Abstract
Extinct megafaunal mammals in the Americas are often linked to seed-dispersal mutualisms with large-fruiting tree species, but large-fruiting species in Europe and Asia have received far less attention. Several species of arboreal Maloideae (apples and pears) and Prunoideae (plums and peaches) evolved large fruits starting around nine million years ago, primarily in Eurasia. As evolutionary adaptations for seed dispersal by animals, the size, high sugar content, and bright colorful visual displays of ripeness suggest that mutualism with megafaunal mammals facilitated the evolutionary change. There has been little discussion as to which animals were likely candidate(s) on the late Miocene landscape of Eurasia. We argue that several possible dispersers could have consumed the large fruits, with endozoochoric dispersal usually relying on guilds of species. During the Pleistocene and Holocene, the dispersal guild likely included ursids, equids, and elephantids. During the late Miocene, large primates were likely also among the members of this guild, and the potential of a long-held mutualism between the ape and apple clades merits further discussion. If primates were a driving factor in the evolution of this large-fruit seed-dispersal system, it would represent an example of seed-dispersal-based mutualism with hominids millions of years prior to crop domestication or the development of cultural practices, such as farming.
Similar content being viewed by others
Introduction
The history of the domesticated apple (Malus pumila (formerly M. domestica)) is closely intertwined with humans. There are 3,000 species in the Rosaceae family, many of which are economically important, such as apples, peaches (Prunus persica), plums (Prunus spp.), and pears (Pyrus spp.). Malus encompasses 55 species (Hancock et al. 2008), of which the apple is the most morphologically diverse, with over 10,000 landraces of apples recognized worldwide (Sau et al. 2018). Human-facilitated gene flow has caused major changes in population genetics within Malus over the past few centuries, leading to immense phenotypic change in fruit structure (Urrestarazu et al. 2016). Recent archaeobotanical and genetic research suggests that the modern apple evolved through hybridization caused by human-induced movement of trees between isolated populations during the late Holocene (Harris et al. 2002; Gross et al. 2013; Cornille et al. 2015). The geographically restricted populations of some large-fruiting Malus trees, such as M. orientalis and M. sieversii, appear today to map over former glacial refugia (Fig. 1) (Spengler 2019). Humans started spreading the seeds beyond these refugial pockets in association with the Neolithization of Europe and the development of transcontinental exchange networks (Cornille et al. 2015; Duan et al. 2017; Spengler 2019). Although this period represented a significant evolutionary change for these plants as they responded to human dispersal and maintenance of the population, the appearance of large sugary fruits actually occurred roughly nine million years ago (Ma) and their interactions with hominid populations may also have a far greater time depth.
Map showing locations of archaeobotanical finds of apple seeds during the mid-Holocene in black from Spengler (2019); red data points represent Pleistocene sites with apples and humans or artifacts found at the same site: (1) Staosnaig, Scotland, Mesolithic (Carruthers 2000; Mithen et al. 2001); (2) Öküzini, Turkey, Epipaleolithic (Martinoli and Jacomet 2004); (3) Neumark Nord, Germany, Middle Pleistocene (Gregor and Vodickova 1983; Schweigert 1991); (4) Ehringsdorf, Germany, 243–200 ka (Vent 1974; Schwarcz et al. 1988; Mallick and Frank 2002); (5) Stuttgart-Untertürkheim, Germany, Pleistocene (Mai 2010); (6) Burgtonna, Germany, 130–115 ka (Vent 1978); (7) Shamb outcropping, Armenia, 53 ka (Ollivier et al. 2010); (8) Azokh 1 Cave, Armenia, 184–100 ka (Allué 2016); and (9) Molí del Salt, Spain, 11,000–12,500 (Allué 2016). Most of the data points fall in Pleistocene forest refugial zones, suggesting that these large-fruiting clades did not colonize new areas during the early Holocene, likely due to a lack of dispersers. Areas of loess deposits modified from Börker et al. (2018) and Bateman and Catt (2007)
Megafruits (defined as any fruit too large for typical avian dispersal, > 25 mm) evolved in parallel at least twice (Prunoideae and Maloideae) across the Rosaceae clade in Eurasia starting in the late Miocene (Xiang et al. 2017). Prominent examples of Maloideae megafruits with highly restricted ranges in the wild include Cydonia spp., Pyrus spp., Malus spp., Mespilus germanica, Sorbus domestica, and Eriobotrya japonica. This leaves open the question as to which seed dispersers the trees originally evolved larger fruits to recruit. It is clear that the mutualistic relationship between humans and apples stretches much further back than the Neolithic. Heavy foraging of wild European apples by pre-farming peoples undoubtedly manipulated gene flow and population dynamics. Some scholars have suggested that these pre-agricultural foragers were directly maintaining apple trees (Clarke 1978). Intentional burning would have increased forest patchiness, facilitating Malus sp. growth and dispersal (Zvelebil 1994; Kaplan et al. 2016). In certain parts of Central Europe, wild apples were an important part of the Neolithic economy, and foragers may have been targeting remnants of refugial apple populations (Antolín et al. 2016). Wild apple foraging was practiced during the Pleistocene; Malus seeds have been recovered from the Staosnaig site on the Isle of Colonsay, Scotland (Carruthers 2000; Mithen et al. 2001), and large-fruiting Rosaceae seeds are recorded as foraged foods across Mesolithic Europe (Zvelebil 1994). In addition, seeds of wild pears (Pyrus pyraster) were recovered from the Epipaleolithic cave site of Öküzini in Anatolia, along with other wild arboreal fruit seeds (Fig. 1) (Martinoli and Jacomet 2004).
Hominin collection and use of these fruits appears to extend even beyond the appearance of our own species in Eurasia, hinting at a much deeper time depth for human/Malus seed-dispersal mutualism. For example, fossil apple impressions in travertine from the Ehringsdorf site (Fig. 2) of central Germany (Vent 1974) place large-fruiting apples in Central Europe between 243,000 and 200,000 years ago during Marine Isotope Stage 7 (Schwarcz et al. 1988; Mallick and Frank 2002). The fossils at Ehringsdorf include several hominin remains, characterized either as early Neanderthal, pre-Neanderthal (Vlcek 1993), or typical Neanderthal with Mousterian stone tools (Hublin 2009). The site contains several occupations, including hearths, preserved in rapidly hardening travertine and provides some of the earliest linkages between Homo and apples (Behm-Blancke 1960; Kot 2017). Apple fossils have also been recovered at the middle Pleistocene interglacial site of Neumark Nord (Geiseltal, Germany) (Mai 2010). Resembling the travertine incrustations from Ehringsdorf (Fig. 2), numerous apple impressions were also preserved in travertine deposits at Stuttgart-Untertürkheim, which were presumed to be gathered by hominins (Gregor and Vodickova 1983; Schweigert 1991).
There are also further, more tentative, deep time links between hominins and Maloideae. Although the identification of apples from leaves was also originally made at Burgtonna, in Eemian travertine of Germany (Vent 1978), this identification has been refuted as Lonicera arborea by Schweigert (Schweigert 1991). Other Pleistocene sites in refugial zones of Europe have provided evidence of large-fruiting Rosaceae, including Mespilus germanica which was also reported at Burgtonna (Vent 1978). Travertine formations dating to both the late Pleistocene (53,000 BP) and early Holocene (12,000 BP) in the Shamb outcropping of southern Armenia, a former forest refugium, contain leaf impressions from Malus sp., Prunus sp., and Pyrus sp. (Ollivier et al. 2010). In the same Pleistocene forest refugia, wood charcoal analyses from Azokh 1 Cave in Armenia (184,000–100,000 years ago), illustrate that 80% of the wood that hominins (both Neanderthal and modern humans are present in varying layers) were burning was from Prunus sp.; among the remaining 20% of charcoal were fragments of Maloideae and other fruit trees, such as Paliurus/Ziziphus and Celtis/Zelkova (Allué 2016). Similarly, Maloideae wood was found with Prunus sp. wood fragments and a carbonized seed of Prunus spinosa at the site of Molí del Salt in northeastern Iberia (Allué et al. 2010).
These publications suggest: (1) wild fruit forests were common in pre-Holocene Eurasia; (2) many of the megafruits with restricted ranges during the mid-Holocene (e.g., Mespilus) may have had much wider ranges across refugial pockets during the Pleistocene; and (3) humans were likely utilizing these resources. In this article, we take this evidence for hominin–Malus spp. interactions a stage further to argue that the evolution of large fruits in the Rosaceae lineage may have been linked to seed-dispersal-based mutualism prior to the Pleistocene. We base our hypothesis on: (1) the fossil and genetic record for Malus spp.; (2) morphological traits of fruits in the clade; (3) the dominance of frugivorous or folivorous primates on the landscape during the development of fleshy fruits in the Miocene; (4) the fossil and extant record for large primate frugivory; and (5) the prominence of seed dispersal in other primate clades.
Seed-Dispersal-Based Mutualisms
Large rosaceous fruits evolved to recruit animals on a different landscape than that of modern Eurasia. Continual dispersal through the Pleistocene and Holocene was likely facilitated by ursids, equids, and elephantids. Bears have long been theorized as one of the dispersers of the Tian Shan wild apples, and we emphasize the likelihood that bears have been a key disperser for these fruits through the Holocene. Personal observations by the lead author attest to the germination of apple seeds after digestion by North American black bears. Many studies have emphasized the success of bears as seed dispersers, although, in these cases, largely for small-fruiting plants (García‑Rodríguez et al. 2021).
Ecologists tend to agree that plants with small fleshy fruits largely evolved for avian dispersal (Tiffney 2004; Sussman et al. 2013), but, with the influx of African frugivorous megafauna in the early Miocene, including large primates and certain groups of proboscideans and perissodactyls, some angiosperms found more effective dispersal mechanisms (Van der Made and Mazo 2003; Begun et al. 2012). European megafaunal mammals prior to the early Miocene migrations were largely adapted for grazing (Steininger et al. 1985; Van der Made 1999). The new megafaunal dispersers that migrated to Europe in the late Miocene, by contrast, would have been able to spread much larger seeds than their avian or small mammalian predecessors. The spread of African frugivorous species into Europe was coupled with a climatic shift or a series of climatic changes over millions of years (Van der Made and Mazo 2003). During the Miocene, in mid-latitudes, north of the Tropic of Cancer, climates fluctuated wildly from much hotter and more humid than today to much drier. The late Miocene saw an encroachment of drier, more open deciduous forests and advances of grassland biomes, and was the key period for the evolution of large-fruiting rosaceous trees and shrubs (Mai 1995; Xiang et al. 2017). M. sieversii trees evolved for an open steppe or savanna landscape and only grow to two to ten meters tall; like most large-fruiting Rosaceae trees, they cannot survive under a forest canopy. This growth habit mandates a long-distance seed-dispersal strategy to ensure directed colonization to suitable gaps in the forest cover. Biotic dispersal often leads to directed dispersal, targeting prime colonization areas (Eriksson 2008). In the case of Rosaceae, recruitment of animals that frequently forage in forest clearings helps the plant to jump between colonization sites.
