Abstract
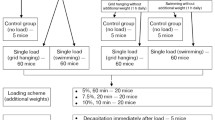
The effect of forced treadmill running on the level of some cytokines in skeletal muscles of mice with a model of type II diabetes mellitus was studied. The mouse model was developed using a 12-week high-fat diet; physical loading in the form of forced treadmill running was applied for 4 weeks. The concentration of myokines in m. gastrocnemius was determined by an enzyme-linked immunosorbent assay (ELISA). Diabetes formation in mice was accompanied by changes in the skeletal muscle concentration of two pro-inflammatory cytokines: an increase in IL-6 and a decrease in IL-15. Forced treadmill running loads had differential effects on the level of myokines in healthy and sick mice. In healthy animals, there was a decrease in IL-6 and IL-15 concentrations and an increase in the leukemia inhibitory factor (LIF) concentration in skeletal muscles after 4 weeks of regular forced running. At the same time, in diabetic mice, IL-6 and IL-15 concentrations increased, while the LIF concentration decreased after forced running loads. The NAP3 concentration in skeletal muscles was found to be insensitive to both the development of type II diabetes mellitus and regular forced treadmill running.




Similar content being viewed by others
REFERENCES
Frontera WR, Ochala J (2015) Skeletal muscle: a brief review of structure and function. Calcif Tissue Int 96:183–195. https://doi.org/10.1007/s00223-014-9915-y
Sprenger H, Jacobs C, Nain M, Gressner A, Prinz H, Wesemann W, Gemsa D (1992) Enhanced release of cytokines, interleukin-2 receptors, and neopterin after long-distance running. Clin Immun Immunopathol 63:188–195. https://doi.org/10.1016/0090-1229(92)90012-d
Drenth JP, Van Uum SH, van Deuren MG, Pesman J, Van der Ven-Jongekrijg J, Van der Meer JW (1995) Endurance run increases circulation IL-6 and IL-1ra but downregulatesex vivo TNF-α and Il-1α production. J Appl Physiol 79:1497–1503. https://doi.org/10.1152/jappl.1995.79.5.1497
Nehlsen-Cannarella SL, Fagoaga OR, Nieman DC, Henson DA, Butterworth DE, Schmitt RL, Bailey EM, Warren BJ, Utter A, Davis JM (1997) Carbohydrate and the cytokine response to 2.5 h of running. J Appl Physiol. 82:1662–1667. https://doi.org/10.1152/jappl.1997.82.5.1662
Ostrowski K, Ronde T, Asp S, Schjerling P, Pedersen BK (1999) Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515:287–291. https://doi.org/10.1111/j.1469-7793.1999.287ad.x
Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen B (2000) Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529:237–242. https://doi.org/10.1111/j.1469-7793.2000.00237.x
Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD (2001) Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J 15:2748–2750. https://doi.org/10.1096/fj.01-0507fje
Kapilevich LV, Kabachkova AV, Zakharova AN, Lalaeva GS, Kironenko TA, Dyakova EYu, Orlov SN (2016) Secretory Function of Skeletal Muscles: Producing Mechanisms and Myokines Physiological Effects. Uspekhi Fiziol Nauk 47(2):7–26. (In Russ). PMID: 27530041
Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88:1379–1406. https://doi.org/10.1152/physrev.90100.2007
Iizuka K, Machida T, Hirafuji M (2014) Skeletal muscle is an endocrine organ. J Pharmacol Sci 125:125–131. https://doi.org/10.1254/jphs.14r02cp
Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secetory organ. Nat Rev Endocrinol 8:457–465. https://doi.org/10.1038/nrendo.2012.49
