- 1Department of Basic Sciences Research, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
- 2Department of Cancer Registry and Clinical Data Management, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
- 3Department of Endocrinology, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
- 4Department of Clinical Research, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
- 5Department of Radiology, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan
Background: Diabetes and cancer are the leading causes of mortality all over the world. Infectious diseases are more common and/or life-threatening in patients with diabetes. Cancer patients with diabetes are individuals that are more susceptible to the current COVID-19 pandemic. We investigated the clinical features of survivor and non-survivor COVID-19-infected cancer patients with diabetes.
Patients and Methods: We did a retrospective study of 43 diabetic cancer patients with PCR-confirmed COVID-19 infection from Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore, Pakistan between March 03, 2020, and May 18, 2021. These patients either were discharged from the hospital or had died by Jun 16, 2021. Clinicopathological and radiological features were compared between survivors and non-survivors by fisher’s exact test and chi-square test.
Results: Forty-three diabetic cancer patients with SARS-CoV-2 infection were enrolled and the majority were males 26 (60.5%). The overall mean age was 61.67 ± 11.80. 39 (90.7%) had solid tumors and 3 (7.0%) had hematological malignancies. Fever (74.4%) and dyspnea (58.1%) were the most common symptoms. Complications were reported in 36 (83.7%) patients; during the course of the disease. Additionally, all the deceased patients (n=15) had acquired the complications. 11 (25.6%) patients were admitted to an intensive care unit (ICU). Furthermore, 29 (67.4%) out of 43 patients showed abnormal features in the radiological findings. We found significantly elevated levels of C-reactive protein (P=0.005), serum lactate (P=0.01), albumin (P=0.02), alkaline phosphate (P=0.03), and neutrophil count (P=0.04) in the non-survivors as compared to the survivors.
Conclusion: Cancer patients with diabetes are a vulnerable population in the current pandemic. Identifying how diabetes in cancer patients affects the severity of SARS-CoV-2 infection is crucial for the clinical management of these patients. Rigorous scrutiny of clinicopathological features of COVID-19 infected cancer patients with diabetes especially values of C-reactive protein, lactate, albumin, alkaline phosphate, neutrophils, and regular monitoring of blood glucose levels may play a critical role in the outcome of the disease.
Introduction
In 2019, Wuhan City, China underwent an outbreak of respiratory disease caused by a novel coronavirus SARS-Cov-2 (1). The disease was consequently named COVID-19 (2, 3). The whole world including Pakistan experienced COVID-19 outbreaks with a devastating force (4, 5). It has been observed that the severity and fatality of COVID-19 are enhanced in the elderly or in patients with underlying comorbidities, specifically cancer, diabetes, cardiovascular diseases, lung and renal diseases (6–9).
It is estimated that Pakistan had 178,388 new cancer cases in the year 2020 (10). Shaukat Khanum Memorial Cancer Hospital and Research Centre (SKMCH & RC) is a state-of-the-art charitable cancer hospital in Lahore, Pakistan (4, 11). Several studies demonstrated that cancer patients infected with COVID-19 had poorer prognoses and outcomes (12–14). We published the first comprehensive data of COVID-19 infected cancer patients from Pakistan and showed that these patients are a high-risk population with an increased mortality rate (15).
Diabetes and cancer are the leading causes of mortality all over the world (16, 17). The susceptibility of diabetic patients to contract the infection is still obscure, but it is definite that once they are infected, the patients are at high risk for severe disease (16). Moreover, it has also been established that the overall survival of diabetic patients, who develop cancer, is worse as compared to non-diabetic patients (17).
We investigated the data from diabetic cancer patients with SARS-CoV-2 infection who were admitted at SKMCH&RC. We analyzed the hematological, pathological, and radiological parameters in these patients. Here, we report the clinical characteristics of survivors and non-survivors of COVID-19-infected diabetic cancer patients in Pakistan, in an attempt to identify the risk factors associated with severe disease in this group of patients.
