Abstract
Objectives. The detection of Coronavirus Disease 2019 (COVID-19) by reverse transcription polymerase chain reaction (RT-PCR) has varying sensitivity. Computed tomography (CT) of the chest can verify infection in patients with clinical symptoms and a negative test result, accelerating treatment and actions to prevent further contagion. However, CT employs ionising radiation. The purpose of this study was to evaluate protocol settings, associated radiation exposure, image quality and diagnostic performance of a low-dose CT protocol in a university hospital setting. Materials and Methods. Chest CT examinations were performed on a single scanner (Somatom Definition Edge, Siemens Healthineers, Germany) in 105 symptomatic patients (60 male, 45 female). Images were evaluated with regard to protocol parameters, image quality, radiation exposure and diagnostic accuracy. Serial RT-PCR served as the standard of reference. Based on this reference standard sensitivity, specificity, positive and negative predictive values of CT with 95% confidence interval were calculated. Results. The mean effective dose was 1.3 ± 0.4 mSv (0.7–2.9 mSv) for the patient cohort (mean age 66.6 ± 16.7 years (19–94 years), mean body mass index (BMI) 26.6 ± 5.3 kg m−2 (16–46 kg/m2)). A sensitivity of 100 [95% CI: 82–100]%, a specificity of 78 [95% CI: 68–86]%, a positive predictive value of 50 [95% CI: 33–67]% and a negative predictive value of 100 [95% CI: 95–100]% were obtained. No COVID-19 diagnoses were missed by CT. Image noise did not strongly correlate with BMI or patient diameter and was rated as average. Conclusions. We presented a robust imaging procedure with a chest CT protocol for confident diagnosis of COVID-19. Even for an overweight patient cohort, an associated radiation exposure of only 1.3 ± 0.4 mSv was achieved with sufficient diagnostic quality to exclude COVID-19.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Computed tomography (CT) can lead the diagnosis of Coronavirus Disease 2019 (COVID-19) with focus on symptomatic patients with negative reverse transcription polymerase chain reaction (RT-PCR) results from swab tests [1–3]. The sensitivity and specificity of COVID-19 diagnosis using swab tests is impeded by inadequate swab procedures, testing outside the diagnostic window or use of inadequately validated assays [4]. A positive RT-PCR result clearly confirms the presence of the inducing SARS-CoV-2 virus, while a negative test result only confirms the absence of the virus in the taken sample. Therefore, a single negative RT-PCR test has not been found to be reliable in COVID-19 diagnosis and is typically followed by serial RT-PCR testing in patients with symptoms compatible with the diagnosis of COVID-19, increasing its diagnostic accuracy. Although the numbers of infections are currently decreasing in Europe, hospitals might have to deal with an increasing number of patients again during a second peak of the COVID-19 pandemic. Furthermore, results of RT-PCR tests are not immediately available and can take up to 24 h in total or even longer for high volumes of tests. Thus, in critically ill patients immediate CT may be mandatory for diagnosis and assessment of disease severity.
X-ray radiographs as possible alternatives to CT are challenging in the diagnosis of COVID-19 as early pulmonary disease may be missed on a chest x-ray [5]. Especially if patients are obese or imaged in a reclining position, diagnosis is impeded. Patterns typically found in COVID-19 patients such as ground glass opacities or consolidation are characteristically detected with CT [1, 5–8]. Magnetic resonance imaging of the lungs as a potential alternative has known limitations in pulmonary disease, is technically more complex and time intensive, and has high complexity of proper disinfection. Problems might also arise with MR-unsafe respirators [9]. A small number of studies have covered the employed CT protocol settings and the associated radiation exposure [6, 10–12], though the majority of publications mainly cover manifestations of COVID-19 in CT or its diagnostic performance [13–16], even for paediatric protocols [17–20]. A number of publications have treated follow-up CT examinations to assess CT findings with regard to the course of COVID-19 and with regard to changes with time, despite the high cumulative radiation exposure associated with repetitive CT examinations [6, 11, 12]. Although the infections usually cause more severe symptoms in elderly patients, the number of young patients in radiology departments might increase in the future. Especially for younger patients, the lifetime risk of developing a radiation-induced cancerous disease is increased, one reason being due to the longer life expectancy of young patients. Another reason is that cells divide faster in young patients compared to older patients, shortening the cell cycle and the time for repair of a radiation-induced injury [21, 22]. Although follow-up examinations are not expected or required for the majority of patients, patients with severe and progressive symptoms might undergo frequent CT examinations to monitor pulmonary changes. Hence, there is a need for a low-dose chest CT protocol that still allows for confident diagnosis and follow-up of COVID-19. The aim of this study was to evaluate a chest CT protocol, the resulting image quality and radiation exposure, and its diagnostic accuracy for COVID-19 detection.
