Abstract
Obesity-related glomerulopathy and diabetic nephropathy (DN) are serious complications to metabolic syndrome and diabetes. The purpose was to study effects of a fat, fructose and cholesterol-rich (FFC) diet with and without salt in order to induce hypertension on kidney function and morphology in Göttingen Minipigs with and without diabetes. Male Göttingen Minipigs were divided into 4 groups: SD (standard diet, n = 8), FFC (FFC diet, n = 16), FFC-DIA (FFC diet + diabetes, n = 14), FFC-DIA + S (FFC diet with extra salt + diabetes, n = 14). Blood and urine biomarkers, glomerular filtration rate (GFR), blood pressure (BP) and resistive index (RI) were evaluated after 6–7 months (T1) and 12–13 months (T2). Histology, electron microscopy and gene expression (excluding FFC-DIA + S) were evaluated at T2. All groups fed FFC-diet displayed obesity, increased GFR and RI, glomerulomegaly, mesangial expansion (ME) and glomerular basement membrane (GBM) thickening. Diabetes on top of FFC diet led to increased plasma glucose and urea and proteinuria and tended to exacerbate the glomerulomegaly, ME and GBM thickening. Four genes (CDKN1A, NPHS2, ACE, SLC2A1) were significantly deregulated in FFC and/or FFC-DIA compared to SD. No effects on BP were observed. Göttingen Minipigs fed FFC diet displayed some of the renal early changes seen in human obesity. Presence of diabetes on top of FFC diet exacerbated the findings and lead to changes resembling the early phases of human DN.
Similar content being viewed by others
Introduction
Diabetic nephropathy (DN) is one of the most serious chronic complications of diabetes and the condition is associated with considerable risk of morbidity and mortality. DN is the leading cause of end-stage renal disease and has a prevalence of up to 30–40% of diabetic patients1,2,3. In addition, obesity-related glomerulopathy (ORG) is observed with increasing frequency in non-diabetic people living with obesity4. The two diseases share many of the same renal morphological and functional changes and the risk factors for disease development are overlapping1,5, indicating a common early pathogenesis.
The complex and multifactorial pathogenesis of both ORG and DN remains to be fully elucidated but involves both metabolic and haemodynamic disturbances, and is associated with risk factors such as obesity, insulin resistance, hyperglycemia, dyslipidemia and hypertension1,5. The two diseases typically evolve over several years, and there is an unmet need for therapies that halt the progression of the early changes into more advanced disease.
It is not possible to diagnose or monitor early stages of ORG and DN by using the circulating biomarkers traditionally used in kidney disease, i.e. blood urea nitrogen and creatinine or creatinine-derived glomerular filtration rate (GFR), since these biomarkers are not specific, do not reflect small or early changes in renal function, and can only be considered reliable biomarkers in the late stages when significant impairment of the renal function is present6,7. Also, microalbuminuria, one of the hallmarks of DN, typically is not present in the very early disease stages and is not a sensitive predictor of disease progression8.
Of the newer biomarkers for DN, the tubulo-interstitial biomarker neutrophil gelatinase-associated lipocalin (NGAL) has been shown to be increased in urine prior to the occurrence of microalbuminuria and may serve as a complementary prognostic marker6,9. Also, the imaging biomarker resistive index (RI) assessed by Doppler ultrasound has been shown to be increased in obesity10 and in the early stages of DN before GFR declines are observed11.
From an animal model perspective, benefits may be gained from supplementing the existing rodent models with a porcine model, since the pig is more human-like on several key parameters such as the structure and function of the kidney12,13, the inflammasome14, and the metabolism and plasma lipid profile15. Minipigs are especially attractive to use in long-term studies due to their small size, and studies in minipigs, indicate that early-stage nephropathy can be induced by long-term hyperglycemia, Western diets or a combination of the two16,17,18. More late-stage models with an accelerated disease development would be attractive, and this may be achieved by combining hyperglycemia with some of the other risk factors for developing diabetic nephropathy, such as diet-induced obesity/metabolic syndrome and hypertension. In the present study, obesity and metabolic syndrome were induced by feeding a fat, fructose and cholesterol-rich (FFC) diet, while hypertension was theoretically induced by including a group with a high dietary salt content.
The aim of this study was thus to evaluate changes in functional and morphological renal parameters in Göttingen Minipigs fed an FFC diet with or without chemically induced diabetes and with or without extra dietary salt/hypertension. The evaluations included plasma and urinary biomarkers relevant for ORG/DN, GFR evaluated by inulin clearance, blood pressure (BP), RI, gene expression analyses and histopathological and electron-microscopical examinations of renal tissue samples.
Methods
Animals, diet and housing
Fifty-two castrated male Göttingen Minipigs (Ellegaard Göttingen Minipigs A/S, Dalmose, Denmark) aged approx. seven months at study start were included in the study. The average body weight was 16 ± 2 kg. The animals were part of a larger study (n = 84 in total) focusing on other end points (see Supplementary information file 1).