Methods
Study design and participants
This is a retrospective cohort study. After the COVID-19 outbreak, SKMCH & RC initiated the diagnosis and treatment of SARS-CoV-2 infected cancer patients based on the guidelines provided by WHO (18). All patients (aged ≥ 18 years) included in the current study had a history of cancer and diabetes. The patients had a diagnosis of either solid or hematological malignancies along with PCR confirmation of SARS-CoV-2 infection. We investigated the clinical features of survivors and non-survivors of COVID-19 infected diabetic cancer patients from Pakistan. The study cutoff date was May 18, 2021. The institutional review board (IRB) of SKMCH & RC approved this study (IRB-EX-20-04-20-01). IRB permitted the waiver of the written informed consent from participants.
Data collection
Demographic data, clinicopathological and radiological characteristics were acquired from the medical records system of SKMCH & RC. Data about gender, age, comorbidities, cancer history, vital signs, body mass index and, symptoms and pathology lab tests were all obtained at the time of diagnosis of COVID-19 in cancer patients. According to the TNM staging system, cancers were defined as stages I, II, III, and IV. We also collected the data about therapies provided to diabetic cancer patients with COVID-19, complications, and outcomes during admission to the hospital.
Statistical analysis
We hypothesized that differences exist in demographic, clinical, and laboratory characteristics, treatments, and cancer history between survivors and non-survivors of COVID-19-infected diabetic cancer patients. Quantitative variables were presented as medians/mean (range: minimum-maximum/standard deviation), and qualitative variables were presented by frequencies and percentages. The Mann-Whitney U test, Fisher’s exact test, and Chi-square test were applied to analyze the differences between groups according to the type of data. Kaplan-Meier analysis was used to check the overall survival. The differences between groups were considered to be significant when the P-value was less than 0.05.
Results
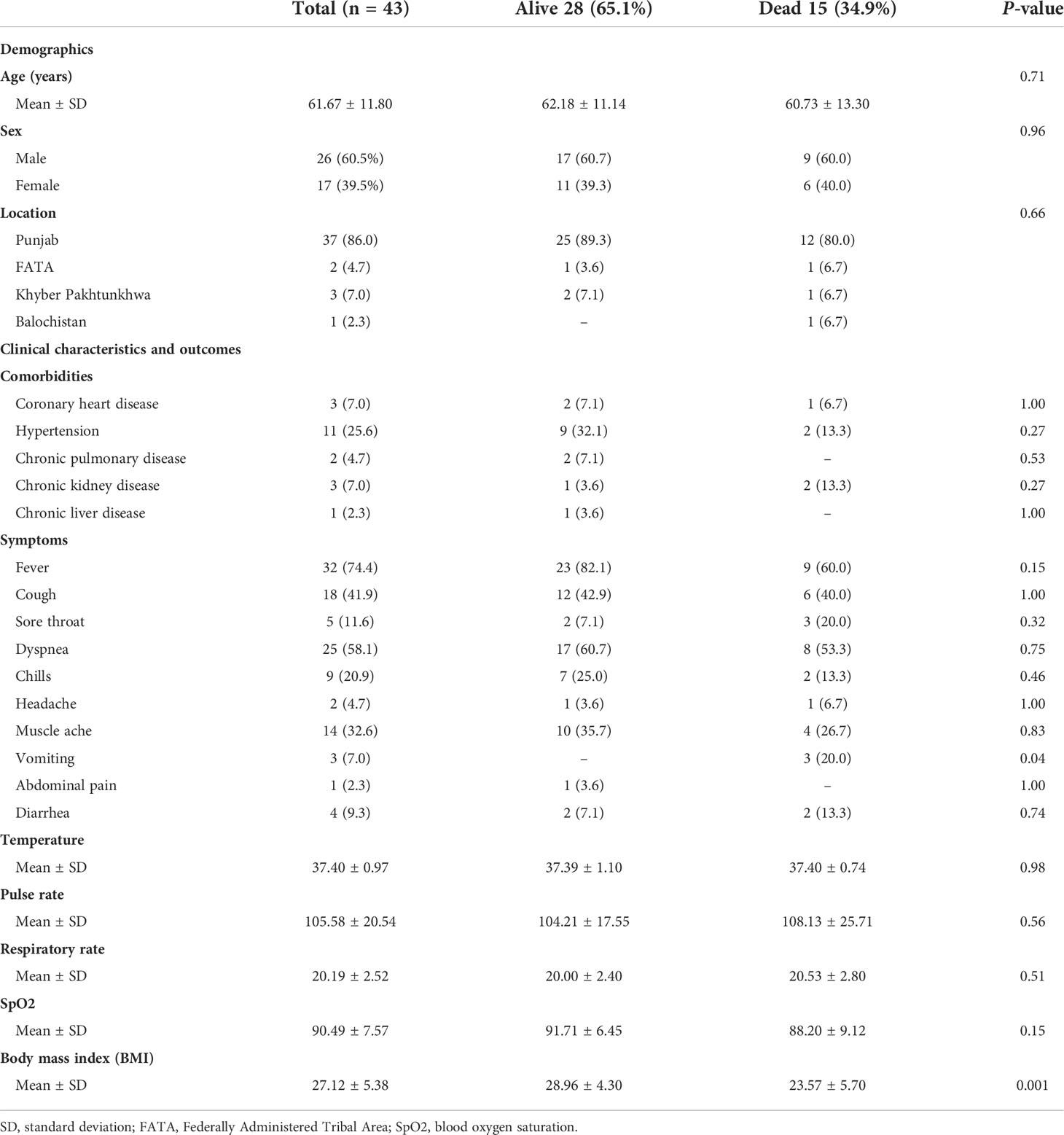
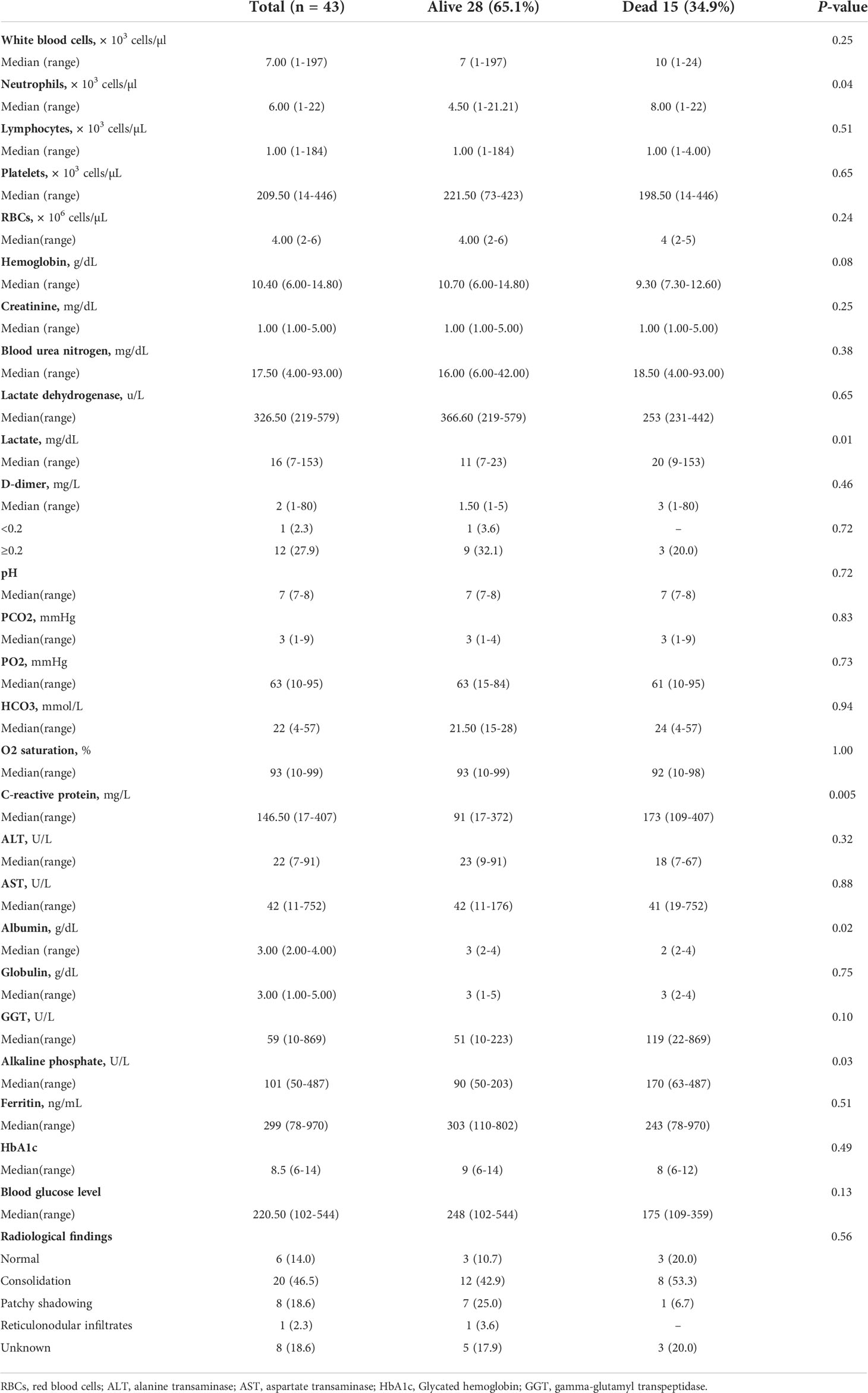
We retrospectively enrolled 43 cancer patients with diabetes and PCR-confirmed COVID-19 infection admitted to SKMCH & RC, Lahore, between March 03, 2020, and May 18, 2021. None of the 43 patients was lost to follow-up during the hospital admission. Of the 43 patients, 15 (34.9%) had died as of Jun 16, 2021. The main cause of death was SARS-CoV-2 infection in these patients. 26 (60.5%) patients were male, 17 (39.5%) patients were female, and the overall follow-up for all the patients was 6 days. The overall mean age was 61.67 ± 11.80 (Table 1). 32.56% of patients were admitted to the hospital within 24 hours after diagnosis. 20 (46.51%) of 43 patients had symptoms during the course of COVID-19. Fever (74.4%), dyspnea (58.1%), cough (41.9%) and muscle ache (32.6%) were the most common symptoms (Table 1). The cancer patients with diabetes also had other comorbidities (Table 1). Non-survivor patients had higher pulse rates (108.13± 25.71) and lower levels of blood oxygen saturation (SpO2) (88.20± 9.12) as compared to the survivors. There was a statistically significant mean difference in body mass index (BMI) versus survival and non-survival (P=0.001). Additionally, in non-survivor patients, 4 (27%) had underweight BMI. No significant differences in gender, age, and other comorbidities were identified among survivors and non-survivors groups. We found significantly elevated levels of C-reactive protein in the non-survivors as compared to the survivors (P=0.005). Furthermore, the non-survivor patients presented with higher levels of neutrophils (P=0.04) and alkaline phosphate (P=0.03), serum lactate (P=0.01), and albumin (P=0.02) (Table 2). Moreover, 29 (67.4%) out of 43 patients showed abnormal features in the radiological findings (Figure 1). 20 (46.5%) of 43 patients presented with consolidation (Table 2).