2. Materials and methods
In this prospective study, we assessed 105 initial chest CT examinations of patients with suspected or diagnosed SARS-CoV-2-infection that underwent a non-enhanced chest CT examination at our department between March 17 and April 17, 2020. The institutional review board approved this study and waived patient informed consent. All patients had respiratory symptoms and possible contact with SARS-CoV-2 infected persons.
2.1. Indications for CT
CT was performed in the following clinical settings:
-
(a)Patients with clinical symptoms of COVID-19 and a negative (89 patients) or unknown (1 patient) PCR test result
-
(b)Patients with known COVID-19 (positive PCR test result) to assess severity of pulmonary disease (15 patients)
2.2. Aim of the CT protocol
Findings compatible with SARS-CoV-2 infection are detectable on CT images without the need for contrast media injection. The chest CT protocol employed in our department was designed to provide diagnostic information on both, lung and soft tissue (e.g. lymph nodes). Hence, we chose a tube potential of 100 kVp, independent of patient body mass index (BMI) or weight, to increase soft tissue contrast in an unenhanced (native) CT acquisition. Only patients with a severe course of disease and clinical indication were examined twice or more.
2.3. Organisational structure and image acquisition
To prevent suspected patients from transmitting the infection to others, several organisational actions have been taken in the radiology department [23, 24]. Patients with suspected COVID-19 were hospitalised through a transit unit, where RT-PCR tests were taken and—in almost all cases—an x-ray examination of the chest was performed. Patients with positive RT-PCR test results but minor symptoms were home quarantined to prevent further transmission.
Patients suspected to suffer from COVID-19 and with clear clinical symptoms were scanned on a single dedicated CT scanner. All staff were specifically trained in handling infectious patients and equipped with protective gear. Furthermore, a fixed team of two radiologists working at the CT scanner were provided with a template for a structured report, which covered typical COVID-19 manifestations on CT. All data were evaluated by one of the two radiologists who were not aware of the RT-PCR result when reading the scans. Scans were assessed according to previously published criteria (1).
All images were acquired on a third-generation CT scanner (Somatom Definition Edge, Siemens Healthineers, Forchheim, Germany). Patients were scanned in supine position with elevated arms when patient condition allowed for proper positioning and with breath-holding after inspiration. The scan range was from the lung apex to the base of the lung. The applied CT protocol parameters are described in table 1. Images were iteratively reconstructed using ADMIRE (Siemens Healthineers, Forchheim, Germany) as described in table 2. After image acquisition and evaluation, patients received treatment based on their symptoms (e.g. administration of oxygen) and their assumed COVID-19 status (COVID-19 ward or general ward; specific medication for viral or other pneumonia).
Table 1. Protocol parameters for the COVID–19 chest CT examination.
| Parameter | |
|---|---|
| tube potential [kVp] | 100 |
| reference TCTP [mAs] | 60 |
| tube current modulation | X-CARE, semi: 100 kVp fixed, CARE Dose4D |
| spiral pitch | 0.6 |
| rotation time [s] | 0.28 |
| collimation | 128 × 0.6 mm |
| total collimation width [mm] | 38.4 |
| FOV [cm] | 50 |
| contrast enhancement | None |
Abbreviations—FOV: field of view; TCTP: tube current–time product
Table 2. Chest CT reconstruction parameters for each acquisition.
