4-Methoxy-2,2′-bipyrrole-5-carbaldehyde, a biosynthetic intermediate of bipyrrole-containing natural products from the Streptomyces culture, arrests the strobilation of moon jellyfish Aurelia coerulea
- 1Program of Biotechnology, Graduate School of Integrated Sciences for Life, Hiroshima University, Higashi-Hiroshima, Hiroshima, Japan
- 2Hiroshima Research Center for Healthy Aging (HiHA), Hiroshima University, Higashi-Hiroshima, Hiroshima, Japan
- 3School of Applied Biological Science, Hiroshima University, Higashi-Hiroshima, Hiroshima, Japan
- 4Program of Food and AgriLife Science, Graduate School of Integrated Sciences for Life, Hiroshima University, Higashi-Hiroshima, Hiroshima, Japan
- 5Department of Food Science and Technology, Graduate School of Marine Science and Technology, Tokyo University of Marine Science and Technology, Tokyo, Japan
- 6Department of Molecular Biotechnology, Graduate School of Advanced Sciences of Matter, Hiroshima University, Higashi-Hiroshima, Hiroshima, Japan
- 7Department of Fermentation Sciences, Faculty of Applied Biosciences, Tokyo University of Agriculture, Tokyo, Japan
- 8Natural Science Center for Basic Research and Development, Hiroshima University, Higashi-Hiroshima, Hiroshima, Japan
Streptomyces spp. are well-known producers of secondary metabolites with diverse biological activities. We screened the substances that regulate polyp-to-jellyfish transition, called strobilation, of the moon jellyfish (Aurelia coerulea) from the Streptomyces culture library. Among the culture extracts of the strains tested, Streptomyces albus HUT6047 inhibited the strobilation of A. coerulea. The active component in strain HUT6047 was purified. Based on structure elucidation, this component was identified as 4-methoxy-2,2′-bipyrrole-5-carbaldehyde (MBC), a possible common biosynthetic intermediate of pyrrole-containing natural products including prodigiosins and tambjamines. Synthetic MBC arrested strobilation without inducing cytotoxicity and generated abnormal tentacle-like structures in a dose-dependent manner. Synthetic MBC also exhibited a minimum activity of 6.3 µM. To our knowledge, this study provides the first example of a biological activity of MBC.
1 Introduction
The filamentous bacterial genus Streptomyces is well characterized as the most prolific producer of secondary metabolites with various significant biological activities, including the production of antibiotics, anticancer agents, antifungal agents, immunosuppressants, and herbicides (Ōmura, 2011). Exhaustive screening of the Streptomyces culture library provides bioactive molecules that control the physiological functions of certain organisms, including plants, animals, and humans (Chen et al., 2021). For example, microbial bioherbicides were screened from 102 Streptomyces strains, of which strain-329 produced two glutarimide derivatives (Bo et al., 2019). Thus, unique biocontrol agents can be discovered through exhaustive screening using a Streptomyces culture library.
The moon jellyfish, Aurelia coerulea (Dawson and Jacobs, 2001; Scorrano et al., 2016), is a marine animal that is widely distributed along coastal oceans worldwide. Jellyfish blooms of A. coerulea and other species often negatively affect marine fisheries and aquaculture (Brotz et al., 2012; Bosch-Belmar et al., 2021). Hence, controlling jellyfish blooms is important for coastal human activities. Owing to their abundance and water retention capacity, jellyfish, including A. coerulea, are a valuable source of collagen, a biomedical material utilized by humans (Hoyer et al., 2014; Sumiyoshi et al., 2021). Jellyfish are thus worth studying because of their basic and applicable properties as bioresources.
The life cycle of A. coerulea consists of two reproductive stages, the asexual polyp stage and the sexual medusa (jellyfish) stage (Arai, 1997). The transition from polyps to jellyfish is called strobilation (Figure 1A). Strobilation is induced by lowering a water temperature (Kroiher et al., 2000; Kuniyoshi et al., 2012; Tsujita et al., 2015). After the initiation of strobilation, the polyp becomes a strobila with several transverse segments on the body column. Strobila segments are sequentially generated in an oral-to-aboral direction (segmentation phase). Thereafter, each segment is metamorphosed into one jellyfish (morphogenesis phase). Finally, several juvenile jellyfish, termed ephyrae, detach from the strobilae. Strobilation can be induced by the exogeneous addition of indomethacin (IM) (Kuniyoshi et al., 2012) or indole derivatives (Fuchs et al., 2014; Helm and Dunn, 2017). Artificial induction of strobilation by chemicals, including IM, is now employed in aquarium displays and collagen biomass production. Notably, the inhibition of strobilation could result in the control of jellyfish blooms, leading to the maintenance of sustainable coastal human activities. However, effective strobilation inhibitors have not been developed.