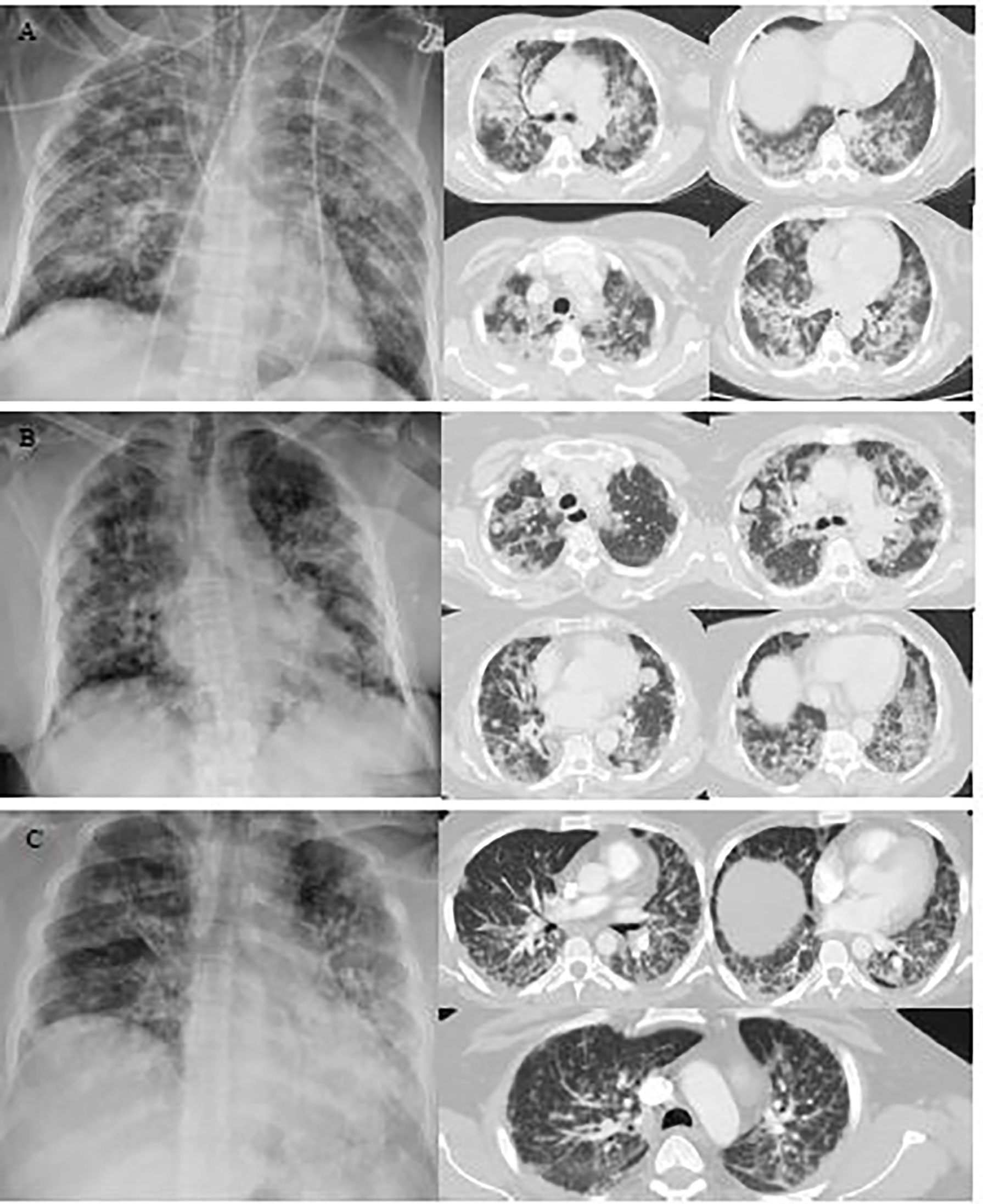
Figure 1 Representative images of the chest X-ray and chest computed tomography (CT) scan of COVID-19 cancer patients with diabetes. (A) A chest X-ray image of a 65-year-old female patient with scattered patchy airspace changes with prominent interstitial markings in bilateral lungs. Bilateral diffuse confluent airspace consolidations with COVID pneumonia. (B) Chest X-ray image of a 64-year-old female patient with interval increase in reticulation and interstitial lung disease with pulmonary nodules. Multifocal pulmonary metastases on the background of severe COVID pneumonia. (C) Chest X-ray image of a 40-year-old male patient with bilateral air space opacification, relatively confluent in the left lung. Peribronchial inflammatory cuffing with thickening of the intralobular septa with extensive pulmonary changes of COVID 19 infection seen bilaterally.