Groop LC, Eriksson JG (1992) The etiology and pathogenesis of non-insulin-dependent diabetes. Ann Med 24(6):483–489. https://doi.org/10.1002/dmr.5610090503
Fujimaki S, Kuwabara T (2017) Diabetes-induced dysfunction of mitochondria and stem cells in skeletal muscle and the nervous system. Int J Mol Sci 18(10):2147. https://doi.org/10.3390/ijms18102147
Højlund K (2014) Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance. Dan Med J 61(7):B4890. PMID: 25123125
Nagy C, Einwallner E (2018) Study of In vivo glucose metabolism in high-fat diet-fed mice using oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). J Vis Exp 7(131):1–12. https://doi.org/10.3791/56672
Huh JY (2018) The role of exercise-induced myokines in regulating metabolism. Arch Pharm Res 41(1):14–29. https://doi.org/10.1007/s12272-017-0994-y
Meneilly GS (2001) Pathophysiology of diabetes in the elderly. In: Diabetes in old age. John Wiley & Sons 155–164. https://doi.org/10.1002/0470842326.ch2
Brandt C, Pedersen BK (2010) The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol 2010:520258. https://doi.org/10.1155/2010/520258
Hansen JS, Zhao X, Irmler M, Liu X, Hoene M, Scheler M, Li Y, Beckers J, Hrabĕ de Angelis M, Häring HU, Pedersen BK, Lehmann R, Xu G, Plomgaard P, Weigert C (2015) Type 2 diabetes alters metabolic and transcriptional signatures of glucose and amino acid metabolism during exercise and recovery. Diabetologia 58:1845–1854. https://doi.org/10.1007/s00125-015-3584-x
Karstoft K, Pedersen BK (2016) Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol 94:146–150. https://doi.org/10.1038/icb.2015.101
Kapilevich L, Zakharova A, Kabachkova A, Kironenko T, Milovanova K, Orlov S (2017) Different impact of physical activity on plasma myokines content in athletes and untrained volunteers. FEBS J 284(1):373–373. WOS:000409918904202
Kapilevich LV, Zakharova AN, Kabachkova AV, Kironenko TA, Orlov SN (2017) Dynamic and static exercises differentially affect plasma cytokine content in elite endurance- and strength-trained athletes and untrained volunteers. Front Physiol 8:35. https://doi.org/10.3389/fphys.2017.00035
Winzell MS, Ahren B (2004) The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53(3):S215–S219. https://doi.org/10.2337/diabetes.53.suppl_3.s215
Kapilevich LV, Zakharova AN, Dyakova EYu, Kalinnikova JG, Chibalin AV (2019) Mice experimental model of diabetes mellitus type ii based on high fat diet. Bull Siberian Med 18(3):53–61. https://doi.org/10.20538/1682-0363-2019-3-53-61
Zakharova AN, Kalinnikova Y, Negodenko ES, Orlova AA, Kapilevich LV (2020) Experimental simulation of cyclic training loads. Teor Prakt Fizich Kult 10:26–27.
Schwartz V (2009) Regulation of metabolic processes by interleukin-6. Cytokines and Inflam 3:3–10. (In Russ).
Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B (2003) Searching for the exercise factor:: is IL-6 a candidate? J Muscle Res Cell Motil 24(2-3):113–119. https://doi.org/10.1023/a:1026070911202
Pedersen BK, Fischer CP (2007) Beneficial health effects of exercise: the role of Il-6 as a myokine. Trends Pharmacol Sci 28:152–156. https://doi.org/10.1016/j.tips.2007.02.002
Malashenkova IK, Casanova GV, Didkovsky NA (2014) Interleukin-15: structure, signaling and role in immune defense. Molec Med 3:9–20. (In Russ).
