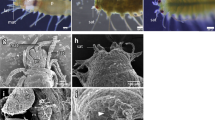
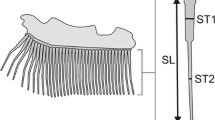
Abstract
Aurelia aurita is a representative of Scyphozoa, one of the classes of Cnidaria, the development and life cycle of which are quite well studied. In recent years, A. aurita has often been used as an object of studying the processes of cnidarian development, including a specific variant of asexual reproduction by strobilation, the formation of ephyrae (jellyfish larvae) due to the transverse fission of the polyp. In most cases, a prolonged decrease in temperature acts as a natural trigger for the initiation of strobilation. The need for prolonged induction and the unpredictability of the result makes it difficult to work with this model object. Therefore, for the induction of strobilation in laboratory conditions, various synthetic chemical compounds, the structure of which is close to that of the alleged “strobilation hormone,” are often used. This paper presents data on the detected deviations in the process of the separation of ephyrae in their external morphology in case of induction of strobilation by 5-methoxy-2-methylindole in A. aurita in laboratory conditions. For the first time, the deviations in the morphology of ephyrae observed in the experiment were compared with the deviations in the morphology of ephyrae and jellyfish from natural populations described in the literature. It is suggested that chemical induction leads to an increase in the frequency of morphological deviations in the ephyrae formed during the strobilation process based on the disorder of spatial patterning and the processes of differentiation of the rudiments.













Similar content being viewed by others
REFERENCES
Berking, S., Czech, N., Gerharz, M., Herrmann, K., Hoffmann, U., Raifer, H., Sekul, G., Siefker, B., Sommerei, A., and Vedder, F., A newly discovered oxidant defense system and its involvement in the development of Aurelia aurita (Scyphozoa, Cnidaria): reactive oxygen species and elemental iodine control medusa formation, Int. J. Dev. Biol., 2005, vol. 49, no. 8, pp. 969–976.
Berking, S. and Herrmann, K., Compartments in Scyphozoa, Int. J. Dev. Biol., 2007, vol. 51, no. 3, pp. 221–228.
Berrill, N.J., Developmental analysis of Scyphomedusae, Biol. Rev., 1949, vol. 24, no. 4, pp. 393–410.
Bondar’, N.I., Comparison of the formation of the polarity of the main body axis during asexual reproduction of Aurelia sp. and Cassiopea sp. (Scyphozoa, Cnidaria), MS Thesis, Moscow: Moscow State Univ., 2019.
Brekhman, V., Malik, A., Haas, B., Sher, N., and Lotan, T., Transcriptome profiling of the dynamic life cycle of the scypohozoan jellyfish Aurelia aurita, BMC Genomics, 2015, vol. 16, no. 1, p. 74.
Browne, E.T., Memoirs: on the variation of the tentaculocysts of Aurelia aurita, J. Cell Sci., 1895, vol. 2, no. 147, pp. 245–251.
Browne, E.T., Variation in Aurelia aurita, Biometrika, 1901, vol. 1, no. 1, pp. 90–108.
Brusca, R.C. and Brusca, G.J., Invertebrates, Sunderland: Sinauer, 2003.
Cabrales-Arellano, P., Islas-Flores, T., Thome, P.E., and Villanueva, M.A., Indomethacin reproducibly induces metamorphosis in Cassiopea xamachana scyphistomae, PeerJ, 2017, vol. 5, art. ID e2979.
Chernyshev, A.V. and Isaeva, V.V., Formation of chaotic patterns of the gastrovascular system in the ontogenesis of the medusa Aurelia aurita, Russ. J. Mar. Biol., 2002, vol. 28, no. 5, pp. 347–351.
Custance, D.R.N., Light as an inhibitor of strobilation in Aurelia aurita, Nature, 1964, vol. 204, no. 4964, pp. 1219–1220.
Dawson, M.N., Macro-morphological variation among cryptic species of the moon jellyfish, Aurelia (Cnidaria: Scyphozoa), Mar. Biol. (Berlin), 2003, vol. 143, no. 2, pp. 369–379.
Dawson, M.N. and Jacobs, D.K., Molecular evidence for cryptic species of Aurelia aurita (Cnidaria, Scyphozoa), Biol. Bull., 2001, vol. 200, no. 1, pp. 92–96.
Dogel, V.A., Zoologiya bespozvonochnykh: Uchebnik dlya universitetov (Invertebrate Zoology: Textbook for Universities), Polyanskii, Yu.I., Ed., Moscow: Vysshaya Shkola, 1981, 7th ed. (ext., rev.).
Fuchs, B., Wang, W., Graspeuntner, S., Li, Y., Insua, S., Herbst, E.-M., Dirksen, P., Hemmrich, G., Sommer, F., Domazet-Loao, T., Klostermeier, Ulrich, C., Anton-Erxleben, F., Rosenstiel, P., Bosch Thomas, C.G., and Khalturin, K., Regulation of polyp-to-jellyfish transition in Aurelia aurita, Curr. Biol., 2014, vol. 24, no. 3, pp. 263–273.