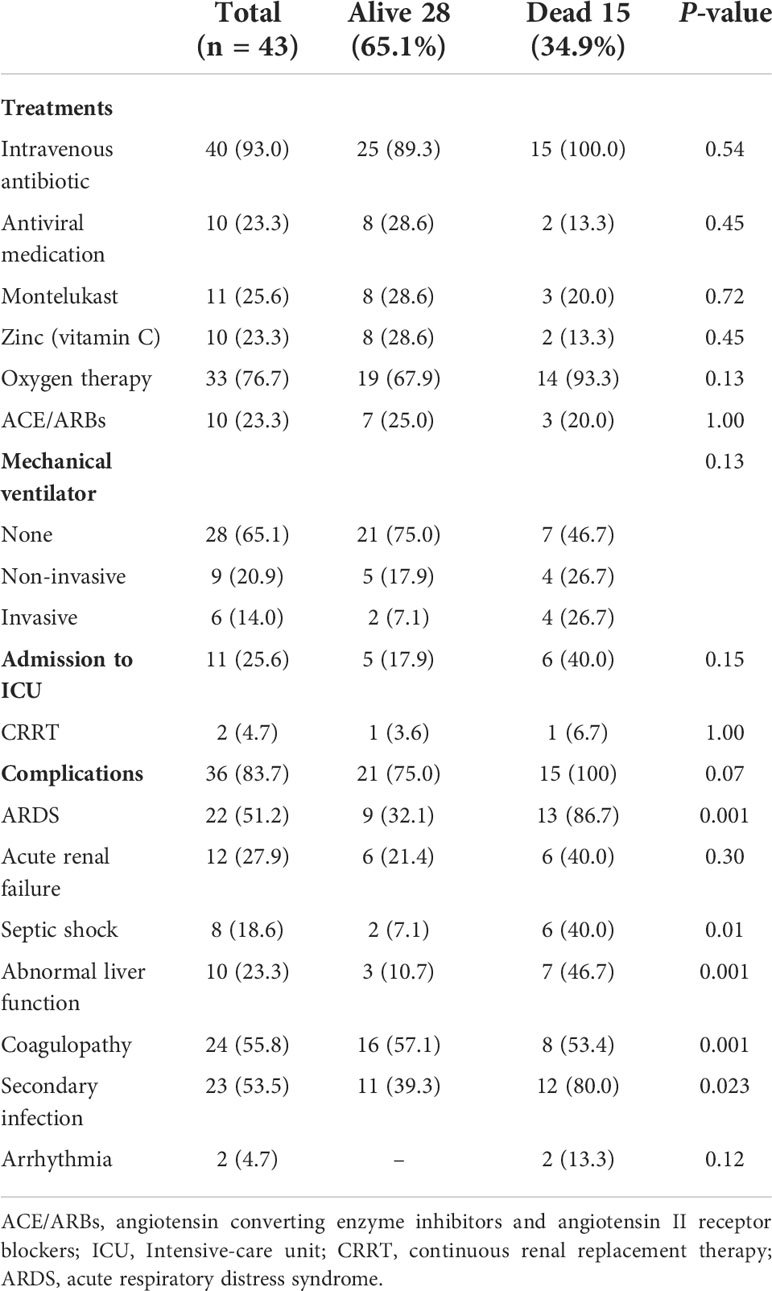
Of the 43 patients included, 40 (93.0%) received intravenous antibiotics and 10 (23.3%) received antiviral medication (Table 3). 37 (86.0%) of 43 were on antidiabetic medication (Supplementary Data Table 1). Oxygen therapy was given to 33 (76.7%) patients. ACE/ARBs were given to 10 (23.3%) patients (Table 3). Invasive and non-invasive mechanical ventilation was provided to 6 (14.0%) and 9 (20.9%) patients, respectively. 11 (25.6%) of 43 patients were admitted to the ICU. 36(83.7%) of 43 patients developed the complications such as ARDS 22 (51.2%), acute renal failure 12 (27.9%), septic shock 8 (18.6%), abnormal liver function 10 (23.3%), coagulopathy 24 (55.8%), secondary infections 23 (53.5%) and arrhythmia 2 (4.7%). Moreover, all the deceased patients (n=15) had acquired the complications (Table 3).