Large mammals, collectively, represent highly effective seed dispersers, due to their ability to disperse large seeds and high abundances of seeds (Tiffney and Mazer 1995; Escribano-Avila et al. 2014; Jara‐Guerrero et al. 2018). Seed dispersal mutualism usually relies on guilds of animals, what Tiffney (2004) refers to as diffuse coevolution (Janzen and Martin 1982; Wenny 2001). When the dispersers in a guild are lost, there can be direct evolutionary consequences on the plant communities that relied on them. To take one example, a recent study by Onstein et al. (2018) demonstrated both a decrease in size and an increased rate of extinction in megafruits in the Arecaceae clade throughout the Holocene. Other studies have demonstrated that the loss of megafaunal dispersers results in a loss of seed dispersal and subsequently extinction or fragmentation of large-fruiting plant populations (Galetti et al. 2006, 2018; Eriksson 2008; Malhi et al. 2016; Pires et al. 2018). Seed-dispersal studies consistently illustrate that perissodactyla (including rhinoceroses and tapirs) are far more likely to disperse large seeds and consume sugary fruits than true ruminants, notably artiodactyla (including cattle, deer, and their relatives), and are more readily featured in zoochory studies (Nathan et al. 2008; O’Farrill et al. 2013; Jara‐Guerrero et al. 2018). Extensive research has also gone into the study of proboscidean seed dispersal rates (Kitamura et al. 2007; Campos-Arceiz et al. 2008; Harich et al. 2016; McConkey et al. 2018).
Malus Radiation and Diversification
While Pleistocene and early Holocene hominins undoubtedly dispersed wild apples, large-fruiting apple populations are unevenly situated across Eurasia, with concentrations in Central Europe, in areas such as the Rhine Valley and around the Caucasus, and in northern Central Asia, in the Tian Shan and Pamir Mountains. Meanwhile, endemic large-fruiting species such as M. tschonoskii and M. floribunda have restricted ranges in East Asia, as far east as Japan. These patches of mid-latitude distribution reflect the remnants left by the late Pleistocene ice sheets and permafrost, suggesting that the large-fruiting species of Malus have seen limited mobility over the past 20,000 years (Spengler 2019). Richards et al. (2009) noted that prior to the Last Glacial Maximum (ca. 20,000 years ago) there was likely a much larger ancestral population covering the range of all of these now-isolated populations. The main progenitor for the domesticated apple comes from a restricted population in a few river valleys in the western Tian Shan Mountains of southeastern Kazakhstan (Fig. 1) (Harris et al. 2002; Velasco et al. 2010; Cornille et al. 2014). Today heavy herd animal grazing has restricted the trees to steep slopes (between 800 and 1500 m above sea level); with low rates of seed dispersal in the current population, their range has dwindled considerably throughout the Holocene. The trees largely propagate today through root shoots and cloning, with most of their prolific generations failing to disperse and energetically costly fruits rotting below the parent trees (Omasheva et al. 2015; Duan et al. 2017; Spengler 2019). The production of large sugary fruits that, on the modern Eurasian landscape, fail to disperse would be highly maladaptive without human intervention today.
Genetic studies show that the pome-fruiting branch of the family diverged in the late Miocene, ca. nine million years ago (Töpel et al. 2012). Most of the morphological change in fruit structure seems to be tied to hybridization and polyploidy (Xiang et al. 2017). A whole genome duplication appears to be shared by all Pyrus and Malus species (Wu et al. 2013) and the formation of the Maloideae subfamily likely resulted from the hybridization of ancestral members of the Spiraeoideae and Amygdaloideae subfamilies (Dickinson et al. 2007; Xiang et al. 2017). Fossils of Rosaceae-type leaves and flowers have been recovered dating back to the early Eocene (Evans and Campbell 2002; Xiang et al. 2017), and fossil and genetic evidence suggest large-fruiting forms evolved well before the Pleistocene, starting in the late Miocene (Jongmans 1915; Reid and Reid 1915; Mai 1995; Gümbel and Mai 2004; DeVore and Pigg 2007). Some fossil studies specifically claim to identify large-fruiting Pyrus in late Miocene and Pliocene deposits from Northern Europe (Mädler 1939; Szafer 1947). Kvaček et al. (2020) note leaves from several different Maloideae species, including Malus remains from Pliocene deposits in central Germany.
The wild progenitor of the peach is regarded as once being widely distributed through northern China but is now extinct (Fedorov et al. 1971; Lu and Bartholomew 2003). Similarly, several large-fruiting species of Prunus exist across Eurasia, predominantly in East Asia. In general, the larger the fruit, the more restricted the modern range, and many species reproduce largely asexually—a few, such as P. mira, have lifespans of up to a millennium. Like large-fruiting Maloideae in Europe, Prunus in Asia have lost their ability to colonize new territory and have constrained gene flow (Spengler 2019). All these population features, shared across large-fruiting Maloideae and Prunoideae, suggest that they lost their ability to disperse seeds before the Holocene. Evolutionary genomics of the peach and almond suggest that the two lineages split around eight million years ago, likely in response to geological uplift of the Himalaya and climate change (Velasco et al. 2016). Assuming both lineages shared a large-seeded fleshy fruiting ancestor, then it would push large fruits in the Prunus clade back to the late Miocene as well. Yu et al. (2018) argue for a more recent origin of large-fruiting varieties of wild peaches, suggesting that they diverged from a shared ancestral relative with the almonds only 4.99 Ma. They argue for a shared ancestor that did not have a thick fleshy endocarp, and therefore suggest that fleshy fruiting forms evolved in southwest China during the terminal Miocene or early Pliocene. They also support Su et al.’s (2015) hypothesis of large primate dispersers (notably Gigantopithecus) driving evolution of large fruits. Velasco et al. (2016) point out a deep time depth for fruit divergence. The ancient origins of large-fruiting Prunus trees are further supported by fossils of P. kunmingensis from Yunnan, China, dating to the Pliocene/Pleistocene boundary 2.6 million years ago (Su et al. 2015).
Hominids of Miocene Eurasia
About 19 million years ago, the “Gomphotherium land bridge” formed between Europe and Africa (Fortelius 2015). Catarrhine primates were among the taxa that dispersed into Eurasia at this time. The oldest fossil hominids in Eurasia, the griphopiths, descend from an African dispersal, had thick dental enamel, and were likely frugivorous “hard-object” feeders (Heizmann and Begun 2001; King 2001). Roughly contemporaneously, another clade of frugivorous primates, the Pliopithecoidea, make their first appearance in Eurasia. Pliopithecoids diversified into three recognized clades (pliopithecids, dionysopithecids, and crouzelids) with distinctive dental morphologies; almost all are considered to have been largely frugivorous (Ungar and Kay 1995; Kay and Ungar 1997; Deane et al. 2013). By about 12.5 Ma the thickly enameled hominids of Europe are replaced by the dryopithecins, a diverse group mainly comprised of more thinly enameled apes (Begun et al. 2012). By about 9.5 Ma most dryopithecins in Europe are extinct, but another group, best represented by the large, thickly enameled frugivorous ape Ouranopithecus, appears in northern Greece. Other thickly enameled apes persist in the Balkans and Anatolia until about 7.2 Ma. Finally, an ape with unique dentition, strongly suggestive of a folivorous diet, Oreopithecus, appears at about 8.3 Ma in Italy (Ungar and Kay 1995; Hammond et al. 2020). Most of the fossil apes from Europe and Western Asia are associated with forested ecological settings, the exceptions being the Balkan/Anatolian apes, which are associated with more open settings.
During the middle to late Miocene, these primates flourished from the area of modern Barcelona to Georgia (Begun et al. 2012) and were associated with the expansion of subtropical forests under increased atmospheric CO2 and high temperatures (Hamon et al. 2012; Bouchal et al. 2018). The period after the mid-Miocene Climatic Optimum (ca. 14 million years ago) was characterized by a significant global cooling (Herbert et al. 2016) associated with increasing aridity and seasonality and resulting in a vegetation shift across Europe, as forests changed from humid subtropical and evergreen to temperate, deciduous, and seasonal (Mai 1995; Agustı 2007; Denk et al. 2018). The thinly enameled dryopithecins, ranging from about 12.5 to 10 Ma, lived during the early stages of this global cooling and are associated with persistent humid forest conditions. By 9.5 Ma the cooling had progressed significantly, especially in the Balkans and Anatolia, where Ouranopithecus and Graecopithecus were found. These large, thickly enameled apes are found in drier, more open woodlands and probably consumed terrestrial as well as arboreal resources (de Bonis and Koufos 1994; Bonis and Koufos 2014; Güleç et al. 2007; Böhme et al. 2017; Koufos and de Bonis 2017).
Both the thinly enameled dryopithecins and thickly enameled Ouranopithecus preserve abundant morphological evidence of frugivorous diets. Dryopithecins possessed molar occlusal morphology most similar to Pan, large, robust upper incisors and relatively gracile masticatory apparatus, all suggestive of a diet primarily involving soft-fruit frugivory (Begun and Kordos 1997; Begun et al. 2012). This is consistent with the results of microwear, shearing quotient, and incisor curvature analysis (Ungar and Kay 1995; Kay and Ungar 1997; King 2001; Deane et al. 2013). Rudapithecus is also klinorynchous (ventrally deflected face), and has an anteroposteriorly elongated temporomandibular joint, both of which have been associated with an enlarging jaw gape—a possible adaptation for processing large food items with the anterior dentition (de Bonis and Koufos 1993; Terhune 2013; Gunz et al. 2020). In addition to thick enamel, Ouranopithecus and Graecopithecus share attributes of gnathic morphology with australopithecines, indicative of powerful mastication typically associated with a hard-object frugivorous diet (de Bonis and Koufos 1994; Begun and Kordos 1997; Begun et al. 2012; Böhme et al. 2017; Fuss et al. 2017).
The mid-Vallesian Crisis (ca. 9.5 Ma; a period marked by the extinction of several mammalian clades) may have caused large-scale species extinction, and both floral and faunal communities were dramatically changed (Agustı et al. 2003; Agustı 2007). Forest-dwelling fauna experienced higher extinction rates, presumably due to forest fragmentation and an increasingly mosaic landscape structure. The increased prevalence of large-bodied herbivores further illustrates a shift towards grassland and patchy deciduous forest ecology. The transition from Pan-like soft-fruit frugivory to hard/tough-object frugivory is probably a direct consequence of the Vallesian Crisis. However, Oreopithecus evolved highly derived dentitions with distinctive high-crested molars, tall cusps separated by narrow, deep basins, and a powerfully developed masticatory apparatus with robust mandibles and prominent muscle attachments, including a sagittal crest. In these attributes Oreopithecus resembles colobine monkeys, the most folivorous of the Old World monkeys. Oreopithecus is easily distinguished from colobines in many details of morphology and each have clearly converged on a superficially similar folivorous functional complex.
Asian Miocene apes, such as Sivapithecus, Indopithecus, Khoratpithecus, and Lufengpithecus, were also adapted to a range of habitats from humid forests to patchy woodland/grassland mosaics. They exhibit a range of morphological similarities seen in European Miocene apes, suggestive of frugivory ranging from soft fleshy fruits to hard/tough ones (Teaford and Walker 1984; Wu et al. 2002; Merceron et al. 2006; Nelson 2007). In addition to the evidence from gnathic morphology and microwear, additional evidence of frugivory in middle and late Miocene apes includes caries, big brains, and circumstantial evidence from genetics. Fuss et al. ( 2018) describe crown caries in the Austrian late middle Miocene (12.5 Ma) ape Dryopithecus carinthiacus. They relate this to the frequent exploitation of sugar-rich fruits. Crown caries also occur in the Hungarian late Miocene ape Rudapithecus. Caries are relatively rare in extant primates, suggesting that Miocene apes were even more committed to sugar-rich fruits than apes are today (Fuss et al. 2018). Paleobotanical evidence of food resources with cariogenic sugars at St. Stefan, Austria, where D. carinthiacus is found, includes plants in the Prunus, Vitis, and Morus clades (Fuss et al. 2018).
