) and a Department of Veterans Affairs grant (to C.J.M.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Ion V. Deaciuc and Zhenyuan Song are contributed equally to this study.
Electronic supplementary material
Below are the link to the electronic supplementary material. Two tables (Microsoft Excel), one comprising all the genes that underwent changes in their expression with statistical processing and another comprising gene ontology for the genes involved in carbohydrate and lipid metabolism.
Rights and permissions
About this article
Cite this article
Deaciuc, I.V., Song, Z., Peng, X. et al. Genome-wide transcriptome expression in the liver of a mouse model of high carbohydrate diet-induced liver steatosis and its significance for the disease. Hepatol Int 2, 39–49 (2008). https://doi.org/10.1007/s12072-007-9025-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-007-9025-2