| R1 | R2 | R3 | R4 | R5 | |
|---|---|---|---|---|---|
| stack orientation | axial | axial | axial | coronal | sagittal |
| slice thickness [mm] | 2.0 | 2.0 | 2.0 | 3.0 | 3.0 |
| increment [mm] | 1.6 | 1.6 | 2.0 | 2.4 | 2.4 |
| kernel (IR-level) | I30 f (2) | I70 f (2) | I70 f (2) | I70 f (2) | I70 f (2) |
| windowing | soft issue | bone | lung | lung | lung |
| FOV | full view | full view | lung | lung | lung |
Abbreviations—IR: iterative reconstruction, R1–R5: reconstructions 1–5
2.4. RT-PCR
RT-PCR tests were performed on respiratory tract specimens (oropharyngeal or nasopharyngeal swab or tracheal aspirate) at the Institute of Virology of the University Hospital Düsseldorf. External PCR test results were additionally taken into account, if available. Repetitive tests were performed in most patients (mean: 3 tests, range 1–17 in negative tested patients). For hospitalised patients with two negative RT-PCR test results, a third RT-PCR test was performed from bronchial lavage specimens. If the CT examination showed typical manifestations of COVID-19, patients were tested with RT-PCR on a daily basis during their hospitalisation. Patients with at least one positive RT-PCR test result were counted as confirmed diseased patients despite other possible negative RT-PCR test results.
2.5. Data collection
CT image data, volumetric CT dose index (CTDIvol), dose length product (DLP), and scan length were collected in the local picture archive and communication system (SECTRA Medical, Sweden). Furthermore, the time between importing the scout acquisition and the last image reconstruction was determined in the picture archive and communication system and evaluated to estimate the duration of an examination. Patient weight and height were documented when patient condition allowed for surveying (body height and weight were not available in 1/105 (1%) patients (a rather lean patient)). In all examinations, the lateral (lat) and anterior–posterior (ap) diameter of the center CT slice were measured to calculate the effective diameter as
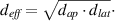
The effective dose was calculated using the conversion factor published by Deak et al (k = 0.0144 mSv/(mGy·cm)) for adults scanned at a tube potential of 100 kVp, using ICRP publication 103 tissue weighting factors [25]. Size-specific dose estimates (SSDEs) were calculated from the effective diameter and the CTDIvol as published in AAPM report no. 220 [26].
RT-PCR test results and the time of testing were taken from the hospital information system.
2.6. Objective and subjective image quality
The objective image quality was determined by drawing regions of interest (size 1 cm2) in the lung parenchyma, aorta and air, and determining the mean CT-value and standard deviation (SD). The signal-to-noise ratio (SNR) was calculated as  and the contrast-to-noise ratio (CNR) was calculated as
and the contrast-to-noise ratio (CNR) was calculated as  .
.
The subjective image quality of the lung reconstructions was rated by means of image noise based on a five-point scale (1: minimal noise, 2: less than average noise, 3: average noise, 4: above average noise, 5: unacceptable noise) by one radiologist with 10 years of experience.
2.7. Statistical analysis
Statistical analysis was performed using SPSS version 26 (SPSS Inc. Chicago, IL). Results are provided as mean ± standard deviation with range. Groups divided by BMI (BMI < 25 kg m−2, n = 43, and BMI ≥ 25 kg m−2, n = 62) were compared regarding the chest CT protocol parameters and radiation exposure using the Kruskal–Wallis test with a significance level of p < 0.05. We further calculated the sensitivity, specificity, positive predictive and negative predictive values using the RT-PCR results as a reference and calculated 95%-confidence intervals using the epiR package in R [27]. Correlations between CTDIvol, DLP, effective dose, SSDE, patient characteristics (BMI, effective diameter, age, size) and image quality were tested using a Spearman correlation coefficient. A two-sided p value of <0.05 was considered statistically significant.
3. Results
3.1. Patient population
Of 105 patients, 60/105 (57%) were male and 45/105 (43%) were female. The average age was 66.6 ± 16.7 years (range 19–94 years) and the average BMI was 26.6 ± 5.3 kg m−2 (range 16–46 kg m−2). Details can be found in table 3(a).