The pathways involved in fructose metabolism vary among primates. In hominids several genetic events have led to a unique pattern of fructose metabolism. Efficient metabolism of fructose into stored fat requires the presence of high levels of serum uric acid. In most mammals the enzyme uricase metabolizes uric acid, resulting in low serum levels and less efficient conversion into fat. In hominids, a series of mutations has suppressed the production of uricase, leading to higher levels of serum uric acid. Uncontrolled levels of uric acid in the blood stream can have negative consequences, the best known of which is gout. An overproduction of fat has many more well-known negative health consequences (Johnson et al. 2020). A second set of mutations, involving the production of the URAT 1 enzyme, enhances the regulation of serum uric acid levels and normally maintains a balance between serum uric acid, fat production, and their health consequences (Tan et al. 2016). There are many advantages to efficiently converting fructose to fat and to maintaining high serum uric acid. Metabolism of fat into energy (glycolysis) requires less oxygen than mitochondrial (ATP) energy production. Glycolysis also makes metabolic water available during periods of resource scarcity. In addition to buffering during stressful periods, fructose metabolism favors an increase in glucose levels to fuel the brain and has positive effects on immunity and blood pressure (Johnson et al. 2020). The efficient storage and metabolism of fat is crucial for organisms with high energy demands, particularly related to brain size, that are subjected to periodic (seasonal) shortages of food. There is clear evidence of seasonal ecological and dietary stress in European fossil great apes. Kelley (2008) and Skinner et al. (1995) describe patterns of dental enamel hypoplasia that are consistent with seasonal stress.
Brain function is heavily dependent on fructose-glucose metabolism. Enhanced ability to process fructose to produce glucose for brain metabolism is thought to be related to the development of enlarged brains in hominids (Johnson et al. 2020). Only three Miocene ape specimens provide anatomical evidence of adult brain size. Ekembo nyanzae, an early Miocene ape from Kenya, had a relative brain size similar to hylobatids and baboons; the latter is the most encephalized Old World monkey (Falk 1983; Begun and Kordos 2004). In contrast, Rudapithecus hungaricus had a relative brain size in the range of great apes (Begun and Kordos 2004; Gunz et al. 2020). The brain size of Oreopithecus has been estimated indirectly from the size of the foramen magnum as well as its relatively small braincase (Harrison 1989; Alba et al. 2001; Begun and Kordos 2004). It falls among smaller brained cercopithecoids. This is consistent with the pattern of frugivory and fructose metabolism outlined above. Ekembo lived after the URAT 1 mutation regulating uric acid metabolism but before the suppression of the uricase gene. This resulted in some enhancement of fructose metabolism. By the time Rudapithecus evolved, additional mutations had cleared the way for further enhancement of fructose metabolism that made it possible to energize a significantly enlarged brain. This larger brain in turn enhanced the ability of fossil great apes to exploit resources under challenging conditions, and the feedback loop was set in place that would lead to the immensely enlarged brains of modern humans (Begun and Kordos 2004). Estimates of the timing of the two critical mutations in hominid evolution (uricase suppression and URAT 1 metabolism) vary. The enhanced role of URAT 1 to regulate uric acid serum levels results from a series of mutations, the last of which occurred about 27 Ma (Tan et al. 2016). The mutation leading to the suppression of uricase is timed at about 17 Ma.
To summarize, researchers agree that Eurasian Miocene fossil primates inhabited a wide range of environments from swampy forests to patchy woodland/grassland mosaics. All, except Oreopithecus, were primarily frugivorous. Frugivory encompasses a broad range of dietary strategies and there has been little discussion of the types of fruits exploited by different Miocene apes, other than a characterization of their mechanical properties. The humid forests of Europe before about 9.5 Ma included resources such as Ficus, Prunus, and Malus. The open deciduous forests in the Balkans after 9.5 Ma, contained shrubby trees that produced nuts or non-fleshy fruits (Mai 1995; Denk et al. 2018). However, there is little evidence for the actual floral composition of Balkan hominid localities, in particular, the presence of fleshy fruits. Several members of the Rosaceae clade evolved during this period and their radiation further illustrates the shift towards open forb and grassland ecology. Many woody species evolved typical shrubby tree habits, characteristic of open savanna or patchy forest landscapes. Likewise, fleshy fruit production allowed for more successful directed seed dispersal and the colonization of open patches.
Megafaunal Primate Seed Dispersal
Modern ecological studies show how effective primates are at dispersing seeds, an observation that can be extrapolated back to their frugivorous ancestors. Extensive studies across the tropics and beyond have illustrated the prevalence of seed dispersal by primates (Leighton and Leighton 1982; Lambert and Garber 1998; Cowlishaw and Dunbar 2000; Worman and Chapman 2006; Fourrier 2013; McConkey et al. 2015; Fuzessy et al. 2017). Lambert and Graber (1998) state that it “is apparent that many primate lineages exhibit dental, digestive, and/or sensory adaptations that aid in the exploitation of particular food types and that many lineages of flowering plants have evolved characteristics of fruits and seeds that facilitate seed dispersal.” Primates express more frugivorous behavior and evolutionary adaptation to frugivory than any other mammalian clade (Fleming and Kress 2011; Russo and Chapman 2011). Many primates, apes in particular, gain the majority of their caloric intake through fruit consumption (Ban et al. 2016). Primatologists estimate that 95% of tree species in tropical forests are dispersed via endozoochory (Terborgh et al. 2002). Observational studies have correlated high densities of primates, especially large-bodied species, and rich forest foraging patches, notably tree species with large fleshy fruits (Lambert and Garber 1998; Lambert 2001; Stevenson 2001). Fossil studies of Pleistocene and Holocene primates also illustrate the effective mutualism for large-fruit seed dispersal. Evidence for giant subfossil lemurs driving fruit evolution in Madagascar comes from the loss of range in all large-fruiting arboreal species following their extinction. The gradual loss of fruiting trees after the loss of the great lemurs indicates that these trees originally evolved larger fruits to attract the lemurs. Likewise, Gigantopithecus has been implicated in the dispersal of large-fruiting Prunus species in South Asia (see the following section for more details on these case studies).
Large-Bodied Primates and Seed Dispersal
Extant Primates
Ecologists recognize the importance of primates to tree species’ richness and abundance; in many tropical forests, fruit trees are largely limited to primate dispersers, as other large mammalian dispersers are functionally extinct (Clark et al. 2001; Koné et al. 2008). Often germination rates are extremely low without primate fruit consumption, due to heavy seed predation and fungal attack (Lambert 2001). Additionally, studies have consistently demonstrated that the loss of primate dispersal services in a forest can lead to reductions in vegetation richness and an inability for forests to regenerate (Beckman and Muller-Landau 2007; Nuñez‐Iturri and Howe 2007; Stoner et al. 2007; Wright et al. 2007; Stevenson and Aldana 2008). While some other mammalian clades, such as equids or proboscideans, opportunistically consume fruit as part of a browsing habit, primates are the only group of mammals where strict frugivory is a common practice (Turner 2001). Many studies have shown that seed dispersal is the most important ecological service provided by primates and that they increase overall biodiversity (Cowlishaw and Dunbar 2000; Russo and Chapman 2011). Primates not only consume large-seeded fruits, they also carry fruits with the seeds in them, and in some tropical forests they are one of the primary drivers of forest vegetation communities (Lambert and Garber 1998). The early fossil record suggests that primates may have diverged from other mammalian lineages as a response to obligate frugivory, notably targeting colorful avian-dispersed fruits (Sussman 1991; Reagan et al. 2001).
Large-bodied frugivorous mammals disperse a greater abundance and more diverse variety of seeds than other tropical mammals; they also spread seeds of larger sizes with more offspring provisioning (Peres 2002; Wotton & Kelly 2011). Fossil evidence illustrates that there is a correlation between bigger seeds and zoochoric dispersal (Tiffney 2004; Eriksson 2008). Larger animals have longer gut passages and, therefore, can disperse seeds to far-flung colonization locations (Ruxton and Schaefer 2012; Wotton and Kelly 2012). Several studies have demonstrated high post-digestion germination rates and extensive dispersal distances in orangutans (Pongo abelii and P. pygmaeus), chimpanzees (Pan troglodytes; Idani 1986; Wrangham et al. 1994; Gross-Camp and Kaplin 2005), bonobos (Pan paniscus), and gorillas (Gorilla gorilla). The seeds dispersed by chimpanzees and gorillas ranged in size from 0.1 to 2.7 cm, and seeds larger than 2.0 cm were readily dispersed over great distances (Wrangham et al. 1994). Some studies show that hundreds of seeds can be dispersed over great distances daily (Lambert 1999).
Some primate studies go so far as to imply social decision-making and reasoning in relation to access to feeding patches (Leighton and Leighton 1982), accounting for complex weather and temperature variables when choosing fruit foraging routes (Janmaat et al. 2006), and access to higher quality fruits as a preference to fruit abundance (Ban et al. 2014, 2016). Nonhuman great apes have proven to be particularly effective seed dispersers and actively select fruits based on high sugar concentration (Fuzessy et al. 2016, 2017). Many large mammals have coevolved seed-dispersal-based mutualism with large-fruiting trees, but primates are particularly successful at driving the evolution of larger fruits (Sussman et al. 2013). Primates essentially create orchards of primate-dispersed fruit trees, by consuming and carrying seeds to ideal growing sites (Lambert and Garber 1998; McConkey and Brockelman 2011; McConkey et al. 2014, 2015). Rindos (2013, p. 132) refers to these monkey hot spot forests as “monkey gardens,” noting that Canarium trees in the tropics tend to be central points for primate activity. Many other studies of primate-planted fruit forests show similar results (Hladik and Hladik 1967; Gartlan 1968; Glander 1975; Van der Pijl 1982).
It is difficult to ascertain what the diet of European late Miocene primates might have looked like, given that there are few extant primates that experience significant seasonality and because we lack detailed data on food sources available to them. The Japanese macaque (Macaca fuscata) may serve as a rough case study for at least some of the ecologies that European apes lived in, given their existence in deciduous forests that are snow-covered for a portion of the year. This level of seasonality is probably more extreme than what most European late Miocene apes dealt with; nonetheless, seasonal fruit availability would have been key to their survival. Extensive observational studies of semi-captive (in confined preserves) macaques illustrate the seasonal importance of fruits in their diet and the year-round importance of plants (Jaman et al. 2010). Similar observations were made of macaques in the Yakushima Forest (Maruhashi 1980; Hill 1997), and in a larger study spanning the range of the species, which also found variable diets based on ecological constraints (Tsuji et al. 2015). While large-fruiting Rosaceae were not among the arboreal species observed in any of these primate preserves, the macaques were important seed dispersers of fruits between 4 and 16 mm in diameter, but they also consumed smaller fruits (Noma and Yumoto 1997). Seasonally intense consumption of fruits is common in other large primate clades as well, including chimpanzees that occupy mixed ecological settings that include more open savanna landscapes. Observational studies of Bornean orangutans (Pongo pygmaeus ssp. morio) of the Danum Valley, Sabah, Malaysia, show that they will intensively consume fruits of Dipterocarpus during masting periods and regularly consume moderate amounts of continually fruiting Ficus and Spatholobus during periods between (Kanamori et al. 2010).