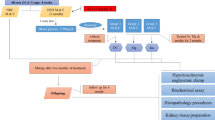
The study was run in 3 staggered cohorts, with animals from all groups equally represented in all cohorts, and animals from each group represented in random order on each study day. After an acclimation period of 2 weeks, the animals were weight-stratified into 3 groups according to diet and diabetic state: SD (fed standard diet, Mini-Pig, SDS, UK) (n = 8), FFC (fed high fat/fructose/cholesterol diet with 2% cholesterol (5B4L) for the first 5 months of the study, then changed to same diet with 1% cholesterol (9G4U) for the rest of the study) (n = 16) and FFC-DIA/FFC-DIA + S (fed FFC 1% cholesterol diet until chemical diabetes induction in week 9, n = 28). After diabetes induction, these animals were allocated into two groups based on their blood sugar levels (aiming at similar blood sugar levels in the two diabetic groups): FFC-DIA (continued on FFC 1% cholesterol diet for the rest of the study) (n = 14) and FFC-DIA + S (switched to FFC 1% cholesterol diet with extra salt (5BTJ, sodium chloride content 2.5%) after diabetes induction) (n = 14) (all FFC diets from TestDiet®, Missouri, US) (Fig. 1). Nutritional compositions of the diets can be found in Supplementary information file 2. The animals were fed once daily and had free access to drinking water; straw was used as bedding and rooting material and apples were given as treats. The animals were housed at the research facilities at the University of Copenhagen and were monitored daily during caretaking and dosing of insulin. They were group housed except for periods with intravenous (IV) catheters implanted and/or wearing blood pressure telemetry equipment.

Modified from Schumacher et al. with permission50.
Study overview. (A) Fifty-two male castrated Göttingen Minipigs were included in the study and 15 were terminated prematurely. The numbers and reasons for premature termination (PT) are described under each group. The procedure-related complications occurred during investigations of other end points in the same animals (unrelated to the present study). (B) Timeline and details of feeding and tests in each of the four groups over the study period. FFC: high fat/fructose/cholesterol diet, STZ: induction of diabetes with streptozotocin, T1: mid-study in vivo evaluations, T2: end-study in vivo evaluations. Grey arrow indicates termination and tissue sampling.
Study overview, in vivo evaluations and tissue sampling
In vivo evaluations were performed at mid study (T1, from 6–7 months after study start) and repeated at end study (T2, from 12–13 months after study start) (Fig. 1B). In vivo evaluations included weighing, measurement of body composition by dual energy x-ray absorptiometry (DEXA) scanning, blood and urine sampling, GFR measurement by inulin clearance test, BP measurements by telemetry, and RI assessment by ultrasound scanning. All in vivo methods are described in detail in Supplementary information file 3.
Study duration was 13 months and at the end of the study the animals were euthanised by exsanguination during deep surgical anaesthesia using tiletamin and zolazepam mixture (Zoletil 50 Vet, ChemVet, Silkeborg, Denmark) with added ketamine (Ketaminol Vet (100 mg/mL) Intervet, Skovlunde, Denmark), xylazine (0.84 mg/kg) (Rompun Vet (20 mg/mL) Bayer, Lyngby, Denmark) and buthorphanol (0.16 mg/kg) (Torbugesic (10 mg/mL) Scanvet, Fredensborg, Denmark), after which the kidneys were harvested for histopathological and electron microscopical evaluations and gene expression analysis as described in Supplementary information file 3. All tissue analyses were blinded (investigators were unaware of group allocations).
Catheter implantation
A central-venous catheter was implanted under general anesthesia at least 1 week before procedures requiring venous access (diabetes induction or test periods) using one of the 3 methods described in Supplementary information file 3. The catheter was used for diabetes induction, stress-free blood sampling and infusion of glucose and inulin during tests.
Induction of diabetes
Six to eight weeks into the study, after 4–6 weeks on the experimental diets, a type 1-like (insulin-dependent) diabetes was induced in 28 animals (FFC-DIA and FFC-DIA + S) by streptozotocin (STZ, Sigma-Aldrich, Denmark), 60 mg/kg given intravenously (IV) once daily on three consecutive days (protocol modified from Gerrity et al.19). Fasting blood glucose level was measured daily for the first 8 days after induction and then 1–2 times weekly (glucometer Accu-Chek® Aviva, Roche Diabetes Care, Roche Danmark). Daily subcutaneous injections with insulin glargine (Lantus®, Sanofi-Aventis, Frankfurt am Main, Germany) were given in the morning in relation to the meal to keep the fasting morning blood glucose at approx. 15 mM. Approximately three weeks after diabetes induction, the animals were allocated into two diabetic groups FFC-DIA (n = 14) and FFC-DIA + S (n = 14) with similar mean plasma glucose level.
Serum, plasma and urine analysis
Serum, plasma and urine analyses were performed as described in Supplementary information file 3.
Calculations and statistical analyses
Data are presented as median and interquartile range (Q1-Q3). . GFR (inulin curves used for calculation are presented in Supplementary information file 4) was given as absolute value (ml/min/pig). Albumin, protein and NGAL concentrations in urine were corrected for urine creatinine concentrations, giving the following derived parameters: uACR (urine albumin/urine creatinine), uPCR (urine protein/urine creatinine) and uNGALCR (urine NGAL/urine creatinine).