Genikhovich, G., How do developmental programs evolve?, in Old Questions and Young Approaches to Animal Evolution, Martin-Duran, J.M. and Vellutini, V.C., Eds., Cham: Springer, 2019, pp. 73–106.
Gershwin, L.A., Clonal and population variation in jellyfish symmetry, J. Mar. Biol. Assoc. UK, 1999, vol. 79, pp. 993–1000.
Gold, D.A., Nakanishi, N., Hensley, N.M., Cozzolino, K., Tabatabaee, M., Martin, M., Hartenstein, V., and Jacobs, D.K., Structural and developmental disparity in the tentacles of the moon jellyfish Aurelia sp. 1, PLoS One, 2015, vol. 10, no. 8, art. ID e0134741.
Gold, D.A., Katsuki, T., Li, Y., Yan, X., Regulski, M., Ibberson, D., Holstein, T., Steele, R.E., Jacobs, D.K., and Greenspan, R.J., The genome of the jellyfish Aurelia and the evolution of animal complexity, Nat. Ecol. Evol., 2019, vol. 3, no. 1, pp. 96–104.
Hamner, W.M. and Jenssen, R.M., Growth, degrowth, and irreversible cell differentiation in Aurelia aurita, Am. Zool., 1974, vol. 14, no. 2, pp. 833–849.
Hargitt, C.W., Variation among hydromedusae, Biol. Bull., 1901, vol. 2, no. 5, pp. 221–255.
Helm, R.R. and Dunn, C.W., Indoles induce metamorphosis in a broad diversity of jellyfish, but not in a crown jelly (Coronatae), PLoS One, 2017, vol. 12, no. 12, art. ID e0188601.
Holst, S., Effects of climate warming on strobilation and ephyra production of North Sea scyphozoan jellyfish, in Developments in Hydrobiology, vol. IV: Jellyfish Blooms, Purcell, J., et al., Eds., Dordrecht: Springer, 2012, pp. 127–140.
Ivanova-Kazas, O.M., Bespoloe razmnozhenie zhivotnykh (Asexual Reproduction of Animals), Leningrad: Leningrad. Univ., 1977.
Kakinuma, Y., An experimental study of the life cycle and organ differentiation of Aurelia aurita Lamarck, Bull. Mar. Biol. Stat. Asamushi, 1975, vol. 15, no. 3, pp. 101–113.
Khalturin, K., Shinzato, C., Khalturina, M., Hamada, M., Fujie, M., Koyanagi, R., Kanda, M., Goto, H., Anton-Erxleben, F., Toyokawa, M., Toshino, S., and Satoh, N., Medusozoan genomes inform the evolution of the jellyfish body plan, Nat. Ecol. Evol., 2019, vol. 3, no. 5, pp. 811–822.
Kroiher, M., Siefker, B., and Berking, S., Induction of segmentation in polyps of Aurelia aurita (Scyphozoa, Cnidaria) into medusae and formation of mirror-image medusa anlagen, Int. J. Dev. Biol., 2000, vol. 44, no. 5, pp. 485–490.
Kuniyoshi, H., Okumura, I., Kuroda, R., Tsujita, N., Arakawa, K., Shoji, J., Saito, T., and Osada, H., Indomethacin induction of metamorphosis from the asexual stage to sexual stage in the moon jellyfish, Aurelia aurita, Biosci. Biotechnol. Biochem., 2012, vol. 76, no. 7, pp. 1397–1400.
Miyake, H., Iwao, K., and Kakinuma, Y., Life history and environment of Aurelia aurita, South Pacific Study, 1997, vol. 17, no. 2, pp. 273–285.
Nakanishi, N., Yuan, D., Jacobs, D.K., and Hartenstein, V., Early development, pattern, and reorganisation of the planula nervous system in Aurelia (Cnidaria, Scyphozoa), Dev. Genes Evol., 2008, vol. 218, pp. 511–524.
Nakanishi, N., Yuan, D., Hartenstein, V., and Jacobs, D.K., Evolutionary origin of rhopalia: insights from cellular-level analyses of Otx and POU expression patterns in the developing rhopalial nervous system, Evol. Dev., 2010, vol. 12, no. 4, pp. 404–415.
R Core Team R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria. 2021. https://www.R-project.org/.
Spangenberg, D.B., A study of strobilation in Aurelia aurita under controlled conditions, J. Exp. Zool., 1965a, vol. 160, no. 1, pp. 1–9.
Spangenberg, D.B., Cultivation of the life stages of Aurelia aurita under controlled conditions, J. Exp. Zool., 1965b, vol. 159, no. 3, pp. 303–318.
Spangenberg, D.B., Iodine induction of metamorphosis in Aurelia, J. Exp. Zool., 1967, vol. 165, no. 3, pp. 441–449.
Spangenberg, D.B., Thyroxine induced metamorphosis in Aurelia, J. Exp. Zool., 1971, vol. 178, no. 2, pp. 183–194.