Extinct Megafaunal Primates
Beyond a review of the hominids of Miocene Europe, another way to probe the likelihood that Miocene apes were major fruit seed dispersers is to explore what happened to fruit distributions in other cases where megafaunal primates went extinct. As an example, in South and Southeast Asia, large-bodied primates may have been a disperser of large fruits in the Prunus spp. clade, notably the extinct Prunus kunmingensis (Su et al. 2015); Indopithecus, at 6.5 million years ago, may or may not be related to Gigantopithecus, the latter mostly known from fossils between about 1 million and 300,000 years ago. Gigantopithecus, mostly known from China, may have also been present in Vietnam, Thailand, and Indonesia (Bocherens et al. 2017). The onset of the Pleistocene and expansion of savannas further south, eliminating the arboreal food sources, may have contributed to the eventual extinction of the clade. Reconstructions of the ecological habitat of this massive ape suggest that it occupied warm-temperate to subtropical climates, in mixed deciduous and evergreen broad-leaved forests (Jin et al. 2008; Li et al. 2014). Paleontological studies suggest that these apes shared the forested landscape with a range of other seed-dispersing primates, including Macaca (several species), Rhinopithecus, Pygathrix, Trachypithecus, Nomascus, and Pongo (Takai et al. 2014). Studies of their dental morphology and phytoliths in dental calculus suggest a largely plant-based diet, with fruits, nuts, roots, and possibly bamboo shoots (McKee et al. 2015). Stable isotope analysis of enamel in Gigantopithecus indicates that it was a forest dweller with a diet most like that of Pongo (Zhao et al. 2011; Qu et al. 2014). Gigantopithecus also has a relatively high frequency of carious lesions, both crown and marginal, ranging from 9.8 to 19.5% of teeth in three different samples (Han and Zhao 2002; Wang 2009). This is significantly higher than in any other sample of extant or extinct hominoids and suggests a diet that included sugary carbohydrates, notably fruits, consistent with other lines of evidence (Ciochon et al. 1990; Zhao et al. 2011; Nelson 2014; Qu et al. 2014; Bocherens et al. 2017; Zhang & Harrison 2017). Other late Pleistocene megafaunal opportunistic fruit-eating seed dispersers of Asia included the straight-tusked elephant (Palaeoloxodon spp.), boar (Sus spp.), and bears (e.g., Ursus tibethanus, Helarctos malayanus, and Melursus ursinus)—collectively suggesting that forests were rich in fruiting trees.
Another informative case of extinct megafaunal primates serving as the primary seed dispersers for trees with megafruits comes from the giant lemurs of Madagascar (e.g., Megaladapis edwardsi). Paleoprimatologists estimate that as many as ten species of lemur went extinct as recently as two millennia ago; this recent extinction allows scholars to study the effects of the loss of seed-dispersal services (Burney et al. 2004; Crowley et al. 2011). While two of these extinct species have specifically been identified as seed dispersers, Archaeolemur majori and Pachylemur insignis (Godfrey et al. 2008), several others have been identified as large-bodied fruit eaters (Crowley et al. 2011). At least eleven genera of trees with large fleshy fruits exist in southern Madagascar; all of which were likely reliant upon the giant lemurs. However, seed dispersal in some of these species does continue on a limited scale through extant smaller-bodied lemurs (Crowley et al. 2011). Genetic studies illustrate that many of these tree populations, without their giant carriers, are now fragmenting and becoming genetically isolated (Voigt et al. 2009), a similar pattern to what we see around the world in other megafruit trees during the Holocene.
Several paleontologists have noted that a general diversification of angiosperms, and specifically the development of larger fruits, occurred globally during the late Paleocene and early Eocene (Tiffney 2004; Sussman et al. 2013). The role of primates in driving the evolution of angiosperms, especially fruiting trees, was clearly laid out by Sussman (Sussman 1991), in his angiosperm/primate coevolution theory. His interest rested on the Eocene evolution of arboreal traits, color vision, and other features specifically derived in primates largely for the acquisition of fruits (Velasco et al. 2016; Yu et al. 2018). In this theoretical framework, the mutualistic link drove angiosperms to develop larger and sweeter fruits as an evolutionary adaptation for recruiting primate dispersers. Hence, the evolution of larger fruits in Europe during the late Miocene may represent a continuation of the trajectory towards diversification and radiation of angiosperms starting in the Eocene. However, like most seed dispersal, the bonds of mutualism appear to have been tied into a guild of species and mammals, including the fruit-focused lineages of primates and bats, and birds both radiate around this period (Tiffney 2004; Meredith et al. 2011). Valenta et al. ( 2018) have recently readdressed the question of whether primates were responsible for a suite of dispersal traits shared among arboreal tropical species.
Color Vision and Visual Ripeness Displays in Fruits
Scholars have debated the likelihood that primates were a driving factor in the evolution of angiosperm fruit features (Sussman et al. 2013; Valenta et al. 2018). Some scholars refer to this as the “frugivory hypothesis” (Nevo et al. 2018; Onstein et al. 2020), and Sussman et al. (2013) emphasized the role of Eocene primates in their angiosperm/primate coevolution theory. Supporting this theory, increasing evidence seems to point to a suite of traits that have evolved in parallel among tropical trees in order to recruit primate (and similarly avian and chiropteran) dispersers (Janson 1983; Stevens et al. 2009; Valenta et al. 2013, 2015; Melin et al. 2014; Valenta 2014; Nevo et al. 2018). Discussions continue regarding which fruit characteristics are closely associated with primates as opposed to other seed-dispersing animals, and studies illustrate that primates have preferences towards fruits with certain colors (Valenta et al. 2016, 2018; Valenta and Chapman 2018). Many of the traits associated with primate-dispersed fruits would have attracted a larger guild of species, including many now-extinct megafaunal mammals, making tight coevolutionary linkages unlikely. Nonetheless, as the only mammals with trichromatic color vision, the visual displays of ripeness that most catarrhine primate-dispersed fruits utilize for signaling are a strong contender for a clade-specific recruitment feature. This view is further supported by the fact that fruits that are more closely tied with non-primate megafaunal dispersers tend to remain green, yellow, or brown at ripeness. Additionally, a recent study that mapped the distribution of bright-fruiting Arecaceae and primates with color vision noted a strongly linked relationship (Onstein et al. 2020). The authors of that study suggest that the availability of palm fruits resulted in a coevolutionary relationship between the species leading to the dynamics of primate color vision systems and palm fruit colors. They argue that the rapid radiation of primates with color vision and brightly colored palm species in Africa ten million years ago are correlated.
Avian-dispersed plants, such as the rose, have fruits that turn red when fully ripe to signal birds. Birds are attracted to bright colors, notably red, which is why so many avian-dispersed berries are red, for example, Rubus, Sorbus, Vaccinium, Viburnum, and Rosa. It is reasonable to assume that the red color of a ripe apple is a plesiomorphic trait given that Rosaceae fruits of the early Miocene were likely avian dispersed. However, its persistence as a trait or possible derived presence in Malus could be linked to disperser recruitment. Red fruits, as in wild Malus spp., are rare among large fruits. Most plants that relied on now-extinct megafauna that were color blind and utilized an olfactory foraging system have fruits with high sugar or oil content, and are green, yellow, or brown when ripe. “Contrary to fruit assemblages from different communities, the range of fruit colors of megafaunal species is very restricted” (Guimarães et al. 2008).
Most mammals have dichromatic vision; scholars have theorized that this basal trait is a relic of a mammalian bottleneck during the K-Pg extinction event at the Cretaceous–Paleogene boundary around 65 million years ago (Wu et al. 2017). If small subterranean and nocturnal mammals were the only clades to survive the mass extinction, then all resulting lineages would have dichromatic vision, which is advantageous under low-light conditions. Trichromatic vision evolved independently in several primate lineages; notably the great apes and howler monkeys as well as females of certain species of New World monkeys (Rowe 2018). These primate lineages likely coevolved with fruiting tree species that used bright colors to signal ripeness of fruits in order to attract frugivorous birds. Having the ability to identify red ripe fruits from a distance gave a selective advantage to both the tree and the primate, supporting the idea of a coevolutionary linkage between angiosperms and primates.
Olfactory foraging is the basal state for most mammals; however, in Old World primates, especially the apes, olfactory abilities have been de-emphasized as trichromatic color vision and better depth perception developed (Fobes 1982; Osorio and Vorobyev 1996). Primates are the only eutherian mammals with full trichromatic vision (Nevo and Heymann 2015). The unique quality of color vision in Old World primates and howler monkeys is linked to several types of cone photopigments in the eye and special neural processors (Leonhardt et al. 2009; Nevo and Heymann 2015). The loss of scent perception in favor of visual abilities is usually thought to be a major component in the evolution of the hominin lineage (Smith 1927). Studies of primates have illustrated the importance of visual cues in foraging and food acquisition, specifically demonstrating that in many primate lines olfactory-guided long-distance food acquisition is challenging (Leonhardt et al. 2009; Rushmore et al. 2012; Nevo and Heymann 2015). Interestingly, nocturnal primates tend to rely more on scent tracking, and howler monkeys (Alouatta spp.), the only New World primates (platyrrhines) with color vision, have reduced olfactory perception as well (Leonhardt et al. 2009; Nevo and Heymann 2015). Genetic studies of apes, Old World monkeys, and howler monkeys have shown that they possess a significantly higher proportion of olfactory pseudogenes than other New World monkeys (Gilad et al. 2004). Geneticists have linked the pseudogenes to a degradation of the olfactory receptors and an increased reliance on visual cues (Dominy and Lucas 2001). Studies suggest that Old World monkeys developed color vision 23 million years ago (Yokoyama and Yokoyama 1989), which would support the model that they developed this trait in order to obtain angiosperm fruits that were intended for birds. There is also reason to believe that the loss of olfactory reception is tied in to the loss of pheromone signaling in primates (Gilad et al. 2004).
Megafaunal Monkeys and Megafloral Roses
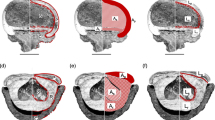
Many of the megafaunal-dispersed fruits that survived into the first half of the Holocene have become important components in the human diet and benefit from anthropogenic seed-dispersal services (Levis et al. 2017, 2018; van Zonneveld et al. 2018). Tropical “megafruits” have maintained their gene flow regimes, due to the continual presence of at least some megafauna, including humans and bears, certain large clasping birds such as parrots, and bats. However, in temperate zones, most members of the former disperser guilds are now extinct and much of the extant megafauna is composed of grazing animals. The lack of Gomphotheridae, Xenarthra, and lower densities of Mammutidae in Europe during the Pleistocene as opposed to the Americas further complicates the question of what species served as seed dispersers. Ruminant grazers and animals with multichambered stomachs tend to avoid sugary fruits, which ferment in the gut and cause high rates of methane production; additionally, their restricted caecum blocks the rapid processing of large seeds (Fig. 3). While medium-sized omnivores, notably boars, bears, and humans, opportunistically disperse these species, most megafruiting trees in temperate zones and in many tropical regions are currently endangered. While the paleo-distribution ranges in these species require further study, they appear to have slowly lost range throughout the Holocene (Galetti et al. 2018; Pires et al. 2018; Spengler 2019; Onstein et al. 2020).