Statistical analysis, Spearman correlation analysis and principal component analysis (PCA) were performed using SAS version 9.4, SAS Studio (Statistical Analysis Software, SAS Institute Inc., Cary, NC, USA) or RStudio (gene expression analyses, Rstudio Team, 2015). Graphs were made with GraphPad Prism (GraphPad Software, La Jolla, CA, USA). No power calculations were performed, since no a priori knowledge of effect size and variation for the renal end points was available. Further details can be found in Supplementary information file 3.
Ethical approval
The study was approved by the Animal Experiments Inspectorate, Ministry of Food, Agriculture and Fisheries, Denmark (License No. 2012-15-2934-00715). All procedures were performed in accordance with the EU directive on protection of Animals for Research Purposes Directive 2010/63/EU. The study is reported in accordance with the ARRIVE guidelines.
Results
Thirty-seven animals out of 52 completed the study (see Fig. 1 for causes of premature termination). The key data and statistics are presented in Tables and Supplementary information file 5.
BW and body composition
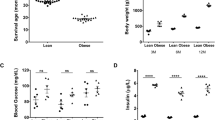
Body weight and Fat% were significantly higher in the three FFC-groups compared to SD at both T1 and T2. At T2, the FFC group had higher Fat% than FFC-DIA + S whereas no significant differences were observed between the two FFC-DIA groups. Both BW and Fat% increased significantly from T1 to T2 (Table 1).
Circulating and urinary biomarkers
As expected, plasma glucose and fructosamine were significantly increased and insulin significantly decreased in the two diabetic groups compared to both the FFC and SD groups, and at both T1 and T2. Total cholesterol (TC) was significantly higher in all three FFC-groups compared to SD at both time points. Triglyceride (TG), urea and glucagon were significantly higher in the two diabetic groups compared to both FFC and SD. Furthermore, TG and glucagon were significantly higher in the FFC group compared to SD. Plasma creatinine was significantly lower in the two diabetic groups compared to both FFC and SD, and in the FFC group compared to SD at both T1 and T2. For plasma creatinine, the values were overall higher at T2 compared to T1, and for glucagon the values were higher at T1 compared to T2. No significant differences were observed in plasma NGAL concentrations at either time point.
At both T1 and T2, UPCR was higher in FFC-DIA compared to the three other groups, whereas no significant differences between the groups were found in UACR. UNGALCR was higher in the two diabetic groups compared to SD and FFC at both time points (Table 2).
Haematology and clinical chemistry
FFC-DIA and FFC-DIA + S had lower sodium concentrations compared to FFC and SD. No significant differences were found in any of the other parameters (Supplementary information file 6).
GFR by inulin clearance test
The absolute GFR was significantly higher in FFC animals compared to SD and FFC-DIA at T1, and in all three FFC groups compared to SD at T2 (Fig. 2D). In addition, the GFR increased in all three FFC groups from T1 to T2 (Table 3, Supplementary information file 5).
Data related to renal function and morphology. Glomerular size (A), mesangial expansion score (B), examples of mesangial expansion scores also illustrating the differences in glomeruli sizes (C). Score 0: no mesangial expansion, score 1: mild mesangial expansion, score 2: moderate mesangial expansion and score 3: marked mesangial expansion. Periodic acid-Schiff (PAS). × 40 original magnification. GFR estimated by inulin clearance test (D), resistive index (RI) (E), illustration of the RI measure (F), normal range glomerular basement membrane (*) in SD minipig, × 4800 original magnification (G) moderate thickening of basement membrane (*) in FFC minipig, × 4800 original magnification (H) severe thickening of basement membrane (*) and podocyte foot process fusion in FFC-DIA minipig, × 4800 original magnification (I). Castrated male Göttingen Minipigs fed with standard diet (SD) or fat, fructose and cholesterol rich diet (FFC) with or without additional salt (S) and with or without streptozotocin-induced diabetes (DIA). n = 6–14. *p < 0.05, **p < 0.01, ***p < 0.001.
24 hour systemic blood pressure and heart rate
Thirty-nine animals were included in the mid study BP evaluations and 29 animals were included in the end study evaluations (see number of animals included in each group in Table 1). For all of DIA-BP, SYS-BP and MEAN-BP there were no significant differences between the groups; all values were higher at T2 compared to T1, and in the day-time compared to the night-time (Table 1, Supplementary information file 7). For HR, the values were also higher in the day-time versus the night-time, but lower at T2 compared to T1. In addition, the two FFC-DIA groups had significantly higher HR compared to both FFC and SD.
Resistive index (RI)
There was a significant difference in RI between groups both at T1 and T2, with all three FFC groups having higher values than SD (Table 3, Fig. 2E). In addition, there was a significant overall increase in RI from T1 to T2.
Kidney weight and histopathological evaluations of kidney tissue
The three FFC-groups had significantly higher absolute KW compared to SD (Table 3).
Based on Periodic acid–Schiff (PAS) staining, the kidneys in the three FFC groups showed varying degrees of histopathological changes such as hypertrophic and hyperplastic glomerular lesions and basement membrane thickening. Occasionally, glomerulosclerosis, periglomerular fibrosis, tubular atrophy and tubular basal membrane thickening associated to focal interstitial fibrosis were observed (n = 2 FFC; n = 2 FFC-DIA, Supplementary information file 8), but quantification of fibrosis using image analysis revealed no significant difference between the groups. Specifically, the glomerular size was significantly higher in all three FFC groups compared to SD (Fig. 2) and mesangial expansion evaluated as mesangial score was higher in all 3 FFC groups compared to SD (Fig. 2B). The lesions were not followed by clear immune cell infiltration or other characteristic features observed in diabetic nephropathy.