Figure 1 Schematic view of strobilation and the effect of MBC (see Figure 2) on strobilation in Aurelia coerulea. (A) Schematic view of strobilation. (B–I) Morphological images of Aurelia strobilation. (B–E) Control experiments in the Aurelia bioassay described in the text. Images (B–E) were taken at 1 h, 1 day, 2 days, and 6 days after the start of bioassay, respectively. (B) Earlier segmentation-phase strobila with three segments. (C) Later segmentation-phase strobila. (D) Earlier morphogenesis-phase strobila. (E) Later morphogenesis-phase strobila. An ephyra (white arrow) has just been detached from the strobila. (F, G) Abnormal strobila caused by purified natural MBC. Images (F, G) were taken at 3 days and 6 days after administration, respectively. Arrowheads in (F) indicate abnormal tentacle-like structures. (H, I) Abnormal strobila caused by synthetic MBC (12.5 µM). Images (H, I) were taken at 3 days and 6 days after administration, respectively. Scale bars, 1 mm.
Here, we performed the Streptomyces culture screening to identify the inhibitor(s) involved in the strobilation of A. coerulea. Among these culture extracts, the Streptomyces albus strain HUT6047 arrested strobilation without inducing cytotoxicity and generated abnormal tentacle-like structures. The active component was purified by chromatographies. Thereafter, through structural elucidation, the component was identified as 4-methoxy-2,2′-bipyrrole-5-carbaldehyde (MBC) (Figure 2), the results of which were described in this paper.

Figure 2 Structure of 4-methoxy-2,2′-bipyrrole-5-carbaldehyde (MBC) isolated from Streptomyces albus HUT6047. Structures of undecylprodigiosin, prodigiosin, and tambjamine BE-18591, derivatives from the common biosynthetic intermediate MBC, were also displayed.
2 Materials and methods
2.1 Bacterial strain and preparation of the culture library
Various Streptomyces strains, including strain HUT6047, were cultured in YM medium (0.4% yeast extract, 1.0% malt extract, and 0.4% D-glucose, pH 7.3) at 28°C with 120 rpm (revolutions per minute) for 3 days, according to our standard protocol (Arakawa et al., 2005). The Streptomyces culture library was prepared as described below. Briefly, 100 ml of culture broth was extracted twice with EtOAc, and then the combined organic phase was dried (Na2SO4), filtered, and concentrated in vacuo. The residues were dissolved in MeOH (1 ml), and their aliquots (10 µl each) were collected for the bioassay.
2.2 Spectroscopic instruments
The compounds (active component against A. coerulea polyps and synthetic MBC) were analyzed by electrospray ionization-mass spectrometry (ESI-MS) and nuclear magnetic resonance (NMR). ESI-MS was performed using an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). NMR spectra were recorded on a JEOL ECA-600 spectrometer equipped with a field gradient accessory (JEOL, Ltd., Tokyo, Japan). The NMR chemical shifts were recorded as δ values in ppm. The coupling constants in 1H-NMR were shown as J value in Hz. Dimethylsulfoxide-d6 (99.8 atom %; Kanto Chemical, Co., Inc., Tokyo, Japan) was used as the solvent for 1H- and 13C-NMR, while tetramethylsilane (δH = 0) was used as the internal standard for 1H-NMR and 13C-NMR.