Modern observations of apple consumption and seed dispersal suggest that birds and rodents either avoid the seeds or destroy them through seed predation. Likewise, ungulate grazers largely avoid or only selectively consume the fruits. Counterclockwise from bottom left: (A) a squirrel gnawing through an apple in order to consume the seeds; (B) two images of partially consumed fruit—birds ate the fruit but failed to disperse the seeds; and (C) apples lying in a cattle field and avoided by the grazers
The rose hip is small and red, in most cases small enough for birds, which do not masticate their food. The apple is clustered with other mega-rosaceous fruits, including the pear (Pyrus) and quince (Cydonia). These mega-fruits evolved to attract a seed disperser much larger than a bird; therefore, we can think of an apple tree as a mega-floral rose bush. Looking at the temperate forests of Eurasia, there are fewer potential seed dispersers than in the tropical belt. Likewise, many animals that may seem like candidates for a dispersal guild either ignore or only selectively consume sugary fruits, such as ruminants (see Fig. 3, illustrating feral apples rotting in a cow pasture). Additionally, many small mammals serve as seed predators and birds fail to disperse the seeds (Fig. 3). Hominids were the only megafaunal dispersers in late Miocene Eurasia with trichromatic vision, allowing them to observe, and select for, the evolved red color of ripe Malus spp. fruits. They were also the only large arboreal animals. Ripe apples often remain on the tree even after the leaves fall due to a lack of function in pedicle abscission zones, a typical adaptation for megafaunal-dispersed fruits that helps them avoid rodent seed predation.
Comprehensive studies of the fossil record of Miocene Eurasia suggest that fleshy-fruit-based endozoochory was rare overall on a landscape dominated by grazers and open woodlands (Fortelius et al. 2006). However, simultaneously, fleshy-fruiting members of the Rosaceae clade radiated and diversified. At least two independent lineages, Maloideae and Prunoideae, developed larger fruits in parallel, simultaneously evolving higher sugar concentrations. Seed dispersal is important in maintaining diversity and genetic connectivity within and across populations (Nathan and Muller-Landau 2000; Jara-Guerrero et al. 2018). Seed dispersal is also essential for colonization, especially in arboreal species (Escribano‐Avila et al. 2014). Therefore, identifying the members of a dispersal guild can inform scholars about the evolutionary processes at play on the largely savanna or patchy forest landscape of Eurasia during the late Miocene. While this landscape would have contained high densities of animal species, many of them, such as small mammals and birds, would have failed to disperse seeds, either due to seed predation or seed avoiding (Fig. 3). Additionally, grazing animals mostly avoid fleshy fruits, and in the case of ruminant digesters would have destroyed seeds of large Maloideae species. Although primates are not the only potential species that may have dispersed rosaceous seeds in Eurasia during the late Micocene, they were likely a prominent disperser in the guild (Table 1).
Conclusion
Most examples of endozoochoric megafruits have muted colors, often yellow, green, or brown. Asimina triloba of North America or related Annona fruits of Central and South America are good examples, with large hard seeds, soft sweet flesh, and remaining green when ripe. Additionally, many, such as the cucurbits and Maclura pomifera, do not possess high sugar concentrations. Wild apples contain red morphotypes, have high sugar levels, and they contain small easily crushed seeds. Fruits that were likely dispersed by elephantine or xenarthran species often contain large seeds or pits, easily mashed fruit coats, muted colors, and oily or low-sugar fruits. Smaller fruiting members of Rosaceae attract avian dispersers; however, the fruits of many Maloideae and Prunoideae are too large for frugivorous birds to swallow. It is probable that red fruits of M. sylvestris and M. sieversii, which are too large for avian dispersal, evolved to attract a megafaunal disperser that possessed trichromaticism, likely one that cannot swallow large pits. Additionally, there were fewer frugivores on the pre-Holocene landscape of Europe than in the Americas, where gomphotheres and other proboscideans, and xenarthrans likely drove the evolution of larger fruits. Ruminant dispersers and other grazing animals avoid sweet fleshy fruits and tend to be destructive of larger seeds.
A fleshy fruit dispersal guild in late Miocene Europe likely included bear, rhinoceros, proboscideans, equids, and suids, all of which would have selected for larger fruiting hybrid examples of Malus trees. Large primates were prominent on the European landscape during the Miocene. Fossil evidence seems to suggest that these primates evolved new traits to adapt to ecological changes during the late Miocene, including dental features that suggest specialized frugivory. The limited availability of other large-fruiting trees in late Miocene Europe points to a strong focus on Rosaceae. Similarly, studies of fruit consumption among both extant and extinct apes further supports the present hypothesis. Identifying the exact drivers of evolution is always difficult, and organisms often evolve in a complex milieu of selective forces, many of which are complementary. Likewise, seed dispersal often relies on guilds rather than a specific species; however, humans appear to have been the main dispersers for M. sylvestris in Europe since at least the Mesolithic and of M. sieversii and M. orientalis during the past few millennia. Given the close relationship between people and the apple over the past few hundred thousand years, as evidenced in the archaeobotanical record, it is reasonable to assume a deeper time depth for this mutualism. Further paleontological research, especially targeting rare fleshy fruit fossils, may validate or refute the theories that we present here, but in either case, it is clear that collaborations between domestication scholars and evolutionary ecologists can prove to be fruitful. Ultimately, this study demonstrates that the coevolutionary process of human-driven evolution that we colloquially call “domestication” has deeper roots in our hominin lineage.
References
Agustı J (2007) The biotic environments of the late miocene hominids. Handbook of Paleoanthropology. Springer, Berlin, pp 979–1010
Agustı J, De Siria AS, Garcés M (2003) Explaining the end of the hominoid experiment in Europe. J Hum Evol 45(2):145–153
Alba D, Moyà-Solà S, Köhler M, Rook L (2001) Heterochrony and the cranial anatomy of Oreopithecus: some cladistic fallacies and the significance of developmental constraints in phylogenetic analysis. In: Bonis L, Koufos G, Andrews P (eds) Hominoid evolution and climatic change in Europe: phylogeny of the Neogene hominid primates of Eurasia, vol 2. Cambridge University Press, Cambridge, pp 284–315
Allué E (2016) Charcoal remains from Azokh 1 cave: preliminary results. In: Fernández-Javlo Y, King T, Yepiskoposyan L, Andrews P (eds) Azokh Cave and the Transcaucasian Corridor. Vertebrate Paleobiology and Paleoanthropology. Springer, Cham, pp 297–304
Allué E, Ibáñez N, Saladié P, Vaquero M (2010) Small preys and plant exploitation by late pleistocene hunter–gatherers. a case study from the Northeast of the Iberian Peninsula. Archaeol Anthropol Sci 2(1):11–24
Antolín F, Bleicher N, Brombacher C, Kühn M, Steiner BL, Jacomet S (2016) Quantitative approximation to large-seeded wild fruit use in a late Neolithic lake dwelling: new results from the case study of layer 13 of Parkhaus Opéra in Zürich (Central Switzerland). Quaternary Int 404:56–68
Ban SD, Boesch C, Janmaat KR (2014) Taï chimpanzees anticipate revisiting high-valued fruit trees from further distances. Anim Cogn 17(6):1353–1364
Ban SD, Boesch C, N’Guessan A, N’Goran EK, Tako A, Janmaat KR (2016) Taï chimpanzees change their travel direction for rare feeding trees providing fatty fruits. Anim Behav 118:135–147
Bateman RM, Catt JA (2007) Provenance and palaeoenvironmental interpretation of superficial deposits, with particular reference to post-depositional modification of heavy mineral assemblages. Dev Sedimentol 58:151–188
Beckman NG, Muller-Landau HC (2007) Differential effects of hunting on pre‐dispersal seed predation and primary and secondary seed removal of two Neotropical tree species. Biotropica 39(3):328–339
Begun DR, Kordos L (1997) Phyletic affinities and functional convergence in Dryopithecus and other Miocene and living hominids. In: Begun DR, Ward CV, Rose M, De (eds) Function, phylogeny, and fossils. Advances in Primatology. Springer, Boston, pp 291–316
Begun DR, Kordos LO (2004) Cranial evidence of the evolution of intelligence in fossil apes. In: Russon A, Begun DR (eds) The evolution of thought: evolutionary origins of great ape intellegence. Cambridge University Press, Cambridge, pp 260–279
Begun DR, Nargolwalla MC, Kordos L (2012) European Miocene hominids and the origin of the African ape and human clade. Evol Anthropol 21(1):10–23
Behm-Blancke G (1960) Altsteinzeitliche Rastplätze im Travertingebiet von Taubach, Weimar, Ehringsdorf. VEB Landesdruckerei Thüringen, Weimar
Bocherens H, Schrenk F, Chaimanee Y, Kullmer O, Mörike D, Pushkina D, Jaeger J-J (2017) Flexibility of diet and habitat in Pleistocene South Asian mammals: implications for the fate of the giant fossil ape Gigantopithecus. Quaternary In 434:148–155
Böhme M, Spassov N, Ebner M, Geraads D, Hristova L, Kirscher U et al (2017) Messinian age and savannah environment of the possible hominin Graecopithecus from Europe. PloS One, 12(5): e0177347
Börker J, Hartmann J, Amann T, Romero-Mujalli G (2018) Terrestrial sediments of the Earth: development of a global unconsolidated sediments map database (GUM). Geochem Geophys Geosyst 19:997–1024. https://doi.org/10.1002/2017GC007273
Bouchal JM, Güner TH, Denk T (2018) Middle Miocene climate of southwestern anatolia from multiple botanical proxies. Clim Past 14:1427–1440
Burney DA, Burney LP, Godfrey LR, Jungers WL, Goodman SM, Wright HT, Jull AT (2004) A chronology for late prehistoric Madagascar. J Hum Evol 47(1–2):25–63
Campos-Arceiz A, Larrinaga AR, Weerasinghe UR, Takatsuki S, Pastorini J, Leimgruber P et al (2008) Behavior rather than diet mediates seasonal differences in seed dispersal by Asian elephants. Ecology 89(10):2684–2691
Carruthers W (2000) The charred hazelnut shell and other plant remains. In: Mithen SJ (ed) Hunter-gatherer landscape archaeology: the Southern Hebrides Mesolithic Project 1988–1998, vol 2. MacDonald Institute for Archaeological Research, Cambridge, pp 407–415