TEM
The glomerular basement membrane thickening, observed by PAS staining in the histopathological evaluation, was confirmed by TEM (Fig. 2). Basement membrane thickening was observed both in the FFC and FFC-DIA groups but was most pronounced in the FFC-DIA group alongside with podocyte foot process fusion and nodular masses of mesangial matrix.
Correlations
In Spearman correlation analysis of selected parameters including plasma and urinary biomarkers, renal histology, KW, RI, BW, Fat%, mean 24 h BP and mean 24 h HR, only a few correlations remained significant after Bonferroni correction (Fig. 3C and Supplementary information file 9).
PCA plot (A), PCA variable correlation plot (B), and Spearman correlation analysis (C). *p < 0.05, number in parenthesis is the Spearman correlation coefficient for significant correlations. RI: resistive index, p-NGAL: plasma neutrophil gelatinase-associated lipocalin, p-Glu: plasma glucose, P-crea: plasma creatinine, TG: triglycerides, TC: total cholesterol, GFR: glomerular filtration rate, UACR: urinary albumin to creatinine ratio, UPCR: urinary protein:creatinine ratio, NGALCR: urinary neutrophil gelatinase-associated lipocalin to creatinine ratio, Fat%: body fat percentage, KW: Kidney weight, Glom. Size: glomerular size, BP: blood pressure, HR: heart rate.
Gene expression
After qPCR data processing, 73 kidney related genes and 4 reference genes were expressed within the dynamic range in kidney tissue from the three diet groups SD, FFC and FFC-DIA and were accepted for further statistical analysis (see Supplementary information file 10 for raw data and data log2 FC for these 73 assays). Statistical analysis resulted in 4 genes being significantly de-regulated between groups after multiple test correction: Angiotensin I converting enzyme (ACE), cyclin dependent kinase inhibitor 1A (CDKN1A), solute carrier family 2 member 1 (SLC2A1) and podocin (NPHS2) (Fig. 4 and Supplementary information file 10).
Gene expression data. Relative quantities of the 4 significantly deregulated genes (passing Bonferroni correction) in male, castrated Göttingen Minipigs fed with standard diet (SD, N = 7) or fat, fructose and cholesterol rich diet without induction of diabetes (FFC, N = 13) or with induction of diabetes (FFC-DIA, N = 8). Data are shown as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001. Angiotensin I converting enzyme (ACE), cyclin dependent kinase inhibitor 1A (CDKN1A), solute carrier family 2 member 1 (SLC2A1) and podocin (NPHS2).
Principal components analysis (PCA)
The PCA plot revealed three major groups: diabetic minipigs, the FFC group and the SD group, respectively (Fig. 3A,B).
Discussion
The present study investigated the effects of an FFC diet with and without chemically induced diabetes on renal functional and morphological features in a Göttingen Minipig model. Minipigs fed a FFC diet displayed some of the characteristic features of human ORG, such as glomerulomegaly, early hyperfiltration, moderate mesangial expansion and moderate thickening of the glomerular basement membrane, whereas induction of diabetes on top of the FFC diet, lead to further changes resembling the early stages of human DN, e.g. exacerbation of the glomerulomegaly and the glomerular basement membrane thickening, increased mesangial expansion, increased GFR, proteinuria and fusion of podocyte foot processes.
As expected, the FFC diet induced obesity in all diet-fed groups, as signified by higher BW and fat percentage. In addition, dyslipidemia with increased TG and TC was observed in all diet-fed groups as previously described in Ossabaw pigs fed a similar diet21. The presence of diabetes further exacerbated the TG as is also the case in humans22 and other pig models23. Hyperglycemia and increased fructosamine were only evident in the two diabetic groups, in line with many other reports from pigs showing no or only mild diet-induced increases in plasma glucose24. In humans, hypertension is often present in obese and diabetic individuals25 and is partly related to the dietary salt intake26,27. In the present study, feeding an FFC diet with or without concomitant presence of diabetes and with or without additional dietary salt did not lead to increased BP, which is in contrast to what has previously been observed in pigs fed a high fat, high sugar and/or salt-containing diet, indicating that the salt content and/or feeding period used here may have been insufficient to induce chronic hypertension28,29. However, in the current study, the presence of diabetes led to increased HR independent of the salt content of the diet and independent of increases in BP. Resting HR is described to be positively correlated to BP in young people30, but in this case the increased HR in the diabetic groups could be a result of autonomic neuropathy as has been suggested in humans31, although this needs further investigation.