2.3 Metabolites in strain HUT6047
Metabolite production in strain HUT6047 was analyzed using high-performance liquid chromatography (HPLC) and thin-layer chromatography (TLC). The EtOAc extract from 100-ml culture of strain HUT6047 (average 39 mg extracts from the 430-mg dry cell per 100-ml culture) was dissolved in MeOH (1 ml), and then an aliquot (10 µl) was passed through a COSMOSIL Cholester column (4.6 × 250 mm, Nacalai Tesque, Kyoto, Japan) and eluted with 40% aqueous acetonitrile containing 0.1% TFA at a flow rate of 1.0 ml/min. The eluate was monitored using a JASCO MD-2010 multi-wavelength photodiode array detector (JASCO Corporation, Tokyo, Japan), and active component was detected at 360 nm (Figure 3). Purified natural MBC (see section 2.4) and synthetic MBC (see section 2.5) were also analyzed in the same manipulation. TLC analysis of the EtOAc extract of strain 6047 prepared as above-mentioned was performed using a mixture of CHCl3 and MeOH (15:1, v/v) and exposed to iodine vapor.

Figure 3 Analysis of MBC in strain HUT6047. (A) TLC of (i) the EtOAc extract of strain HUT6047, (ii) purified natural MBC, and (iii) synthetic MBC. TLC was developed with CHCl3–MeOH (15:1, v/v). Spots were visualized by UV irradiation at 254 nm (left panel) or iodine staining (right panel). (B) HPLC chromatogram of (i) the EtOAc extract of strain HUT6047, (ii) purified natural MBC, and (iii) synthetic MBC. Elution profiles were monitored by UV absorbance at 360 nm (left panels) and 250 nm (right panels). (C) 1H-NMR spectra of (i) natural MBC and (ii) synthetic MBC.
2.4 Isolation of MBC from Streptomyces albus HUT6047
The culture supernatant of strain HUT6047 (26 L) was extracted twice with an equal volume of EtOAc. The combined organic phases were dried (Na2SO4), filtered, and concentrated in vacuo. The crude extract was purified using Sephadex LH-20 (GE Healthcare, Chicago, IL, USA) gel filtration chromatography with MeOH. All fractions (1 ml each; total 50 fractions) eluted with MeOH were subjected to a bioassay using A. coerulea polyps (detailed protocol is described in section 2.5). The fractions containing the active component(s) were combined, and the resulting residue was further purified using silica gel chromatography with two different solvent systems, CHCl3–MeOH = 50:1–10:1 (v/v) and hexane–EtOAc = 2:1 (v/v). All the fractions (3 ml each; total 50 fractions) were dried in vacuo, and redissolved in MeOH (1 ml) and also subjected to a bioassay described as above to obtain an active component, MBC (5.7 mg from 26-L culture broth). 1H-NMR (DMSO-d6) δ = 3.84 (3H, s), 6.12 (1H, d, J = 1.5 Hz), 6.27 (1H, s), 6.75 (1H, brs), 6.91 (1H, brs), 9.30 (1H, s), 11.24 (1H, brs), 11.42 (1H, brs). 13C-NMR (DMSO-d6) δ = 57.8 (q; OCH3), 90.9 (d), 108.2 (d), 109.3 (d), 117.3 (s), 120.4 (d), 123.4 (s), 133.2 (s), 158.6 (s), 171.6 (d).
HRMS (positive ESI): m/z calculated for C10H10N2O2Na: 213.0640 [M+Na]+; observed: 213.0631.
2.5 Bioassay for the strobilation-inhibiting activity in A. coerulea
A clonal polyp strain KH1A, which was established from A. coerulea jellyfish caught in the Seto Inland Sea, Japan (Tsujita et al., 2015), was used for the bioassay. Polyps were reared in filtered seawater (FSW) at 22–25°C.