Caton J, Hume I, Hill D, Harper P (1999) Digesta retention in the gastro-intestinal tract of the orang utan (Pongo pygmaeus). Primates 40(4):551–558
Ciochon RL, Olsen JW, James J (1990) Other origins: the search for the giant ape in human prehistory. Bantam Dell, New York
Clark C, Poulsen J, Parker V (2001) The role of arboreal seed dispersal groups on the seed rain of a lowland tropical forest. Biotropica 33(4):606–620
Clarke DL (1978) Mesolithic Europe: the economic basis. Duckworth, London
Cornille A, Giraud T, Smulders MJ, Roldán-Ruiz I, Gladieux P (2014) The domestication and evolutionary ecology of apples. Trends Genet 30(2):57–65
Cornille A, Feurtey A, Gélin U, Ropars J, Misvanderbrugge K, Gladieux P, Giraud T (2015) Anthropogenic and natural drivers of gene flow in a temperate wild fruit tree: a basis for conservation and breeding programs in apples. Evol Appl 8(4):373–384
Cowlishaw G, Dunbar RI (2000) Primate conservation biology. University of Chicago Press, Chicago
Crowley BE, Godfrey LR, Irwin MT (2011) A glance to the past: subfossils, stable isotopes, seed dispersal, and lemur species loss in southern Madagascar. Am J Primatol 73(1):25–37
de Bonis L, Koufos GD (1993) The face and the mandible of Ouranopithecus macedoniensis: description of new specimens and comparisons. J Hum Evol 24(6):469–491
de Bonis L, Koufos GD (1994) Our ancestors’ ancestor: ouranopithecus is a greek link in human ancestry. Evol Anthropol 3(3):75–83
de Bonis L, Koufos GD (2014) First discovery of postcranial bones of Ouranopithecus macedoniensis (Primates, Hominoidea) from the late Miocene of Macedonia (Greece). J Hum Evol 74:21–36
Deane AS, Nargolwalla MC, Kordos L, Begun DR (2013) New evidence for diet and niche partitioning in Rudapithecus and Anapithecus from Rudabánya, Hungary. J Hum Evol 65(6):704–714
Denk T, Zohner CM, Grimm GW, Renner SS (2018) Plant fossils reveal major biomes occupied by the late Miocene Old-World Pikermian fauna. Nat Ecol Evol 2(12):1864–1870
DeVore M, Pigg K (2007) A brief review of the fossil history of the family Rosaceae with a focus on the Eocene Okanogan Highlands of eastern Washington State, USA, and British Columbia, Canada. Plant Syst Evol 266(1–2):45–57
Dickinson T, Lo E, Talent N (2007) Polyploidy, reproductive biology, and Rosaceae: understanding evolution and making classifications. Plant Syst Evol 266(1–2):59–78
Dominy NJ, Lucas PW (2001) Ecological importance of trichromatic vision to primates. Nature 410(6826):363–366
Duan N, Bai Y, Sun H, Wang N, Ma Y, Li M et al (2017) Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nat Commun 8(1):1–11
Elliot Smith G (1927) The evolution of man. Oxford University Press, London
Eriksson O (2008) Evolution of seed size and biotic seed dispersal in angiosperms: paleoecological and neoecological evidence. Int J Plant Sci 169(7):863–870
Escribano-Avila G, Calviño‐Cancela M, Pías B, Virgós E, Valladares F, Escudero A (2014) Diverse guilds provide complementary dispersal services in a woodland expansion process after land abandonment. J Appl Ecol 51(6):1701–1711
Evans RC, Campbell CS (2002) The origin of the apple subfamily (Maloideae; Rosaceae) is clarified by DNA sequence data from duplicated GBSSI genes. Am J Bot 89(9):1478–1484
Falk D (1983) A reconsideration of the endocast of Proconsul africanus. In: Ciochon RL, Corruccini RS (eds) New interpretations of ape and human ancestry. Advances in Primatology. Springer, Boston, pp 239–248
Fedorov A, Lavoott R, Komarova L (1971) Flora of the USSR Rosaceae-Rosoideae-Prunoideae. Israel Program for Scientific Translations 10:380–448
Fleming TH, Kress WJ (2011) A brief history of fruits and frugivores. Acta Oecol 37(6):521–530
Fobes J (1982) Vision: the dominant primate modality. In: Fobes JL, King JE (eds) Primate behavior. Academic Press, New York, pp 219–243
Fortelius M (2015) New and Old Worlds database of fossil mammals (NOW). https://nowdatabase.org/. Accessed 7 Sept 2022
Fortelius M, Eronen J, Liu L, Pushkina D, Tesakov A, Vislobokova I, Zhang Z (2006) Late Miocene and pliocene large land mammals and climatic changes in Eurasia. Palaeogeogr Palaeoclimatol Palaeoecol 238(1–4):219–227
Fourrier MS (2013) The spatial and temporal ecology of seed dispersal by gorillas in Lopé National Park, Gabon: linking patterns of disperser behavior and recruitment in an Afrotropical forest. Dissertation, Washington University in St. Louis. All Theses and Dissertations (ETDs) https://doi.org/10.7936/K7SJ1HJN
Fuss J, Spassov N, Begun DR, Böhme M (2017) Potential hominin affinities of Graecopithecus from the Late miocene of Europe. PLoS ONE 12(5):e0177127
Fuss J, Uhlig G, Böhme M (2018) Earliest evidence of caries lesion in hominids reveal sugar-rich diet for a middle miocene dryopithecine from Europe. PLoS ONE 13:8
Fuzessy LF, Cornelissen TG, Janson C, Silveira FA (2016) How do primates affect seed germination? A meta-analysis of gut passage effects on neotropical plants. Oikos 125(8):1069–1080
Fuzessy LF, Janson CH, Silveira FA (2017) How far do neotropical primates disperse seeds? American J Primatol 79(7):e22659
Galdikas B (1982) Orangutans as seed dispersers at Tanjung Puting, Central Kalimantan: implications for conservation. In: de Boer LEM (ed) The orangutan: its biology and conservation. Dr. W. Junk B.V. Publishers, The Hague, p 285
Galetti M, Donatti CI, Pires AS, Guimarães JR, P J (2006) Seed survival and dispersal of an endemic Atlantic forest palm: the combined effects of defaunation and forest fragmentation. Bot J Linn Soc 151(1):141–149
Galetti M, Moleón M, Jordano P, Pires MM, Guimaraes PR Jr, Pape T et al (2018) Ecological and evolutionary legacy of megafauna extinctions. Biol Rev 93(2):845–862
García-Rodríguez A, Selva N, Zwijacz-Kozica T, Albrecht J, Lionnet C, Rioux D et al (2021) The bear-berry connection: ecological and management implications of brown bears’ food habits in a highly touristic protected area. Biol Conserv 264:109376
Gartlan JS, B. C (1968) Ecology and social variability in Cercopithecus aethiops and C. mitis. Holt, Rinehart & Winston, New York
Gilad Y, Wiebe V, Przeworski M, Lancet D, Pääbo S (2004) Loss of olfactory receptor genes coincides with the acquisition of full trichromatic vision in primates. PLoS Biol 2(1):e148
Glander KE (1975) Habitat description and resource utilization: a preliminary report on mantled howling monkey ecology. Socioecology and Psychology of Primates. Mouton, The Hauge, pp 37–57
Godfrey LR, Jungers WL, Schwartz GT, Irwin MT (2008) Ghosts and orphans: Madagascar’s vanishing ecosystems. In: Fleagle GC (ed) Elwyn Simons: A search for origins. Springer, New York, pp 361–395
Gregor H-J, Vodickova V (1983) Paläokarpologische Charakteristik der pleistozänen Travertine des Neckartales bei Stuttgart. Stuttgarter Beiträge zur Naturkunde, Seria B (Geologie und Paläontologie) 94. Institut für Geologie und Paläontologie, Stuttgart
Gross BL, Volk GM, Richards CM, Reeves PA, Henk AD, Forsline PL et al (2013) Diversity captured in the USDA-ARS National Plant Germplasm system apple core collection. J Am Soc Hortic Sci 138(5):375–381
Gross-Camp N, Kaplin BA (2005) Chimpanzee (Pan troglodytes) seed dispersal in an afromontane forest: microhabitat influences on the postdispersal fate of large seeds. Biotropica 37(4):641–649
Guimarães PR Jr, Galetti M, Jordano P (2008) Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS ONE 3(3):e1745
Güleç ES, Sevim A, Pehlevan C, Kaya F (2007) A new great ape from the late Miocene of Turkey. Anthropol Sci 115(2):153–158
Gümbel F, Mai DH (2004) Neue Pflanzenfunde aus dem Tertiär der Rhön. Teil 2: Pliozäne Fundstellen. Fossil Record 7(1):175–220
Gunz P, Kozakowski S, Neubauer S, Le Cabec A, Kullmer O, Benazzi S et al (2020) Skull reconstruction of the late Miocene ape Rudapithecus hungaricus from Rudabánya, Hungary. J Hum Evol 138:102687
Hammond AS, Rook L, Anaya AD, Cioppi E, Costeur L, Moyà-Solà S, Almécija S (2020) Insights into the lower torso in late Miocene hominoid Oreopithecus bambolii. PNAS 117(1):278–284
Hamon N, Sepulchre P, Donnadieu Y, Henrot A-J, François L, Jaeger J-J, Ramstein G (2012) Growth of subtropical forests in Miocene Europe: the roles of carbon dioxide and Antarctic ice volume. Geology 40(6):567–570
Han K, Zhao L (2002) Dental caries of Gigantopithecus blacki from Hubei Province of China. Acta Anthropol Sin 21(3):191–196
Hancock J, Luby JJ, Brown S, Lobos G (2008) Apples. In: Hancock J (ed) Temperate fruit crop breeding: germplasm to genomics. Springer, The Netherlands, pp 1–38
Harich FK, Treydte AC, Ogutu JO, Roberts JE, Savini C, Bauer JM, Savini T (2016) Seed dispersal potential of Asian elephants. Acta Oecol 77:144–151
Harris SA, Robinson JP, Juniper BE (2002) Genetic clues to the origin of the apple. Trends Genet 18(8):426–430
Harrison T (1989) New estimates of cranial capacity, body size and encephalization in Oreopithecus bambolii. Am J Phys Anthropol 78:237
Harrison ME (2009) Orang-utan feeding behaviour in Sabangau, Central Kalimantan. Doctoral thesis, University of Cambridge
Heizmann EP, Begun DR (2001) The oldest Eurasian hominoid. J Hum Evol 41(5):463–481
Herbert TD, Lawrence KT, Tzanova A, Peterson LC, Caballero-Gill R, Kelly CS (2016) Late Miocene global cooling and the rise of modern ecosystems. Nat Geosci 9(11):843–847
Hill DA (1997) Seasonal variation in the feeding behavior and diet of Japanese macaques (Macaca fuscata yakui) in lowland forest of Yakushima. Am J Primatol 43(4):305–320
Hladik CM, Hladik A (1967) Observations sur le rôle des primates dans la dissémination des végétaux de la forêt gabonaise. Biol Gabonica 3:43–58
Hublin J-J (2009) The origin of Neandertals. PNAS 106(38):16022–16027
Idani G (1986) Seed dispersal by pygmy chimpanzees (Pan paniscus): a preliminary report. Primates 27(4):441–447
Jaman MF, Huffman MA, Takemoto H (2010) The foraging behavior of Japanese macaques Macaca fuscata in a forested enclosure: effects of nutrient composition, energy and its seasonal variation on the consumption of natural plant foods. Curr Zool 56(2):198–208
Janmaat KR, Byrne RW, Zuberbühler K (2006) Primates take weather into account when searching for fruits. Curr Biol 16(12):1232–1237
Janson CH (1983) Adaptation of fruit morphology to dispersal agents in a neotropical forest. Science 219(4581):187–189
Janzen DH, Martin PS (1982) Neotropical anachronisms: the fruits the gomphotheres ate. Science 215(4528):19–27
Jara-Guerrero A, Escribano‐Avila G, Espinosa CI, De la Cruz M, Méndez M (2018) White‐tailed deer as the last megafauna dispersing seeds in neotropical dry forests: the role of fruit and seed traits. Biotropica 50(1):169–177
Jin CZ, Qin DG, Pan WS, Wang Y, Zhang Y, Deng CL, Zheng JJ (2008) Micromammals of the Gigantopithecus fauna from Sanhe cave, Chongzuo, Guangxi. Quaternary Sci 28(6):1129–1137