In humans, obesity without diabetes leads to ORG which includes some of the same renal changes as seen in DN, e.g. glomerulomegaly, hyperfiltration, focal thickening of glomerular and tubular basement membranes, moderate mesangial expansion and podocyte effacement4,5,32, likely reflecting the many common risk factors. This is not surprising given that some of the main contributors to renal disease like obesity, insulin resistance, dyslipidemia, hypertension and low-grade inflammation are shared between obese and especially type 2 diabetic individuals1,5. In the present minipig model, glomerulomegaly was observed in all three FFC groups independent of diabetes, and in addition, the mesangial expansion score was higher in all the FFC groups compared to the SD group and with a tendency for more severe changes in the two diabetic groups. Mesangial expansion has been observed in other both obese and diabetic pig models16,18, which is in line with observations in humans, where moderate ME is observed in ORG32 and more severe ME is observed in DN33. Cyclin dependent kinase inhibitor 1A gene (CDKN1A) was found to be significantly upregulated in the diabetic animals compared to FFC and SD. CDKN1A regulates the cell cycle progression at the G1 and S-phase, and by inhibiting cyclin A, D and E it is able to arrest the cell in the G1 phase, leading to cell hypertrophy34. Specifically in DN, increased expression of CDKN1A has been related to glomerular/mesangial cell hypertrophy35 and podocyte apoptosis36, which aligns well with the glomerulomegaly and increased mesangial matrix observed primarily in the diabetic groups. None of the genes related to fibrosis or inflammation were significantly differentially regulated in line with the overall absence of fibrosis and inflammation in the renal sections.
In a few animals, more advanced diabetoid changes such as glomerulosclerosis, periglomerular fibrosis, tubular atrophy and tubular basal membrane thickening associated to focal interstitial fibrosis was observed, but the sporadic nature of these findings limits the value from a model perspective.
Glomerular filtration rate (Table 3), was increased in all FFC groups compared to SDat T2. Furthermore, this increase in GFR was correlated to the increased KW and glomerular size as judged by the positive correlations between KW, glomerular size and GFR (Fig. 3). The increased rather than decreased GFR has been observed in other obese and hyperglycemic pig models18,37 and in humans with obesity38 and early DN39, thus suggesting that the pigs in the present study are in the early stages of the disease.
The RI was increased to a similar extent in all the FFC groups, indicating a main effect of the diet rather than the diabetes. The increased RI may be related to the renal morphological and functional changes observed in all the FFC groups, but could also be related to systemic haemodynamic factors40, like e.g. the concurrent presence of atherosclerosis in the model41.
Like in other obese pig models, plasma creatinine was decreased in all FFC groups compared to SD24 which may be related to hyperfiltration, hypersecretion, decreased tubular reabsorption or differences in creatinine production from muscle creatine and/or differences in dietary creatine intake7. Since the FFBM was largely similar in all groups, reduced creatinine production from the muscles is an unlikely explanation, and the negative correlation between KW/glomerular size/GFR and plasma creatinine rather supports hyperfiltration as a cause of the lower plasma creatinine levels. This is also in line with the hyperfiltration observed both in ORG and in early stages of DN in humans38,39. Urea was increased in the two diabetic groups, which could indicate a significantly reduced renal function, although other factors like dietary protein intake together with net protein utilisation may also affect urea concentrations42. In dysregulated diabetic conditions, whole-body protein breakdown is increased43, and in light of the normal to increased GFR in the two diabetic groups, changes in protein metabolism may be a more plausible cause of the increased urea concentrations than severe renal dysfunction. This is supported by the higher plasma glucagon concentrations in the diabetic groups that correlated significantly with plasma urea concentrations (p < 0.01 at both time points, data not shown), an association that has also been reported in patients with type 1 diabetes44.
The plasma NGAL did not change in the current study, which may be due to the limited tubulointerstitial changes observed or to the timing of the blood samples since the plasma NGAL values tend to be primarily increased in the acute stages of renal disease9. Urinary NGALCR, on the other hand, was increased in the two diabetic groups at both time points compared to both FFC and SD, which indicate increased glomerular filtration and/or decreased tubular reabsorption of NGAL9,45. UPCR was only significantly increased in the FFC-DIA group compared to SD at T2 whereas UACR was not significantly different between any of the groups. This difference between levels of UNGALCR and UPCR/UACR may be explained by the different pathogenic processes causing their increase or simply by the high variability in especially the urinary albumin values. Some changes in the podocyte morphology, e.g. fusion of podocyte foot processes, were observed on EM in the FFC-DIA animals but since the podocyte changes were not quantified no correlations to the degree of albuminuria/proteinuria could be performed, which is a limitation of this study. In diabetic humans, podocyte dysfunction is associated with decreased expression of the two podocyte proteins, nephrin and podocin that are both involved in maintaining the glomerular filtration barrier46. In contrast, in this study, the NPHS2 gene encoding podocin was significantly upregulated in both FFC and FFC-DIA vs. SD and the NPHS1 gene encoding nephrin tended to be upregulated in the FFC vs. the SD but both with moderate fold changes. This could be due to the different stages of renal disease in the minipigs vs. the examined humans, since the humans were all in the later stages of the disease judged by the more consistent presence of proteinuria and the significantly decreased GFR46.
ACE and ACE2 are both involved in regulation of the renin-angiotensin system, with ACE2 being protective and ACE having deleterious effects with respect to development and progression of DN47. In the minipigs, ACE and ACE2 expression was significantly higher in the FFC group compared to both SD and FFC-DIA whereas in human DN patients, ACE expression was found to be increased and ACE2 expression to be decreased both in the glomerular and the proximal tubular compartments48. Overall, it may be the balance between the expression of these two genes and their downstream effectors that are more important than the expression of the individual genes per se. The reduction in SLC2A1 in the diabetic minipigs is most likely a direct consequence of the increased plasma glucose levels49.