Table 3. Final results of the 105 CT examinations, for the total patient cohort (a) and grouped by BMI (b). *Note that the BMI was not available for one patient (male, lean figure with assumed BMI < 25 kg m−2).
| (a) Total patient cohort | |||||
|---|---|---|---|---|---|
| Total patient cohort, N = 105 | |||||
| Mean | SD | Minimum | Maximum | ||
| age [years] | 66.6 | 16.7 | 19.0 | 94.0 | |
| BMI [kg m−2] | 26.6 | 5.3 | 16.3 | 45.8 | |
| effective TCTP [mAs] | 70.4 | 22.3 | 35.0 | 169.0 | |
| scan length [cm] | 30.0 | 3.3 | 21.0 | 38.4 | |
| effective diameter [cm] | 29.6 | 3.1 | 24.2 | 41.9 | |
| CTDIvol [mGy] | 2.8 | 0.9 | 1.4 | 6.7 | |
| DLP [mGy·cm] | 89.3 | 27.7 | 48.5 | 201.7 | |
| effective dose [mSv] | 1.3 | 0.4 | 0.7 | 2.9 | |
| SSDE [mGy] | 3.4 | 0.8 | 2.1 | 7.6 | |
| image noise grade | 2.95 | 0.56 | 2.00 | 4.00 | |
| SNR lung | 12.5 | 2.7 | 5.5 | 19.4 | |
| SNR heart | 0.4 | 0.1 | 0.1 | 0.8 | |
| SNR air | 23.5 | 4.2 | 14.8 | 41.9 | |
| CNR | 9.8 | 1.8 | 5.4 | 14.7 | |
| (b) Grouped by BMI | |||||
| BMI < 25 [kg m−2] N = 43* | BMI ≥ 25 [kg m−2] N = 62 | ||||
| Mean | SD | Mean | SD | p < 0.05 | |
| age [years] | 65.9 | 17.1 | 67.2 | 16.6 | |
| BMI [kg m−2] | 21.8 | 2.5 | 29.9 | 4.0 | X |
| effective TCTP [mAs] | 56.7 | 13.0 | 79.9 | 22.6 | X |
| scan length [cm] | 31.1 | 3.4 | 29.2 | 3.0 | X |
| effective diameter [cm] | 27.7 | 2.2 | 31.1 | 2.9 | X |
| CTDIvol [mGy] | 2.3 | 0.5 | 3.2 | 0.9 | X |
| DLP [mGy·cm] | 74.2 | 16.7 | 99.8 | 29.1 | X |
| effective dose [mSv] | 1.1 | 0.2 | 1.4 | 0.4 | X |
| SSDE [mGy] | 3.0 | 0.5 | 3.7 | 0.9 | X |
| image noise grade | 2.81 | 0.59 | 3.05 | 0.53 | X |
| SNR lung | 13.3 | 2.7 | 12.0 | 2.6 | X |
| SNR heart | 0.4 | 0.1 | 0.4 | 0.1 | |
| SNR air | 24.2 | 3.7 | 23.0 | 4.4 | X |
| CNR | 10.4 | 1.8 | 9.4 | 1.8 | X |
Abbreviations—TCTP: tube current–time product; BMI: body mass index; SSDE: size-specific dose estimate; CTDIvol: volumetric computed tomography dose index; DLP: dose–length product; SD: standard deviation.
3.2. Radiation exposure associated with chest CT
For the 105 CT examinations, the effective TCTP was 70.4 ± 22.3 mAs (35–169 mAs) at 100 kVp resulting in a mean CTDIvol of 2.8 ± 0.9 mGy (1.4–6.7 mGy). The mean effective patient diameter at the central CT slice was 29.6 ± 3.1 (24.2–41.9 cm). With a scan length of 30.0 ± 3.3 cm (21–38 cm), a DLP of 89.3 ± 27.7 mGy·cm (48.5–201.7 mGy·cm) was reached. Combining the effective diameter and the CTDIvol, a mean SSDE of 3.4 ± 0.8 mGy (2.1–7.6 mGy) was obtained. The associated effective dose from the CT examinations amounted to 1.3 ± 0.4 mSv (0.7–2.9 mSv). See figures 1–2 for a depiction of the image quality in a patient with a BMI of 45.8 kg m−2 (figure 1), and with a BMI of 28.4 kg m−2 (figure 2).
Figure 1. 55-year-old male patient (BMI 45.8 kg m−2) with mainly rounded ground glass opacities, consolidation, thickened septa. All lobes were affected, although the lower lobes were focused. The first RT-PCR test was positive for this patient. The effective dose of the chest CT examination amounted to 2.2 mSv (CTDIvol 5.4 mGy). (a) Axial view, (b) coronal view and (c) created 3D view with the function CT Pulmo 3D (syngo.via, Siemens Healthineers, Forchheim, Germany). Window: 1500 HU, level: −500 HU.