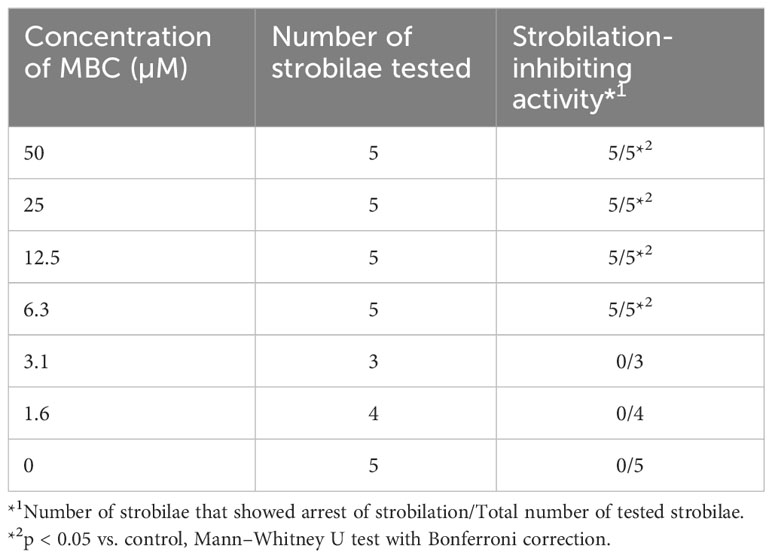
For screening and purification, strobilation was induced by lowering the culture temperature to 10°C from 22–25°C [cold shock (CS)]. Strobilae at the earlier segmentation phase with one to five segments were collected 46–60 days after the temperature drop and kept in a 14-cm dish at 10°C until transferred to 24-well microtiter plates. Three strobilae were placed in individual wells of a 24-well microtiter plate and then cultured in 1 ml of FSW containing 10 µl of the aliquots of either the culture extracts or fractions separated by chromatography at 22°C. Control strobilae were cultured in FSW at 22°C. Their strobilation was monitored over a 7-day period. For the dose–response analysis, strobilation was induced via incubation with 10 µM IM at 22°C. After a 7-day incubation period, segmentation-phase strobilae with one to three segments were collected, and then rinsed with 50 ml of FSW to remove the IM. Three to five strobilae were incubated in individual 9-cm dishes in 40 ml of FSW containing 1.6, 3.1, 6.3, 12.5, 25, or 50 µM MBC at 22°C. Control strobilae were cultured in FSW containing 0.025% DMSO at 22°C. Strobilation was monitored over a 7-day period.
2.6 DNA sequencing and assembly
Genomic DNA of strain HUT6047 was subjected to paired-end sequencing using an Illumina NextSeq sequencing system (San Diego, CA, USA) according to the manufacturer’s protocol. De novo assembly of the raw genome sequencing data was performed using SPAdes 3.13.0 (Bankevich et al., 2012), and Illumina read data were deposited as Bioproject: PRJDB15399, Biosample: SAMD00585629. The MBC biosynthetic gene cluster was identified using antiSMASH ver. 6.0.1 (Blin et al., 2021), and its sequence was also deposited (GenBank Accession number: LC760459).
3 Results and discussion
3.1 Extensive screening for the strobilation of A. coerulea using the Streptomyces culture library
Previously, we revealed that IM induces strobilation in a dose-dependent manner through a chemical library screening using the 456 substances provided by RIKEN Natural Products Depository (RIKEN NPDepo, RIKEN Advanced Science Institute, Wako, Japan) (Kuniyoshi et al., 2012). In addition, we found that the lysosomal acidification inhibitors, chloroquine and bafilomycin A1, partially inhibited strobilation (Tsujita et al., 2017). Owing to these findings, we proceeded to further investigate the unique chemical substances with notable biological activity against strobilation in A. coerulea. Recently, we independently constructed a Streptomyces culture library and performed a pilot screening to explore various biological activities, including antimicrobial, antitumor, and other activities, of these crude extracts.
Strobilation is induced by CS at 10°C, and CS-induced strobilae metamorphose into ephyrae even at 25°C (Tsujita et al., 2015). Similarly, IM-induced strobilae metamorphose into ephyrae in the absence of IM (Kuniyoshi et al., 2012). Thus, once initiated, strobilation is autonomously completed independent of temperature and chemicals. In this study, we aimed to identify substances that inhibit this autonomous process of strobilation.
To determine the effect of the Streptomyces culture library on the autonomous process of strobilation, the culture extracts were administered to earlier segmentation-phase strobilae. In the control experiments, strobilae with three segments (Figure 1B) became fully segmented strobilae (Figure 1C) after 1 day and then morphogenesis-phase strobilae (Figure 1D) after 2 days. Finally, the ephyrae were detached after 6 days (Figure 1E). Among the 38 culture extracts tested (Table S1), three strains displayed remarkable inhibition of autonomous process of strobilation. Hereafter, we focused on the strain HUT6047, which exhibited the highest strobilation-inhibiting activity among the three strains.