As described above, some parameters seemed to be influenced mainly by the diet and others mainly by the presence of diabetes, whereas in other cases the presence of diabetes seemed to exacerbate the effects of the diet. Despite this overlap between diet- and diabetes induced renal changes, the PCA plots with three distinct groups support a differentiation between the dietary effects and the diabetes effects. Extra dietary salt did not increase BP or exacerbate the renal changes, and thus no clear differentiation between the two diabetic groups was observed in the PCA plot. The lack of salt-induced hypertension is a limitation of the study, since part of the aim was to investigate if salt-induced hypertension exacerbated the renal changes. In addition, the inclusion of a lean hyperglycemic group would have completed the study, but since literature data on renal changes in purely hyperglycemic pigs indicate that only very early changes develop within a reasonable time frame, it was prioritised to combine the hyperglycemia with some of the other risk factors for developing diabetic nephropathy, such as diet-induced obesity/metabolic syndrome and hypertension, in order to accelerate the disease development.
Fifteen animals, primarily in the FFC-DIA groups, died spontaneously or were prematurely euthanised during the study which challenges the use of this model in long-term pharmacological intervention studies. However, the pigs in the present studies were used for other purposes and a more simple study design may prevent the issues.
Conclusions
Göttingen Minipigs fed an FFC diet displayed some of the characteristic features of human ORG, such as glomerulomegaly, moderate ME and hyperfiltration and moderate thickening of the glomerular basement membrane. Induction of diabetes on top of the FFC diet, lead to further changes resembling the early stages of human DN, e.g. exacerbation of the glomerulomegaly, the ME and the glomerular basement membrane thickening, increased GFR, proteinuria and fusion of podocyte foot processes. In addition, UNGALCR was shown to be a relevant early biomarker of especially diabetes-related renal disease. The diet-fed Göttingen Minipig thus seems to be a relevant model for the early changes seen in both ORG and DN. Further studies are needed to show whether inclusion of e.g. hypertension in the model will induce some of the later disease stages.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request. Gene expression datasets generated and analysed during the current study are available in the Gene Expression Omnibus (GEO) repository, accession number GSE215843.
Abbreviations
- BP:
-
Blood pressure
- BW:
-
Body weight
- DEXA:
-
Dual energy x-ray absorptiometry
- DIA-BP:
-
Diastolic blood pressure
- DN:
-
Diabetic nephropathy
- Fat%:
-
Total body fat percentage
- FFBM:
-
Fat-free body mass
- FFC:
-
Fat, fructose and cholesterol diet
- FFC-DIA:
-
Minipigs fed FFC diet and chemically induced with diabetes
- FFC-DIA + S:
-
Minipigs fed FFC diet with 2.5% salt and chemically induced with diabetes
- GFR:
-
Glomerular filtration rate
- HR:
-
Heart rate
- KW:
-
Kidney weight
- LLOQ:
-
Lower limit of quantification
- LOCI:
-
Luminescent oxygen channeling assay
- ME:
-
Mesangial expansion
- MEAN-BP:
-
Mean blood pressure
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- NGALCR:
-
Urinary NGAL to creatinine ratio
- ORG:
-
Obesity-related glomerulopathy
- PAS:
-
Periodic Acid-Schiff
- PCA:
-
Principal components analysis
- PSR:
-
Picro-Sirius Red
- RI:
-
Resistive index
- ROI:
-
Region of interest
- STZ:
-
Streptozotocin
- SYS-BP:
-
Systolic blood pressure
- TEM:
-
Transmission electron microscopy
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- UACR:
-
Urinary albumin to creatinine ratio
- UPCR:
-
Urinary protein to creatinine ratio
References
Harjutsalo, V. & Groop, P. H. Epidemiology and risk factors for diabetic kidney disease. Adv. Chronic. Kidney Dis. 21, 260–266 (2014).
Bennett, K. & Aditya, B. S. An overview of diabetic nephropathy: Epidemiology, pathophysiology and treatment. J. Diabetes Nurs. 19, 7 (2015).
Marshall, S. M. Recent advances in diabetic nephropathy. Postgrad. Med. J. 80, 624–633 (2004).
Kambham, N., Markowitz, G. S., Valeri, A. M., Lin, J. & D’Agati, V. D. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 59, 1498–1509 (2001).
Xu, T., Sheng, Z. & Yao, L. Obesity-related glomerulopathy: pathogenesis, pathologic, clinical characteristics and treatment. Front. Med. 11, 340–348 (2017).
Satirapoj, B. Tubulointerstitial biomarkers for diabetic nephropathy. J. Diabetes Res. 1, 1–6 (2018).
Levey, A. S., Perrone, R. D. & Madias, N. E. Serum creatinine and renal function. Annu. Rev. Med. 39, 465–490 (1988).
Perkins, B. A., Ficociello, L. H., Roshan, B., Warram, J. H. & Krolewski, A. S. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 77, 57–64 (2010).
Yang, Y. H. et al. Changes of serum and urine neutrophil gelatinase-associated lipocalin in type-2 diabetic patients with nephropathy: one year observational follow-up study. Endocrine 36, 45–51 (2009).