Johnson RJ, Stenvinkel P, Andrews P, Sánchez-Lozada LG, Nakagawa T, Gaucher E et al (2020) Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J Intern Med 287(3):252–262
Jongmans W (1915) Fossilium catalogus: II Plantae. W. Junk, Berlin
Kanamori T, Kuze N, Bernard H, Malim TP, Kohshima S (2010) Feeding ecology of Bornean orangutans (Pongo pygmaeus morio) in Danum Valley, Sabah, Malaysia: a 3-year record including two mast fruitings. Am J Primatol 72(9):820–840
Kaplan JO, Pfeiffer M, Kolen JC, Davis BA (2016) Large scale anthropogenic reduction of forest cover in last glacial maximum Europe. PLoS ONE 11(11):e0166726
Kay RF, Ungar PS (1997) Dental evidence for diet in some Miocene catarrhines with comments on the effects of phylogeny on the interpretation of adaptation. In: Begun D, Ward C, Rose M (eds) Function, phylogeny, and fossils. Springer, Boston, pp 131–151
Kelley J (2008) Identification of a single birth cohort in Kenyapithecus kizili and the nature of sympatry between K. kizili and Griphopithecus alpani at Paşalar. J Hum Evol 54(4):530–537
King T (2001) Dental microwear and diet in Eurasian Miocene catarrhines. In: de Bonis L, De KG, Andrews P (eds) Hominoid evolution and climatic change in Europe, volume 2. Phylogeny of the Neogene hominoid primates of Eurasia. University Press Cambridge, Cambridge, pp 102–117
Kitamura S, Yumoto T, Poonswad P, Wohandee P (2007) Frugivory and seed dispersal by Asian elephants, Elephas maximus, in a moist evergreen forest of Thailand. J Trop Ecol 23(3):373–376
Koné I, Lambert JE, Refisch J, Bakayoko A (2008) Primate seed dispersal and its potential role in maintaining useful tree species in the Taï region, Côte-d’Ivoire: implications for the conservation of forest fragments. Trop Conserv Sci 1(3):293–305
Kot M (2017) Bifacial and unifacial technology: a real difference or a problem of typo–technological approach? the example of the Ehringsdorf assemblage. Quat Int 428:66–78
Koufos GD, de Bonis L (2017) Upper incisor morphology of the late Miocene hominoid Ouranopithecus macedoniensis from Axios Valley (Macedonia, Greece). Anthropol Sci 125(3):141–151
Kvaček Z, Teodoridis V, Denk T (2020) The Pliocene flora of Frankfurt am Main, Germany: taxonomy, palaeoenvironments and biogeographic affinities. Palaeobio Palaeoenv 100:647–703
Lambert JE (1999) Seed handling in chimpanzees (Pan troglodytes) and redtail monkeys (Cercopithecus ascanius): implications for understanding hominoid and cercopithecine fruit-processing strategies and seed dispersal. Am J Phys Anthropol 109(3):365–386
Lambert JE (2001) Red-tailed guenons (Cercopithecus ascanius) and Strychnos mitis: evidence for plant benefits beyond seed dispersal. Int J Primatol 22(2):189–201
Lambert JE, Garber PA (1998) Evolutionary and ecological implications of primate seed dispersal. Am J Primatol 45(1):9–28
Leighton M, Leighton DR (1982) The relationship of size of feeding aggregate to size of food patch: howler monkeys (Alouatta palliata) feeding in Trichilia cipo fruit trees on Barro Colorado Island. Biotropica 14:81–90
Leonhardt S, Tung J, Camden J, Leal M, Drea C (2009) Seeing red: behavioral evidence of trichromatic color vision in strepsirrhine primates. Behav Ecol 20(1):1–12
Levis C, Costa FR, Bongers F, Peña-Claros M, Clement CR, Junqueira AB et al (2017) Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science 355(6328):925–931
Levis C, Flores B, Moreira P, Luize B, Alves R, Franco-Moraes J et al (2018) How people domesticated Amazonian forests. Front Ecol Evol 5:171. doi: https://doi.org/10.3389/fevo.2017.00171
Li S-P, Li J-F, Ferguson DK, Wang N-W, He X-X, Yao J-X (2014) Palynological analysis of the late Early Pleistocene sediments from Queque Cave in Guangxi, south China. Quaternary Int 354:24–34
Lu L, Bartholomew B (2003) Amygdalus L. In: Wu R, Ye Z, Hi eP (eds) Flora of China. Missouri Botanical Garden Press, St Louis
Mädler K (1939) Die pliozäne flora von Frankfurt am main, vol 446. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, Frankfurt
Mai DH (1995) Tertiäre Vegetationsgeschichte Europas: Methoden und Ergebnisse. Gustav Fischer Verlag, Jena
Mai DH (2010) Karpologische untersuchungen in einem interglazial von Neumark-Nord (Geiseltal). Palaeontographica Abteilung B 222:99–187
Malhi Y, Doughty CE, Galetti M, Smith FA, Svenning J-C, Terborgh JW (2016) Megafauna and ecosystem function from the Pleistocene to the Anthropocene. PNAS 113(4):838–846
Mallick R, Frank N (2002) A new technique for precise uranium-series dating of travertine micro-samples. Geochim Cosmochim Acta 66(24):4261–4272
Martinoli D, Jacomet S (2004) Identifying endocarp remains and exploring their use at Epipalaeolithic Öküzini in southwest Anatolia, Turkey. Veg Hist Archaeobot 13(1):45–54
Maruhashi T (1980) Feeding behavior and diet of the Japanese monkey (Macaca fuscata yakui) on Yakushima Island, Japan. Primates 21(2):141–160
McConkey KR, Brockelman WY (2011) Nonredundancy in the dispersal network of a generalist tropical forest tree. Ecology 92(7):1492–1502
McConkey KR, Brockelman WY, Saralamba C (2014) Mammalian frugivores with different foraging behavior can show similar seed dispersal effectiveness. Biotropica 46(6):647–651
McConkey KR, Brockelman WY, Saralamba C, Nathalang A (2015) Effectiveness of primate seed dispersers for an “oversized” fruit, Garcinia benthamii. Ecology 96(10):2737–2747
McConkey KR, Nathalang A, Brockelman WY, Saralamba C, Santon J, Matmoon U et al (2018) Different megafauna vary in their seed dispersal effectiveness of the megafaunal fruit Platymitra macrocarpa (Annonaceae). PLoS ONE 13(7):e0198960
McKee JK, Poirier FE, Mcgraw WS (2015) Understanding human evolution. Pearson Prentice Hall, New Jersey
Melin AD, Hiramatsu C, Parr NA, Matsushita Y, Kawamura S, Fedigan LM (2014) The behavioral ecology of color vision: considering fruit conspicuity, detection distance and dietary importance. Int J Primatol 35(1):258–287
Merceron G, Taylor S, Scott R, Chaimanee Y, Jaeger J-J (2006) Dietary characterization of the hominoid Khoratpithecus (Miocene of Thailand): evidence from dental topographic and microwear texture analyses. Naturwissenschaften 93(7):329–333
Meredith RW, Janečka JE, Gatesy J, Ryder OA, Fisher CA, Teeling EC et al (2011) Impacts of the cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334(6055):521–524
Mithen S, Finlay N, Carruthers W, Carter S, Ashmore P (2001) Plant use in the Mesolithic: evidence from Staosnaig, Isle of Colonsay, Scotland. J Archaeol Sci 28(3):223–234
Nathan R, Muller-Landau HC (2000) Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol Evol 15(7):278–285
Nathan R, Schurr FM, Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A (2008) Mechanisms of long-distance seed dispersal. Trends Ecol Evol 23(11):638–647
Nelson SV (2007) Isotopic reconstructions of habitat change surrounding the extinction of Sivapithecus, a Miocene hominoid, in the Siwalik Group of Pakistan. Palaeogeogr Palaeoclimatol Palaeoecol 243(1–2):204–222
Nelson SV (2014) The paleoecology of early Pleistocene Gigantopithecus blacki inferred from isotopic analyses. A J Phys Anthropol 155(4):571–578
Nevo O, Heymann EW (2015) Led by the nose: olfaction in primate feeding ecology. Evol Anthropol 24(4):137–148
Nevo O, Valenta K, Razafimandimby D, Melin AD, Ayasse M, Chapman CA (2018) Frugivores and the evolution of fruit colour. Biol Lett 14(9):20180377
Nielsen NH, Jacobsen MW, Graham LL, Morrogh-Bernard HC, D’Arcy LJ, Harrison ME (2011) Successful germination of seeds following passage through orang-utan guts. J Trop Ecol 27(4):433–435
Noma N, Yumoto T (1997) Fruiting phenology of animal-dispersed plants in response to winter migration of frugivores in a warm temperate forest on Yakushima Island, Japan. Ecol Res 12(2):119–129
Nuñez-Iturri G, Howe HF (2007) Bushmeat and the fate of trees with seeds dispersed by large primates in a lowland rain forest in western Amazonia. Biotropica 39(3):348–354
O’Farrill G, Galetti M, Campos-Arceiz A (2013) Frugivory and seed dispersal by tapirs: an insight on their ecological role. Integr Zool 8(1):4–17
Ollivier V, Nahapetyan S, Roiron P, Gabrielyan I, Gasparyan B, Chataigner C et al (2010) Quaternary volcano-lacustrine patterns and palaeobotanical data in southern Armenia. Quat Int 223:312–326
Omasheva M, Chekalin S, Galiakparov N (2015) Evaluation of molecular genetic diversity of wild apple Malus sieversii populations from Zailiysky Alatau by microsatellite markers. Russ J Genet 51(7):647–652
Onstein RE, Baker WJ, Couvreur TL, Faurby S, Herrera-Alsina L, Svenning J-C, Kissling WD (2018) To adapt or go extinct? The fate of megafaunal palm fruits under past global change. Proc R Soc B-Biol Sci 285(1880):20180882
Onstein RE, Vink DN, Veen J, Barratt CD, Flantua SG, Wich SA (2020) Kissling WD (2020) Palm fruit colours are linked to the broad-scale distribution and diversification of primate colour vision systems. Proc R Soc B Biol Sci 287(1921):20192731
Osorio D, Vorobyev M (1996) Colour vision as an adaptation to frugivory in primates. Proc R Soc Lond. Ser B: Biol Sci 263(1370):593–599
Peres CA, Van Roosmalen M (2002) Primate frugivory in two species-rich neotropical forests:implications for the demography of large-seeded plants in overhunted areas. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory: ecology, evolution and conservation. CABI Publishing, Wallingford, pp 407–421
Petre C-A, Tagg N, Haurez B, Beudels-Jamar R, Huynen M-C, Doucet J-L (2013) Role of the western lowland gorilla (Gorilla gorilla gorilla) in seed dispersal in tropical forests and implications of its decline. Biotechnol Agron Soc Envir 17(3):517–526
Pires MM, Guimarães PR, Galetti M, Jordano P (2018) Pleistocene megafaunal extinctions and the functional loss of long-distance seed‐dispersal services. Ecography 41(1):153–163
Qu Y, Jin C, Zhang Y, Hu Y, Shang X, Wang C (2014) Preservation assessments and carbon and oxygen isotopes analysis of tooth enamel of Gigantopithecus blacki and contemporary animals from Sanhe Cave, Chongzuo, South China during the Early Pleistocene. Quaternary Int 354:52–58
Reagan B, Julliot C, Simmen B, Viénot F, Charles-Dominique P, Mollon F (2001) Fruits, foliage, and the evolution of primate color vision. Philosophical Trans Royal Soc Lond Ser B: Biol Sci 356:229–283
Reid C, Reid EM (1915) The Pliocene floras of the Dutch-Prussian border. M. Nijhoff, The Hague
Richards CM, Volk GM, Reilley AA, Henk AD, Lockwood DR, Reeves PA, Forsline PL (2009) Genetic diversity and population structure in Malus sieversii, a wild progenitor species of domesticated apple. Tree Genet Genomes 5(2):339–347
Rijksen HD (1978) A field study on Sumatran orangutans (Pongo pygmaeus abelii Lesson 1827): ecology, behaviour and conservation. H. Veenman, Wageningen