3.2 Isolation and structural elucidation of the active component from strain HUT6047
The large-scale fermentation of strain HUT6047 was performed and its metabolites were purified using Sephadex LH20 and silica gel chromatography homogeneity (Figure 3A). All fractions were screened through strobilation-inhibiting activity using A. coerulea polyps. The active compound appeared as a gray green spot on TLC after iodine staining at Rf = 0.55 [CHCl3–MeOH = 10:1 (v/v)] (Figure 3A), and displayed a distinct molecular ion peak at m/z 213.0631 [M+Na]+ (calcd. for C10H10N2O2Na, m/z 213.0640) on high-resolution ESI-MS. In the 13C-NMR spectrum of this compound, one methyl, five methine, and four quaternary carbons were identified. All spectral data including 1H-NMR spectrum agreed well with the reported data for MBC (Figure 2) (Dairi et al., 2006; Rastogi et al., 2013). MBC is a well-characterized biosynthetic intermediate of bipyrrole-containing natural products, including prodigiosins and tambjamines (Figure S1) (Cerdeño et al., 2001; Stanley et al., 2006; Burke et al., 2007; Hu et al., 2016; Grenade et al., 2023).
To confirm its structure and prepare a considerable amount for the bioassay, MBC was synthesized from the commercially available 4-methoxy-3-pyrolin-2-one in two steps (Supplementary Materials and Figure S2) according to previous reports (Dairi et al., 2006; Rastogi et al., 2013). HPLC chromatogram of both purified and synthetic MBC showed a distinct peak at 5.8 min, which was also detected in culture extract of strain HUT6047 (Figure 3B). The spectral data of natural MBC were in good agreement with those of synthetic MBC (1H-NMR in Figure 3C and ESI-MS in Figure S3), supporting its chemical structure (Figure 2).
The genetic feature of strain HUT6047 was assessed using next-generation sequencing. A biosynthetic gene cluster for MBC (mbc) was identified in the genome of strain HUT6047, and it showed a significant similarity to the biosynthetic gene cluster (tab cluster) for tambjamine BF-18591 (Figure 2) (Grenade et al., 2023) as shown in Table S2 and Figure S4, supporting the production of MBC in this strain.
3.3 MBC specifically affects strobilation in A. coerulea
The strobilation-inhibiting activity of the purified natural MBC was examined. As described above, CS-induced strobilae autonomously metamorphose into ephyrae at 22°C. When purified natural MBC was administered to strobilae with one to five segments, the autonomous process of strobilation was arrested (Figures 1F, G). In contrast, the strobilation proceeded until the end of 6 days in the control experiment (Figures 1B–E). Remarkably, abnormal tentacle-like structures were observed around these constrictions (Figure 1F, arrowheads). These abnormal strobilae did not undergo morphogenesis stage during the 7-day observation period.
To confirm the strobilation-inhibiting activity of MBC, the synthetic MBC was subjected to an Aurelia bioassay. As shown in Figures 1H, I, the synthetic MBC reproduced the same activity as purified natural MBC: arrest of the autonomous process of strobilation and generation of abnormal tentacle-like structures. Hence, MBC was confirmed as the active component produced by strain HUT6047. Dose–response analysis using synthetic MBC revealed that the minimum activity was 6.3 µM concentration (Table 1). No apparent death was observed in the bioassay. Despite the abnormal morphology, the MBC-treated strobilae remained alive for more than 1 month, even at a concentration of 50 µM (Table 1), suggesting that MBC had no remarkable cytotoxic activity against A. coerulea. In our independent analysis, MBC also showed no remarkable cytotoxicity against brine shrimp, a model marine organism (unpublished results).
MBC halts the autonomous process of strobilation without causing cytotoxicity, and generates abnormal tentacle-like structures. Abnormal tentacle-like structures appeared to protrude from each constriction between strobilae segments (Figure 1F, arrowheads). However, the developmental origin of the abnormal tentacle-like structures and their similarity with the normal tentacles of polyps remain unknown. Detailed histological observations are required to clarify these issues.