Trovato, G. M. et al. Renal resistive index and parathyroid hormone relationship with renal function in nondiabetic patients. Endocr Res. 37, 47–58 (2012).
Masulli, M. et al. Measurement of the intrarenal arterial resistance index for the identification and prediction of diabetic nephropathy. Nutr. Metab. Cardiovasc. Dis. 19, 358–364 (2009).
Schmidt-Nielsen, B. & O’Dell, R. Structure and concentrating mechanism in the mammalian kidney. Am. J. Physiol. 200, 1119–1124 (1961).
Sachs, D. H. The pig as a potential xenograft donor. Vet. Immunol. Immunopathol. 43, 185–191 (1994).
Dawson, H. D., Smith, A. D., Chen, C. & Urban, J. F. Jr. An in-depth comparison of the porcine, murine and human inflammasomes; lessons from the porcine genome and transcriptome. Vet. Microbiol. 202, 2–15 (2017).
Koopmans, S. J. & Schuurman, T. Considerations on pig models for appetite, metabolic syndrome and obese type 2 diabetes: From food intake to metabolic disease. Eur. J. Pharmacol. 759, 231–239 (2015).
Liu, Y. et al. Severe insulin resistance and moderate glomerulosclerosis in a minipig model induced by high-fat/ high-sucrose/ high-cholesterol diet. Exp. Anim. 56, 11–20 (2007).
Marshall, M., Oberhofer, H. & Staubesand, J. Early micro- and macro-angiopathy in the streptozotocin diabetic minipig. Res. Exp. Med. Zeitschrift für die gesamte experimentelle Medizin einschliesslich experimenteller Chirurgie. 177, 145–158 (1980).
Maile, L. A. et al. Blocking ligand occupancy of the αVβ3 integrin inhibits the development of nephropathy in diabetic pigs. Endocrinology 155, 4665–4675 (2014).
Gerrity, R. G., Natarajan, R., Nadler, J. L. & Kimsey, T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes 50, 1654–1665 (2001).
Swindle, M. M., Makin, A., Herron, A. J., Clubb, F. J. Jr. & Frazier, K. S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 49, 344–356 (2012).
Lim, R. R. et al. Young Ossabaw Pigs fed a western diet exhibit early signs of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 59, 2325–2338 (2018).
Barrett-Connor, E., Grundy, S. M. & Holdbrook, M. J. Plasma lipids and diabetes mellitus in an adult community. Am. J. Epidemiol. 115, 657–663 (1982).
Badin, J. K. et al. Alloxan-induced diabetes exacerbates coronary atherosclerosis and calcification in Ossabaw miniature swine with metabolic syndrome. J. Transl. Med. 16, 58 (2018).
Renner, S. et al. Metabolic syndrome and extensive adipose tissue inflammation in morbidly obese Göttingen minipigs. Mol. Metab. 16, 180–190 (2018).
Must, A. et al. The disease burden associated with overweight and obesity. JAMA 282, 1523–1529 (1999).
Blaustein, M. P. et al. How NaCl raises blood pressure: A new paradigm for the pathogenesis of salt-dependent hypertension. Am. J. Physiol. Heart Circ. Physiol. 302, H1031–H1049 (2012).
Pilic, L., Pedlar, C. R. & Mavrommatis, Y. Salt-sensitive hypertension: Mechanisms and effects of dietary and other lifestyle factors. Nutr. Rev. 74, 645–658 (2016).
Van Vliet, B. N. & Montani, J. P. The time course of salt-induced hypertension, and why it matters. Int. J. Obes. 32(Suppl 6), S35-47 (2008).
Myrie, S. B. et al. Effects of a diet high in salt, fat, and sugar on telemetric blood pressure measurements in conscious, unrestrained adult Yucatan miniature swine (Sus scrofa). Comp. Med. 62, 282–290 (2012).
Christofaro, D. G. D., Casonatto, J., Vanderlei, L. C. M., Cucato, G. G. & Dias, R. M. R. Relationship between resting heart rate, blood pressure and pulse pressure in adolescents. Arq. Bras. Cardiol. 108, 405–410 (2017).
Singh, N. Diabetes, heart rate, and mortality. J. Cardiovasc. Pharmacol. Ther. 7, 117–129 (2002).
Goumenos, D. S. et al. Early histological changes in the kidney of people with morbid obesity. Nephrol. Dial. Transpl. 24, 3732–3738 (2009).
Tervaert, T. W. et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 21, 556–563 (2010).
Wolf, G. Cell cycle regulation in diabetic nephropathy. Kidney Int. Suppl. 77, S59-66 (2000).
Al-Douahji, M. et al. The cyclin kinase inhibitor p21WAF1/CIP1 is required for glomerular hypertrophy in experimental diabetic nephropathy. Kidney Int. 56, 1691–1699 (1999).
Wada, T., Pippin, J. W., Terada, Y. & Shankland, S. J. The cyclin-dependent kinase inhibitor p21 is required for TGF-beta1-induced podocyte apoptosis. Kidney Int. 68, 1618–1629 (2005).
Li, Z. et al. Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am. J. Physiol. Renal Physiol. 301, F1078–F1087 (2011).