Spangenberg, D.B., Rhopalium development in Aurelia aurita ephyrae, Hydrobiologia, 1991, vol. 216, pp. 45–49.
Straehler-Pohl, I., Widmer, C.L., and Morandini, A.C., Characterizations of juvenile stages of some semaeostome Scyphozoa (Cnidaria), with recognition of a new family (Phacellophoridae), Zootaxa, 2011, vol. 2741, no. 1, pp. 1–37.
Sukhoputova, A.V., Histological examination of the modes of asexual reproduction in Aurelia aurita (Scyphozoa), MS Thesis, Moscow: Moscow State Univ., 2013.
Sukhoputova, A.V. and Kraus, Y.A., Environmental factors inducing the transformation of polyp into medusae in Aurelia aurita (Scyphozoa), Russ. J. Dev. Biol., 2017a, vol. 48, no. 2, pp. 106–116.
Sukhoputova, A.V. and Kraus, Yu.A., Plasticity of the response of Aurelia aurita (Cnidaria, Scyphozoa) polyps to a signaling factor regulating their life cycle, Zool. Zh., 2017, vol. 96, no. 11, pp. 1309–1318.
Sukhoputova, A.V., Kraus, Y.A., Kirillova, A.O., and Markov, A.V., Differentiation of the oral-aboral axis and body parts during life cycle transitions in Scyphozoa, Biol. Bull. Rev., 2019, vol. 9, no. 5, pp. 412–431.
Technau, U., Genikhovich, G., and Kraus, J.E.M., Cnidaria, in Evolutionary Developmental Biology of Invertebrates, Springer, 2015, vol. 1, pp. 115–163.
Technau, U. and Steele, R.E., Evolutionary crossroads in developmental biology: Cnidaria, Development, 20, vol. 138, no. 8, pp. 1447–1458.
Uchida, V. and Nagao, Z., The metamorphosis of the scyphomedusa, Aurelia limbata (Brandt), Annot. Zool. Jpn., 1963, vol. 36, no. 2, pp. 83–91.
Vannucci, M., Double monster ephyrae of Aurelia aurita, Nature, 1957, vol. 179, no. 4554, pp. 326–327.
Wang, W., Regulation of metamorphosis and the evolution of life cycles: insights from the common moon jelly Aurelia aurita, Dissertation, Bosch, E.G.P.D.D.h.c.T., Khalturin, Z.G.P.D.K., Kiel: Universitatsbibliothek Kiel, 2017.
Yamamori, L., Okuizumi, K., Sato, C., Ikeda, S., and Toyohara, H., Comparison of the inducing effect of indole compounds on medusa formation in different classes of Medusozoa, Zool. Sci., 2017, vol. 34, no. 3, pp. 173–178.
Yuan, D., Nakanishi, N., Jacobs, D.K., and Hartenstein, V., Embryonic development and metamorphosis of the scyphozoan Aurelia, Dev. Genes Evol., 2008, vol. 218, pp. 525–539.
Zaika, V.E., The frequency of morphological anomalies in the scyphozoan Aurelia aurita (L.) in the Black Sea, Morsk. Ekol. Zh., 2005, vol. 4, no. 2, pp. 51–57.
Zimmermann, B., Robert, N.S.M., Technau, U., and Simakov, O., Ancient animal genome architecture reflects cell type identities, Nat. Ecol. Evol., 2019, vol. 3, no. 9, pp. 1289–1293.
ACKNOWLEDGMENTS
The study of samples using a scanning electron microscope was carried out in the Electron Microscopy Laboratory, Biological Faculty, Moscow State University. The authors express their gratitude to the reviewers for meaningful comments and recommendations that helped to improve this work significantly.
Funding
The work was carried out with the financial support of Education Center Sirius and the Russian Foundation for Basic Research (project no. 19-315-51015). Obtaining and maintenance of cultures of scyphopolypes was supported by the project of the Russian Foundation for Basic Research no. 19-04-01131a.
Author information
Authors and Affiliations
Contributions
P.S. Mostovshikova and I.A. Kosevich designed and developed the experiment, P.S. Mostovshikova conducted the main experimental study, D.M. Saidov conducted statistical data analysis, P.S. Mostovshchikova and I.A. Kosevich conducted a microscopic examination of the samples. P.S. Mostovshchikova and I.A. Kosevich participated in the preparation of the manuscript. All the authors participated in the discussion of results, preparation of illustrations, and editing of the final version of the manuscript. All the authors approved the final version.
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interests.
Statement on the welfare of animals. All applicable international, national, and/or institutional principles for the use of animals in experiments and conditions of animal care have been met.
Additional information
Translated by A. Ermakov
Rights and permissions
About this article
Cite this article
Mostovshchikova, P.S., Saidov, D.M. & Kosevich, I.A. Morphological Deviations in Ephyrae after Chemical Induction of Strobilation in Aurelia aurita (Scyphozoa, Cnidaria). Russ J Dev Biol 53, 82–98 (2022). https://doi.org/10.1134/S1062360422020084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360422020084