Rindos D (2013) The origins of agriculture: an evolutionary perspective. Academic Press, San Diego
Rowe MH (2018) Trichromatic color vision in primates. News Physiol Sci 17:93–98
Rushmore J, Leonhardt SD, Drea CM (2012) Sight or scent: lemur sensory reliance in detecting food quality varies with feeding ecology. PLoS ONE 7(8):e41558
Russo S, Chapman C (2011) Primate seed dispersal: linking behavioral ecology with forest community structure. In: Campbell FA, MacKinnon CJ, Panger JC, Bearder M (eds) Primates in perspective. Oxford University Press, Oxford, pp 523–534
Ruxton GD, Schaefer HM (2012) The conservation physiology of seed dispersal. Philos Trans R Soc B: Biol Sci 367(1596):1708–1718
Sau S, Dondini UM, De Franceschi L, D’hallewin P, Bacchetta G G (2018) Seed morphometry is suitable for apple-germplasm diversity-analyses. Comput Electron Agric 151:118–125
Schwarcz HP, Grün R, Latham A, Mania D, Brunnacker K (1988) The Bilzingsleben archaeological site: new dating evidence. Archaeometry 30(1):5–17
Schweigert G (1991) Die flora der Eem-interglazialen Travertine von Stuttgart-Untertürkheim (Baden-Württemberg). Stuttg Beitr zur Naturkunde: Ser B (Geologie und Paläontologie) 178:1–44
Skinner M, Dupras TL, Moyá-Solá S (1995) Periodicity of linear enamel hypoplasia among Miocene Dryopithecus from Spain. J Paleontol 7:195–222
Spengler RN, III (2019) Origins of the apple: the role of megafaunal mutualism in the domestication of Malus and rosaceous trees. Front Plant Sci 10(617):1–18
Spengler RN, Mueller NG (2019) Grazing animals drove domestication of grain crops. Nat Plants 5(7):656–662
Steininger FF, Rabeder G, Rögl F (1985) Land mammal distribution in the Mediterranean Neogene: a consequence of geokinematic and climatic events. In: Stanley DJ, Wezel F-C (eds) Geological evolution of the Mediterranean Basin. Springer, New York, pp 559–571
Stevens M, Stoddard MC, Higham JP (2009) Studying primate color: towards visual system-dependent methods. Int J Primatol 30(6):893–917
Stevenson PR (2001) The relationship between fruit production and primate abundance in Neotropical communities. Biol J Linn Soc 72(1):161–178
Stevenson PR, Aldana AM (2008) Potential effects of ateline extinction and forest fragmentation on plant diversity and composition in the Western Orinoco Basin, Colombia. Int J Primatol 29(2):365–377
Stoner KE, Riba-Hernandez P, Vulinec K, Lambert JE (2007) The role of mammals in creating and modifying seedshadows in tropical forests and some possible consequences of their elimination. Biotropica 39(3):316–327
Su T, Wilf P, Huang Y, Zhang S, Zhou Z (2015) Peaches preceded humans: fossil evidence from SW China. Sci Rep 5:16794
Sussman RW (1991) Primate origins and the evolution of angiosperms. Am J Primatol 23:209–223
Sussman RW, Rasmussen T, Raven PH (2013) Rethinking primate origins again. Am J Primatol 75(2):95–106
Szafer W (1947) The Pliocene flora of Kroscienko in Poland: Osobne Odbicie z Rozpraw Wydzialu matematyczno-przyrodniczego. Pol Akad Umiejetnosci 72:1–213
Takai M, Zhang Y, Kono RT, Jin C (2014) Changes in the composition of the Pleistocene primate fauna in southern China. Quat Int 354:75–85
Tan PK, Farrar JE, Gaucher EA, Miner JN (2016) Coevolution of URAT1 and uricase during primate evolution: implications for serum urate homeostasis and gout. Mol Biol Evol 33(9):2193–2200
Teaford MF, Walker A (1984) Quantitative differences in dental microwear between primate species with different diets and a comment on the presumed diet of Sivapithecus. Am J Phys Anthropol 64(2):191–200
Terborgh J, Pitman N, Silman M, Schichter H, Núñez P (2002) Maintenance of tree diversity in tropical forests. In: Levey SW, Galetti DJ (eds) Seed dispersal and frugivory: ecology, evolution and conservation. CAB International, Wallingford, pp 1–17
Terhune CE (2013) Dietary correlates of temporomandibular joint morphology in the great apes. Am J Phys Anthropol 150(2):260–272
Tiffney BH (2004) Vertebrate dispersal of seed plants through time. Annu Rev Ecol Evol Syst 35:1–29
Tiffney BH, Mazer SJ (1995) Angiosperm growth habit, dispersal and diversification reconsidered. Evol Ecol 9(1):93–117
Töpel M, Antonelli A, Yesson C, Eriksen B (2012) Past climate change and plant evolution in western North America: a case study in Rosaceae. PLoS ONE 7(12):e50358
Tsuji Y, Ito TY, Wada K, Watanabe K (2015) Spatial patterns in the diet of the Japanese macaque Macaca fuscata and their environmental determinants. Mammal Rev 45(4):227-238
Turner IM (2001) The ecology of trees in the tropical rainforest. Cambridge University Press
Ungar PS, Kay RF (1995) The dietary adaptations of European Miocene catarrhines. Proc Nat Acad Sci USA 92(12):5479–5481
Urrestarazu J, Denancé C, Ravon E, Guyader A, Guisnel R, Feugey L et al (2016) Analysis of the genetic diversity and structure across a wide range of germplasm reveals prominent gene flow in apple at the European level. BMC Plant Biol 16(1):130
Valenta K (2014) Endemic fruit signals in Madagascar drive variation in Eulemur fulvus foraging behaviour and efficiency. PhD thesis, Department of Anthropology. University of Toronto
Valenta K, Chapman CA (2018) Primate-plant mutualisms: is there evidence for primate fruit syndromes? In: Kalbitzer JK (ed) Primate life histories, sex roles, and adaptability - essays in honour of Linda M. Fedigan. Springer, New York, pp 245–255
Valenta K, Burke RJ, Styler SA, Jackson DA, Melin AD, Lehman SM (2013) Colour and odour drive fruit selection and seed dispersal by mouse lemurs. Sci Rep 3(1):1–5
Valenta K, Brown KA, Melin AD, Monckton SK, Styler SA, Jackson DA, Chapman CA (2015) It’s not easy being blue: are there olfactory and visual trade-offs in plant signalling? PLoS ONE 10(6):e0131725
Valenta K, Miller CN, Monckton SK, Melin AD, Lehman SM, Styler SA et al (2016) Fruit ripening signals and cues in a Madagascan dry forest: haptic indicators reliably indicate fruit ripeness to dichromatic lemurs. Evol Biol 43(3):344–355
Valenta K, Nevo O, Chapman CA (2018) Primate fruit color: useful concept or alluring myth? Int J Primatol 39(3):321–337
Van der Made J (1999) Intercontinental relationship Europe-Africa and the Indian subcontinent. In: Rössner GE, Heissig K (eds) The Miocene Land Mammals of Europe. F. Pfeil, Munich, pp 457–472
Van der Made J, Mazo A (2003) Proboscidean dispersals from Africa towards Western Europe. Deinsea 9(1):437–452
Van der Pijl L (1982) Principles of dispersal. Berlin: Springer Verlag
van Zonneveld M, Thomas E, Castañeda-Álvarez NP, Van Damme V, Alcazar C, Loo J, Scheldeman X (2018) Tree genetic resources at risk in South America: a spatial threat assessment to prioritize populations for conservation. Divers Distrib 24(6):718–729
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A et al (2010) The genome of the domesticated apple (Malus domestica Borkh.). Nat Genet 42(10):833–839
Velasco D, Hough J, Aradhya M, Ross-Ibarra J (2016) Evolutionary genomics of peach and almond domestication. G3: Genes Genom Genet 6(12):3985–3993
Vent W (1974) Die Flora der ilmtaltravertine von Weimar-Ehringsdorf. Abhandlungen des Zentralen Geologischen Instituts Paläontologische Abhandlungen 21:259–321
Vent W (1978) Die Flora des Travertins von Burgtonna in Thüringen. Quarterpaläontol 3:59–66
Vlcek E (1993) Fossile Menschenfunde von Weimar-Ehringsdorf, vol 30. Theiss, Stuttgart
Voigt FA, Arafeh R, Farwig N, Griebeler EM, Böhning‐Gaese K (2009) Linking seed dispersal and genetic structure of trees: a biogeographical approach. J Biogeogr 36:242–254
Wang W (2009) New discoveries of Gigantopithecus blacki teeth from Chuifeng Cave in the Bubing Basin, Guangxi, south China. J Human Evol 57(3):229–240
Wenny DG (2001) Advantages of seed dispersal: a re-evaluation of directed dispersal. Evol Ecol Res 3(1):37–50
Worman COD, Chapman CA (2006) Densities of two frugivorous primates with respect to forest and fragment tree species composition and fruit availability. Int J Primatol 27(1):203
Wotton DM, Kelly D (2011) Frugivore loss limits recruitment of large-seeded trees. Proc Royal Soc B: Biol Sci 278(1723):3345–3354
Wotton DM, Kelly D (2011) Do larger frugivores move seeds further? Body size, seed dispersal distance, and a case study of a large, sedentary pigeon. J Biogeogr 39(11):1973–1983
Wrangham RW, Chapman CA, Chapman LJ (1994) Seed dispersal by forest chimpanzees in Uganda. J Trop Ecol 10(3):355–368
Wright SJ, Hernandéz A, Condit R (2007) The bushmeat harvest alters seedling banks by favoring lianas, large seeds, and seeds dispersed by bats, birds, and wind. Biotropica 39(3):363–371
Wu L, Feng G, Liang Z (2002) The diet of the Yuanmou hominoid, Yunnan province, China: an analysis from tooth size and morphology. Anthropol Sci 110(2):149–163
Wu J, Wang Z, Shi Z, Zhang S, Ming R, Zhu S et al (2013) The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res 23(2):396–408
Wu Y, Wang H, Hadly EA (2017) Invasion of ancestral mammals into dim-light environments inferred from adaptive evolution of the phototransduction genes. Sci Rep 7:46542
Xiang Y, Huang C-H, Hu Y, Wen J, Li S, Yi T, Chen H, Xiang J, Ma H (2017) Evolution of Rosaceae fruit types based on nuclear phylogeny in the context of geological times and genome duplication. Mol Biol Evol 34(2):262–281
Yokoyama S, Yokoyama R (1989) Molecular evolution of human visual pigment genes. Mol Biol Evol 6(2):186–197
Yu Y, Fu J, Xu Y, Zhang J, Ren F, Zhao H et al (2018) Genome re-sequencing reveals the evolutionary history of peach fruit edibility. Nat Com 9(1):1–13
Zhang Y, Harrison T (2017) Gigantopithecus blacki: a giant ape from the Pleistocene of Asia revisited. Am J Phys Anthropol 162:153–177
Zhao L, Zhang L, Zhang F, Wu Z (2011) Enamel carbon isotope evidence of diet and habitat of Gigantopithecus blacki and associated mammalian megafauna in the Early Pleistocene of South China. Chin Sci Bull 56:3590–3595. https://doi.org/10.1007/s11434-011-4732-4
Zvelebil M (1994) Plant use in the Mesolithic and its role in the transition to farming. Proceedings of the Prehistoric Society 60(1):35–74
Acknowledgments
We thank Dovydas Jurkenas for help producing Fig. 1. We confirm that all data are available in this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the Max Planck Institute for the Science of Human History and the European Research Council, grant number 851102, Fruits of Eurasia: Domestication and Dispersal (FEDD), and the Natural Sciences and Engineering Research Council of Canada (Grant File Number RGPIN-2016-06761).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spengler, R.N., Kienast, F., Roberts, P. et al. Bearing Fruit: Miocene Apes and Rosaceous Fruit Evolution. Biol Theory 18, 134–151 (2023). https://doi.org/10.1007/s13752-022-00413-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13752-022-00413-1