Download figure:
Standard image High-resolution imageFigure 2. 43-year-old male patient (BMI 28.4 kg m−2) with consolidation, thickened septa, occasional ground glass opacities and crazy paving pattern. All lobes were affected, although the lower lobes were pronounced. The RT-PCR test result was positive 9 d prior to the CT date for this patient, but occasionally negative during the course of treatment. The patient first received only x-ray radiographs in the first days. However, due to worsening of the condition, a CT was performed eventually. The effective dose of the chest CT examination amounted to 1.8 mSv (CTDIvol 3.8 mGy). (a) Axial view, (b) coronal view and (c) created 3D view with the function CT Pulmo 3D (syngo.via, Siemens Healthineers, Forchheim, Germany). Window: 1500 HU, level: −500 HU.
Download figure:
Standard image High-resolution image3.3. Objective and subjective image quality
The mean SNRs of the lung, heart and air were 12.5 ± 2.7 (range 5.5–19.4), 0.4 ± 0.1 (range 0.1–0.8) and 23.5 ± 4.2 (range 14.8–41.9), respectively. The mean CNR was 9.8 ± 1.8 (range 5.4–14.7). The mean image noise was rated as 'average' (2.95 ± 0.56 (range 2.00–4.00)); see table 3(a).
3.4. Correlation and group analysis
Between the BMI groups, patients with BMI < 25 kg m−2 had significantly lower TCTPeff, CTDIvol, DLP, effective doses and SSDEs; see table 3(b). The TCTPeff and CTDIvol were 40% higher in the high-BMI group (BMI ≥ 25 kg m−2) compared to the low-BMI group (BMI < 25 kg m−2). The SSDE in the high-BMI group was 23% higher than the SSDE in the low-BMI group, taking patient effective diameter into account. Using a BMI-independent conversion factor, the effective dose increased with effective diameter (1.1 ± 0.2 mSv (BMI < 25 kg m−2) vs 1.4 ± 0.4 mSv (BMI ≥ 25 kg m−2)). Rated image noise increased slightly with increasing BMI (2.81 ± 0.59 for BMI < 25 kg m−2 and 3.05 ± 0.53 for BMI ≥ 25 kg m−2).
TCTPeff, CTDIvol, DLP, SSDE and effective dose significantly correlate with BMI and effective diameter but not with age (table 4(a)). BMI and effective diameter are strongly correlated (rs > 0.5) for TCTPeff, CTDIvol, DLP and the effective dose. Correlation between image quality, demographic and exposure data was low to fair (range 0.02–0.49); see table 4(b). A significant negative correlation between image quality and BMI was noted, though only with a correlation coefficient ranging between 0.26–0.44.
Table 4. Spearman's correlation coefficient (rs) and significance level (p) for exposure data, demographic values, and objective and subjective image quality. (a) Correlation between TCTPeff, CTDIvol, DLP, effective dose and SSDE with demographic values. (b) Correlation between objective and subjective image quality measures with demographic values and exposure data.
effvollungheartair| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| variable | rs | P | rs | P | rs | P | rs | P | rs | p |
| age | −.056 | 0.570 | −.054 | .586 | −.084 | .394 | −.083 | .399 | −.061 | .536 |
| BMI | .663 | <.001 | .665 | <.001 | .560 | <.001 | .559 | <.001 | .560 | <.001 |
| eff. diameter | .719 | <.001 | .772 | <.001 | .713 | <.001 | .711 | <.001 | .489 | <.001 |
| variable | rs | P | rs | P | rs | P | rs | P | rs | p |
| age | .170 | .083 | −.022 | .824 | −.124 | .208 | −.134 | .173 | .029 | .773 |
| BMI | .306 | .002 | −.374 | <.001 | −.255 | .009 | −.338 | <.001 | −.435 | <.001 |
| eff. diameter | .071 | .472 | −.280 | .004 | −.230 | .018 | −.488 | <.001 | −.440 | <.001 |
| CTDIvol | .118 | .231 | −.309 | .001 | −.125 | .203 | −.336 | <.001 | −.386 | <.001 |
| eff. dose | .023 | .820 | −.212 | .030 | .117 | .235 | −.346 | <.001 | −.328 | .001 |
| SSDE | .113 | .252 | −.248 | .011 | −.030 | .763 | −.184 | .060 | −.279 | .004 |
Abbreviations— CTDIvol: volumetric computed tomography dose index; DLP: dose–length product; SSDE: size-specific dose estimate.