Quinn LS, Strait-Bodey L, Anderson BG, Argilés JM, Havel PJ (2005) Interleukin-15 stimulates adiponectin secretion by 3T3-L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int 29:449–457. https://doi.org/10.1016/j.cellbi.2005.02.005
Broholm C, Pedersen BK (2010) Leukemia inhibitory factor – an exercise-induced myokine. Exerc Immunol Rev 16:77–85. PMID:20839492
Srikuea R, Esser KA, Pholpramool C (2011) Lekemia factor is expressed in rat gastrocnemius muscle after contusion and increases proliferation of rat L6 myoblasts via c-Myc signalling. Clin Exp Pharmacol Physiol 38:501–509. https://doi.org/10.1111/j.1440-1681.2011.05537.x
Pedersen BK, Saltin B (2015) Exercise as medicine – evodence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 25:1–72. https://doi.org/10.1111/sms.12581
Pedersen L, Pilegaard H, Hansen J, Brandt C, Adser H, Hidalgo J, Olesen J, Pedersen BK, Hojman P (2011) Exercise-induced liver chemokine CXCL-1 expression is linked to muscle-derived interleukin-6 expression J Physiol 589(6):1409–1420. https://doi.org/10.1113/jphysiol.2010.200733
Pedersen L, Olsen CH, Pedersen BK, Hojman P (2012) Muscle-derived expression of the chemokine CXCL1 attenuates diet-induced obesity and improves fatty acid oxidation in the muscle. Am J Physiol Endocrinol Metab 302(7):831–840. https://doi.org/10.1152/ajpendo.00339.2011
Peake JM, Gatta PD, Suzuki K, Nieman DC (2015) Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exercise Immunol Rev 21:8–25. PMID: 25826432
Fitts RH, Widrick JJ (1996) Muscle mechanics: adaptations with exercise-training. Exerc Sport Sci Rev 24:427–473. PMID: 8744258
Raue U, Trappe T.A, Estrem S.T, Qian H-R, Helvering LM, Smith RC, Trappe S (2012) Transcriptomic signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol 112:1625–1636. https://doi.org/10.1152/japplphysiol.00435.2011
Gundersen K (2011) Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev 86:564–600. https://doi.org/10.1111/j.1469-185X.2010.00161.x
Kapilevich LV, Kironenko TA, Zaharova AN, Kotelevtsev YV, Dulin NO, Orlov SN (2015) Skeletal muscle as an endicrine organ: role of [Na+]i/[K+]i-mediated excitation-transcription coupling. Gen Diseas 2:328–336. https://doi.org/10.1016/j.gendis.2015.10.001
Ma H, Groth RD, Wheeler DG, Barrett CF, Tsien RW (2011) Excitation-transcription coupling in sympathetic neurons and the molecular mechanism of its initiation. Neurosci Res 70:2–8. https://doi.org/10.1016/j.neures.2011.02.004
Holmes AG, Watt MJ, Carey AL, Febbraio MA (2004) Ionomycin, but not physiological doses of epinephrine, stimulates skeletal muscle interleukin-6 mRNA expression and protein release. Metabolism 53:1492–1495. https://doi.org/10.1016/j.metabol.2004.05.015
Whitham M, Chan MHS, Pal M, Matthews VB, Prelovsek O, Lunke S, El-Osta A, Broenneke H, Alber J, Brüning JC, Wunderlich FT, Lancaster GI, Febbraio MA (2012) Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem 287:10771–10779. https://doi.org/10.1074/jbc.M111.31058
Nedachi T, Hatakeyama H, Kono T, Sato M, Kanzaki M (2009) Charactrization of contraction-inducible CXC chemokines and their roles in C2C12 myocytes. Am J Physiol Endocrinol Metab 297:E866–E878. https://doi.org/10.1152/ajpendo.00104.2009
McKenna MJ, Bangsbo J, Renaud JM (2008) Muscle K+, Na+, and Cl– disturbances and Na+-K+ pump inactivation: implications for fatigue. J Appl Physiol 104:288–295. https://doi.org/10.1152/japplphysiol.01037.2007
Koltsova SV, Trushina Y, Haloui M, Akimova OA, Tremblay J, Hamet P, Orlov SN (2012) Ubiquitous [Na+]i/[K+]i-sensitive transcriptome in mammalian cells: evidence for [Ca2+]i-independent excitation-transcription coupling. PLoS One 7:e38032. https://doi.org/10.1371/journal.pone.0038032
Funding
This work was supported by the Russian Science Foundation, grant No. 19-15-00118.
Author information
Authors and Affiliations
Contributions
A.N.Z., E.Yu.D., A.V.C., and L.V.K. contributed to the development of the concept and methodology of this study, data analysis, writing and editing the manuscript; T.A.K., K.G.M., A.A.O., and Yu.G.K. directly participated in experimental studies.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that this study has been performed without any commercial or financial relationships that might be interpreted as a potential conflict of interest.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2021, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2021, Vol. 107, Nos. 6–7, pp. 864–875https://doi.org/10.31857/S0869813921060157.
Rights and permissions
About this article
Cite this article
Zakharova, A.N., Kironenko, T.A., Milovanova, K.G. et al. Effect of Forced Treadmill Running on Skeletal Muscle Myokine Levels in Mice with a Model of Type II Diabetes Mellitus. J Evol Biochem Phys 57, 904–912 (2021). https://doi.org/10.1134/S0022093021040141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021040141