Wuerzner, G. et al. Marked association between obesity and glomerular hyperfiltration: A cross-sectional study in an African population. Am. J. Kidney Dis. 56, 303–312 (2010).
Sasson, A. N. & Cherney, D. Z. Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J. Diabetes. 3, 1–6 (2012).
Di Nicolò, P. & Granata, A. Renal resistive index: Not only kidney. Clin. Exp. Nephrol. 21, 359–366 (2017).
Ludvigsen, T. P. et al. (18)F-FDG PET/MR-imaging in a Göttingen Minipig model of atherosclerosis: Correlations with histology and quantitative gene expression. Atherosclerosis 285, 55–63 (2019).
Bassily, N. S., Michael, K. G. & Said, A. K. Blood urea content for evaluating dietary protein quality. Nahrung 26, 759–764 (1982).
Hebert, S. L. & Nair, K. S. Protein and energy metabolism in type 1 diabetes. Clin Nutr. 29, 13–17 (2010).
Kawamori, D. et al. Consistency of plasma glucagon levels in patients with type 1 diabetes after a 1-year period. J Diabetes Investig. 11, 337–340 (2020).
Satirapoj, B., Pooluea, P., Nata, N. & Supasyndh, O. Urinary biomarkers of tubular injury to predict renal progression and end stage renal disease in type 2 diabetes mellitus with advanced nephropathy: A prospective cohort study. J. Diabetes Complic. 33, 675–681 (2019).
Woroniecka, K. I. et al. Transcriptome analysis of human diabetic kidney disease. Diabetes 60, 2354–2369 (2011).
Mizuiri, S. et al. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am. J. Kidney Dis. 51, 613–623 (2008).
Reich, H. N., Oudit, G. Y., Penninger, J. M., Scholey, J. W. & Herzenberg, A. M. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 74, 1610–1616 (2008).
Dominguez, J. H., Camp, K., Maianu, L., Feister, H. & Garvey, W. T. Molecular adaptations of GLUT1 and GLUT2 in renal proximal tubules of diabetic rats. Am. J. Physiol. 266, F283–F290 (1994).
Schumacher-Petersen, C. et al. Experimental non-alcoholic steatohepatitis in Göttingen Minipigs: consequences of high fat-fructose-cholesterol diet and diabetes. J. Transl. Med. 17, 110 (2019).
Acknowledgements
Technicians and veterinarians involved in the in-life part of the study: Camilla Schumacher-Petersen, Ann-Charlott Kemp, Charlotte Lasak Krøyer Pedersen, Hanne Toftelund, Johnny Kaltoft Nisted, Kamilla Larsen, Lotte Schmidt Marcher, Pernille Kristiane Pedersen, Anders Uhrenfeldt, Ann-Christin Löff. Plasma and urine analyses: Marianne Kjestine Petersen, Anita Svendsen, Pia Skaarup Andersen. Histology and TEM: Helle Wagner, Susanne Juul Rasmussen, Pia Rothe, Malik Nygaard Nielsen. Gene expression: Tina B. N. Mahler, and Minna B. Jakobsen. Donation of NGAL assays: BioPorto Diagnostics A/S, Hellerup, Denmark.
Funding
The study was funded by Novo Nordisk A/S. LIFEPHARM (Novo Nordisk – LIFE In Vivo Pharmacology Centre) at University of Copenhagen funded a PhD grant for RL. Kits for analysis of plasma and urine NGAL were donated by BioPorto Diagnostics A/S, Hellerup, Denmark.
Author information
Authors and Affiliations
Contributions
B.O.C.: Study design, study conduct, data interpretation, writing of manuscript; C.A.K.: Gene expression analysis including statistics and PCA plots; R.L.T.: Study design, study conduct, writing of manuscript; R.K.K.: Histopathological evaluation and quantification, data interpretation; B.M.V.: Electron microscopy and interpretation; P.H.K.: Histopathological evaluation and quantification, data interpretation; H.D.P.: Study design, study conduct, data interpretation, writing of manuscript; T.P.L.: Study design, study conduct; T.S.: Plasma inulin analysis and interpretation, assay validation; J.J.F.: Plasma insulin and glucagon analysis; T.M.: Statistical analysis and interpretation; L.B.C.: NGAL urine and plasma analysis, assay validation; S.C.: Gene expression analysis incl. statistical analysis, writing of manuscript; L.H.O.: Study design, NGAL urine and plasma analysis, statistical analysis, data interpretation writing of manuscript; All authors reviewed the manuscript. A list of publications based on the same animals are available in supplementary information file 1.
Corresponding author
Ethics declarations
Competing interests
BMV, BØC, HDP, JJF, TPL, RKK and PHK are employees and BØC, JJF, PHK, TPL AND RKK are minor shareholders of Novo Nordisk A/S. All other authors have no conflict of interest. Kits for analysis of plasma and urine NGAL were donated by BioPorto Diagnostics A/S, Hellerup, Denmark.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Christoffersen, B.Ø., Kristensen, C.A., Lindgaard, R. et al. Functional and morphological renal changes in a Göttingen Minipig model of obesity-related and diabetic nephropathy. Sci Rep 13, 6017 (2023). https://doi.org/10.1038/s41598-023-32674-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32674-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.