The image noise was rated 2 in 19/105 (18%) patients, 3 in 72/105 (69%) patients and 4 in 14/105 (13%) patients. No images were rated with 0 (minimal image noise) or 5 (unacceptable image noise). Examples of patients with different image quality are presented in figure 3.
Figure 3. Examples of image noise ratings in the CT examinations. (a) 69-year-old female patient (BMI 16.3 kg m−2) with a rated image noise value of 2 (less than average noise). The effective dose of the chest CT examination amounted to 0.7 mSv (CTDIvol 1.4 mGy). (b) 55-year-old male patient (same as in figure 1), rated with an image quality of 3, despite a BMI of 45.8 kg m−2. The effective dose of the chest CT examination amounted to 1.8 mSv (CTDIvol 2.2 mGy). (c) 89-year old female patient with a BMI of 32.4 kg m−2, rated with an image quality of 4. The effective dose of the chest CT examination amounted to 0.8 mSv (CTDIvol 2.2 mGy). Window: 1500 HU, level: −500 HU.
Download figure:
Standard image High-resolution image3.5. Diagnostic accuracy of chest CT
In this study, a single positive RT-PCR test confirmed infection, independent of other negative results. There were 19/105 (18%) true positive, 67/105 (64%) true negative, and 19/105 (18%) false positive results according to the reference standard (RT-PCR results). No SARS-CoV-2 infections were missed by the CT examination (no false negative CT results). A sensitivity of 100% [95% CI: 82–100], a specificity of 78% [95% CI: 68–86], a positive predictive value of 50% [95% CI: 33–67] and a negative predictive value of 100% [95% CI: 95–100] were obtained.
3.6. Image acquisition time and handling
The time between scout acquisition and import of the last image reconstruction ranged between 2 and 3 min. Since no contrast media were applied, patient contact was reduced to a minimum (transport to/from the radiology department, patient positioning in the CT scanner room). Neither radiographers nor medical doctors were exposed to infectious fluids such as blood due to protective equipment. No infections with SARS-CoV-2 were identified among the radiological staff members at the time of writing.
4. Discussion
In this study, we present a robust patient handling and imaging procedure for the diagnosis of COVID-19 using chest CT and provide information on the protocol settings and the resulting associated radiation exposure. With the employed CT protocol, both lung and soft tissue were assessed with sufficient image quality.
Since all radiologists at our institution are working with a structured report template for COVID-19, reporting can be performed quickly but thoroughly [6]. Even for larger volumes of patients, a non-contrast CT protocol is practicable since patient-handling time is reduced and staff are less exposed to the infectious patient. At our institution, all suspected COVID-19 patients are examined on a dedicated CT scanner with staff trained specifically for this situation. Based on the well-structured institutional organisation there are no SARS-CoV-2 infections identified among staff to date.
For the 105 included patients in the analysed study cohort, effective doses of approximately 1.3 mSv were estimated. In the study cohort published by Dangis et al, effective doses of only 0.6 mSv were obtained in the patient cohort with an even higher average BMI (28.9 kg m−2), using a similar conversion coefficient [10]. They examined patients with a sub-mSv chest CT protocol, resulting in a sensitivity of 86.7% and a specificity of 93.6% in a study cohort with 83 confirmed patients out of 192 patients. With their presented sub-mSv protocol, diagnosis of COVID-19 of PCR-confirmed infected patients via CT was missed in 11/83 (13%) cases. Even though we had a higher effective dose in our cohort compared to Dangis et al, there were no infections missed with CT in our cohort (sensitivity of 100%), which is essential to fast diagnosis and prevention of further contagion. Missing infections in CT might be caused due to an inappropriate image quality or early disease status, where the lungs were not affected yet. The specificity and positive predictive values in our cohort (78% and 50%, respectively) were lower compared to Dangis et al (94% and 92%), due to the large number patients with COVID-19 typical manifestations in CT but negative RT-PCR results in our cohort [10]. This might be explained by differences in the patient cohort (low prevalence of COVID-19 compared to other viral pneumonia with similar manifestations). However, we do not expect the high number of false positive patients to originate from the diagnostic image quality. In our patient cohort, image noise was rated as 'average' for the majority of the patients, even for patients with high BMIs. There have been no images with unacceptable image noise, which would indicate insufficient detector signal. On the other hand, none of the images was rated with minimal image noise; hence, patients were not exposed to too high a radiation exposure. The image quality, both qualitative and quantitative, did not correlate strongly with the BMI or effective diameter. Hence, even for large patients, the image quality remains sufficient, despite the constant tube potential of 100 kVp, which was not adapted to patient BMI to improve soft tissue contrast.