In a comparative assay, the culture extract of Streptomyces coelicolor M145, a notable producer of undecylprodigiosin, one of a prodigiosin derivative, had no effect on A. coerulea, indicating that the strobilation-inhibiting activity was caused by the bipyrrole structure and not by the accessory structures including a hydrocarbon chain and an additional pyrrole ring in undecylprodigiosin.
4 Conclusion
Discovery of the effective strobilation inhibitors is worth studying for the control of jellyfish blooms that could contribute to the maintenance of sustainable coastal human activities. We thus performed the Streptomyces culture screening to obtain the inhibitor of strobilation of A. coerulea. Among the 38 culture extracts, three strains showed the inhibitory activity against A. coerulea. Remarkably, the culture extract of Streptomyces albus strain HUT6047 significantly arrested strobilation without inducing cytotoxicity and generated abnormal tentacle-like structures. The active component in this strain was determined to be MBC.
MBC, a biosynthetic intermediate of bipyrrole-containing natural products, specifically inhibits the strobilation of A. coerulea with a minimum activity value of 6.3 µM, and induces no remarkable cytotoxicity against A. coerulea. To our knowledge, this study provides the first example of a biological activity of MBC. At this stage, the mode of action of MBC against A. coerulea strobilation is unclear, which will be clarified through extensive biochemical analysis.
Streptomyces species are well-known sources of natural bioactive products. Together with these bioactive products, they accumulate various biosynthetic building blocks, including bipyrrole, 3-amino-5-hydroxybenzoic acid (Floss et al., 2011), non-proteinogenic amino acids (Luo et al., 2016), and deoxysugars (Thibodeaux et al., 2008), to some extent; however, their notable biological activities have not yet been elucidated. An advantage of the microbial culture library is that we can investigate the biological activity of not only the final products but also their biosynthetic precursors/shunt products accumulated in the culture extracts. Our microbial sample collection of numerous actinomycete strains from soil and marine environments in Japan and tropical areas, including Indonesia and the Philippines, will enable the discovery of unique natural products with extensive biological activities, which is in progress in our group.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
HKo, HKu, and KA designed the experiments. YM, TH, KF, AH, MS, KI, YH, and HKu performed the experiments. SM, TS, KI, HKo, HKu, and KA analyzed the data, and YM, HKo, HKu, and KA wrote the manuscript with input from all of the authors. All the authors approved the final version of the manuscript.
Funding
This work was supported by Grants-in-Aid for Scientific Research (B) (22H02274 to KA), Scientific Research (C) (20K05851 to HKu), and Fund for the Promotion of Joint International Research (Fostering Joint International Research B) (19KK0149 to KA, HKu, and HKo) from JSPS. YM was supported by a JSPS Research Fellowship for Young Scientists (21J14499). KA was supported by the Program for Fostering Globally Talented Researchers, Japan Society for the Promotion of Science (JSPS, Grant number: JPMXS05S2900002).
Acknowledgments
We are grateful to Mrs. Tomoko Amimoto [Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University] for measurement of the high-resolution mass spectra. We also thank NODAI Genome Research Center, Tokyo University of Agriculture for performing the next-generation sequencing. We would like to thank Mr. Ryuji Kawakita (Technical Center, Hiroshima University) for maintenance of the culture library. We would also like to thank Editage (https://www.editage.com/) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could contain a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1198136/full#supplementary-material
References
Arai M. N. (1997). A functional biology of Scyphozoa (London: Chapman & Hall), 137–171. doi: 10.1007/978-94-009-1497-1
Arakawa K., Sugino F., Kodama K., Ishii T., Kinashi H. (2005). Cyclization mechanism for the synthesis of macrocyclic antibiotic lankacidin in Streptomyces rochei. Chem. Biol. 12, 249–256. doi: 10.1016/j.chembiol.2005.01.009
Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477. doi: 10.1089/cmb.2012.0021
Blin K., Shaw S., Kloosterman A. M., Charlop-Powers Z., Van Wezel G. P., Medema M. H., et al. (2021). AntiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res. 49, W29–W35. doi: 10.1093/nar/gkab335
Bo A. B., Kim J. D., Kim Y. S., Sin H. T., Kim H. J., Khaitov B., et al. (2019). Isolation, identification and characterization of Streptomyces metabolites as a potential bioherbicide. PloS One 14, e0222933. doi: 10.1371/journal.pone.0222933
Bosch-Belmar M., Milisenda G., Basso L., Doyle T. K., Leone A., Piraino S. (2021). Jellyfish impacts on marine aquaculture and fisheries. Rev. Fish. Sci. Aquac. 29, 242–259. doi: 10.1080/23308249.2020.1806201
Brotz L., Cheung W. W. L., Kleisner K., Pakhomov E., Pauly D. (2012). Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia 690, 3–20. doi: 10.1007/s10750-012-1039-7
Burke C., Thomas T., Egan S., Kjelleberg S. (2007). The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata. Environ. Microbiol. 9, 814–818. doi: 10.1111/j.1462-2920.2006.01177.x
Cerdeño A. M., Bibb M. J., Challis G. L. (2001). Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3(2): new mechanisms for chain initiation and termination in modular multienzymes. Chem. Biol. 8, 817–829. doi: 10.1016/s1074-5521(01)00054-0
Chen J., Xu L., Zhou Y., Han B. (2021). Natural products from actinomycetes associated with marine organisms. Mar. Drugs 19, 629. doi: 10.3390/md19110629
Dairi K., Tripathy S., Attardo G., Lavallée J. F. (2006). Two-step synthesis of the bipyrrole precursor of prodigiosins. Tetrahedron Lett. 47, 2605–2606. doi: 10.1016/j.tetlet.2006.02.035
Dawson M. N., Jacobs D. K. (2001). Molecular evidence for cryptic species of Aurelia aurita (Cnidaria, Scyphozoa). Biol. Bull. 200, 92–96. doi: 10.2307/1543089
Floss H. G., Yu T. W., Arakawa K. (2011). The biosynthesis of 3-amino-5-hydroxybenzoic acid (AHBA), the precursor of mC7N units in ansamycin and mitomycin antibiotics: a review. J. Antibiot. (Tokyo). 64, 35–44. doi: 10.1038/ja.2010.139
Fuchs B., Wang W., Graspeuntner S., Li Y., Insua S., Herbst E. M., et al. (2014). Regulation of polyp-to-Jellyfish transition in Aurelia aurita. Curr. Biol. 24, 263–273. doi: 10.1016/j.cub.2013.12.003
Grenade N. L., Chiriac D. S., Pasternak A. R. O., Babulic J. L., Rowland B. E., Howe G. W., et al. (2023). Discovery of a tambjamine gene cluster in Streptomyces suggests convergent evolution in bipyrrole natural product biosynthesis. ACS Chem. Biol. 18, 223–229. doi: 10.1021/acschembio.2c00685
Helm R. R., Dunn C. W. (2017). Indoles induce metamorphosis in a broad diversity of jellyfish, but not in a crown jelly (Coronatae). PloS One 12, e0188601. doi: 10.1371/journal.pone.0188601
Hoyer B., Bernhardt A., Lode A., Heinemann S., Sewing J., Klinger M., et al. (2014). Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 10, 883–892. doi: 10.1016/j.actbio.2013.10.022
Hu D. X., Withall D. M., Challis G. L., Thomson R. J. (2016). Structure, chemical synthesis, and biosynthesis of prodiginine natural products. Chem. Rev. 116, 7818–7853. doi: 10.1021/acs.chemrev.6b00024
Kroiher M., Siefker B., Berking S. (2000). Induction of segmentation in polyps of Aurelia aurita (Scyphozoa, Cnidaria) into medusae and formation of mirrorimage medusa anlagen. Int. J. Dev. Biol. 44, 485–490. doi: 10.1387/ijdb.11032183