In a recent study by Pan et al, a mean CTDIvol of 8.4 ± 2.0 mGy (range 5.2–12.6 mGy) was reported for CT examinations in COVID-19, which is considerably higher than the CTDIvol of 2.8 ± 0.9 mGy (range 1.4–6.7 mGy) we found with our protocol [11]. Moreover, patients in their study cohort were examined four times on average which has also been reported by Wang et al [6, 11]. Liu et al performed CT examinations in pregnant women diagnosed with COVID-19 pneumonia, achieving a CTDIvol of 4.1 ± 0.9 mGy (range 2.3–5.8 mGy) [12]. Out of the 15 included patients, 10 were scanned twice (67%), although none of the patients had acute respiratory distress syndrome in the whole course [12]. In contrast to these studies, patients at our institution only undergo follow-up CT in severe courses of the disease. At the point of manuscript preparation, only 3 out of the 105 patients (3%) had one or two follow-up examinations due to worsening of respiratory symptoms. In the setting of repeat CT scans low radiation exposure is key, especially when younger patients are examined. We do not expect patients to be examined four times on average as has been reported by Wang et al and Pan et al. However, their results are important to understanding the time course of the disease when imaged with CT [6, 11].
In the study of Bernheim et al, symptomatic patients with positive RT-PCR tests were included and examined with chest CT [14]. Out of those patients having an early CT scan within two days of symptom onset, 56% had negative CT. With increasing time between symptom onset and CT examination the percentage of typical CTfindings increased. This was also confirmed by Dangis et al, where CT in patients with symptoms >48 h had a 10% higher sensitivity than patients examined in a very early disease state [10]. In our study cohort, patients that were eventually tested positive for a SARS-CoV-2 infection all had typical manifestations in the initial CT images, such as ground glass opacities or consolidation. This shows that our CT procedure, regarding the indication for CT and the CT protocol, allows for confident diagnosis of infected patients with a low effective dose (1.3 ± 0.4 mSv). Compared to the German diagnostic reference level for a chest CT examination (5mSv), the average effective dose of the proposed CT protocol is 74% lower [28]. Its execution is justified as diagnostic tool in symptomatic patients with negative RT-PCR and follow-up examination in severe courses of COVID-19. According to ICRP publication 103, the risk of fatal cancer from a CT examination amounts to 0.0065% (5% per Sv) [29].
This study has some limitations. We only included 105 patients in a prospective study cohort, which is a fairly low number of patients compared to other publications [1, 2, 13, 14]. We used RT-PCR test results as the reference for calculating the sensitivity, specificity, positive and negative predictive values of chest CT. Even serially repeated RT-PCR may be falsely negative thus affecting statistics. To increase reliability of the disease status, the presence of antibodies from patient samples needs to be additionally assessed. Lastly, the conversion factor used to calculate effective doses (k = 0.0144mSv/(mGy·cm)) is independent of patient size, resulting in a possible overestimation of the dose for large patients.
5. Conclusion
To summarise, we present a low-dose chest CT protocol as a robust imaging procedure with sufficient image quality to diagnose COVID-19 with high sensitivity in a setting of symptomatic patients with mainly negative PCR tests. Low-dose, non-contrast enhanced chest CT may evolve as the modality of choice to support diagnosis of COVID-19 in the setting of symptomatic patients with initially negative PCR and for follow-up of clinically severe disease.
Acknowledgments
The authors have nothing to disclose.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical statement
The institutional review board approved this study and waived patient informed consent.