Kuniyoshi H., Okumura I., Kuroda R., Tsujita N., Arakawa K., Shoji J., et al. (2012). Indomethacin induction of metamorphosis from the asexual stage to sexual stage in the moon jellyfish, Aurelia aurita. Biosci. Biotechnol. Biochem. 76, 1397–1400. doi: 10.1271/bbb.120076
Luo X., Zambaldo C., Liu T., Zhang Y., Xuan W., Wang C., et al. (2016). Recombinant thiopeptides containing noncanonical amino acids. Proc. Natl. Acad. Sci. U.S.A. 113, 3615–3620. doi: 10.1073/pnas.1602733113
Ōmura S. (2011). Microbial metabolites: 45 years of wandering, wondering and discovering. Tetrahedron 67, 6420–6459. doi: 10.1016/j.tet.2011.03.117
Rastogi S., Marchal E., Uddin I., Groves B., Colpitts J., McFarland S. A., et al. (2013). Synthetic prodigiosenes and the influence of C-ring substitution on DNA cleavage, transmembrane chloride transport and basicity. Org. Biomol. Chem. 11, 3834–3845. doi: 10.1039/c3ob40477c
Scorrano S., Aglieri G., Boero F., Dawson M. N., Piraino S. (2016). Unmasking Aurelia species in the Mediterranean Sea: an integrative morphometric and molecular approach. Zool. J. Linn. Soc 180, 243–267. doi: 10.1111/zoj.12494
Stanley A. E., Walton L. J., Zerikly M. K., Corre C., Challis G. L. (2006). Elucidation of the Streptomyces coelicolor pathway to 4-methoxy-2,2’-bipyrrole- 5-carboxaldehyde, an intermediate in prodiginine biosynthesis. Chem. Commun. 14, 3981–3983. doi: 10.1039/b609556a
Sumiyoshi H., Okamura Y., Kawaguchi A. T., Kubota T., Endo H., Yanagawa T., et al. (2021). External administration of moon jellyfish collagen solution accelerates physiological wound healing and improves delayed wound closure in diabetic model mice. Regener. Ther. 18, 223–230. doi: 10.1016/j.reth.2021.06.008
Thibodeaux C. J., Melançon C. E. III, Liu H. W. (2008). Natural-product sugar biosynthesis and enzymatic glycodiversification. Angew. Chem. Int. Ed. Engl. 47, 9814–9859. doi: 10.1002/anie.200801204
Tsujita N., Kuroda R., Okumura I., Yoshioka S., Nakatani M., Koyama H., et al. (2015). Characterization of clonal polyp strains established from Aurelia sp. inhabiting the Seto Inland Sea of Japan. Biosphere Sci. 54, 45–54. in Japanese.
Tsujita N., Kuwahara H., Koyama H., Yanaka N., Arakawa K., Kuniyoshi H. (2017). Molecular characterization of aspartylglucosaminidase, a lysosomal hydrolase upregulated during strobilation in the moon jellyfish, Aurelia aurita. Biosci. Biotechnol. Biochem. 81, 938–950. doi: 10.1080/09168451.2017.1285686
Keywords: Aurelia coerulea, Streptomyces, screening, strobilation, 4-methoxy-2,2′-bipyrrole-5-carbaldehyde
Citation: Misaki Y, Hirashima T, Fujii K, Hirata A, Hoshino Y, Sumiyoshi M, Masaki S, Suzuki T, Inada K, Koyama H, Kuniyoshi H and Arakawa K (2023) 4-Methoxy-2,2′-bipyrrole-5-carbaldehyde, a biosynthetic intermediate of bipyrrole-containing natural products from the Streptomyces culture, arrests the strobilation of moon jellyfish Aurelia coerulea. Front. Mar. Sci. 10:1198136. doi: 10.3389/fmars.2023.1198136
Received: 31 March 2023; Accepted: 02 August 2023;
Published: 24 August 2023.
Edited by:
Bin Wu, Zhejiang University, ChinaReviewed by:
Bingnan Han, Zhejiang Sci-Tech University, ChinaYueying Li, University of Oklahoma, United States
Yantao Wang, Chinese Academy of Sciences (CAS), China
Copyright © 2023 Misaki, Hirashima, Fujii, Hirata, Hoshino, Sumiyoshi, Masaki, Suzuki, Inada, Koyama, Kuniyoshi and Arakawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenji Arakawa, karakawa@hiroshima-u.ac.jp; Hisato Kuniyoshi, hkuni@hiroshima-u.ac.jp
 Yuya Misaki
Yuya Misaki Tomomi Hirashima3
Tomomi Hirashima3  Yutaro Hoshino
Yutaro Hoshino Toshihiro Suzuki
Toshihiro Suzuki Kuninobu Inada
Kuninobu Inada Hisato Kuniyoshi
Hisato Kuniyoshi Kenji Arakawa
Kenji Arakawa