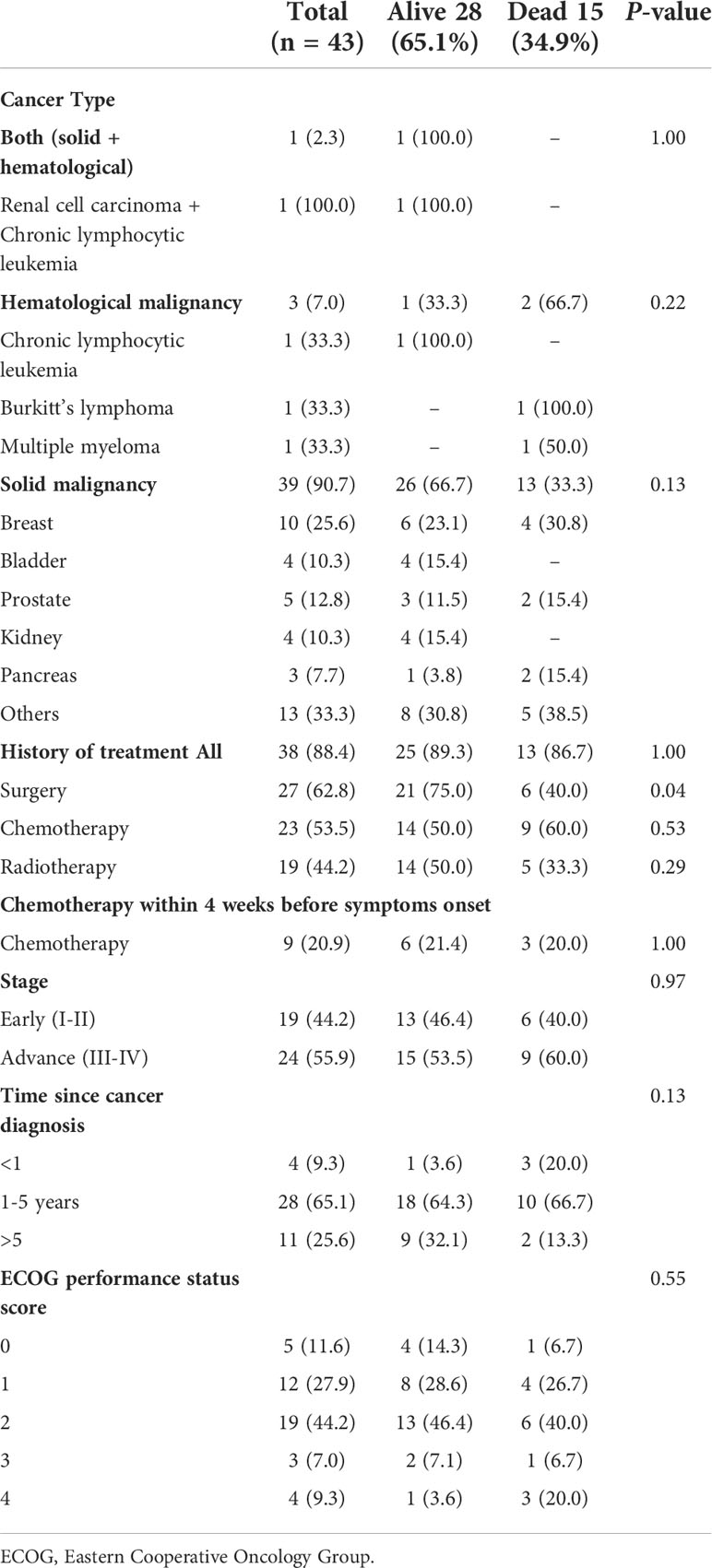
39 (90.7%) of 43 patients were diagnosed with solid tumors and 3 (7.0%) of 43 patients were diagnosed with hematological malignancies. Additionally, 1 (2.3) patient had both solid and hematological malignancy (Table 4). The most common cancers were breast cancer 10 (25.6%) and prostate cancer 5 (12.8%). Among hematological malignancies, there were three types, chronic lymphocytic leukemia 1(33.3%), Burkitt’s lymphoma 1(33.3%), and multiple myeloma 1(33.3%). One had both renal cell carcinoma and chronic lymphocytic leukemia 1 (2.3%). 23 (53.5%) of 43 patients received chemotherapy. Radiotherapy was given to 19 (44.2%) of 43 patients. 27 (62.8%) patients underwent the surgery. 9 (20.9%) of 43 patients received chemotherapy within 4 weeks before the onset of symptoms.
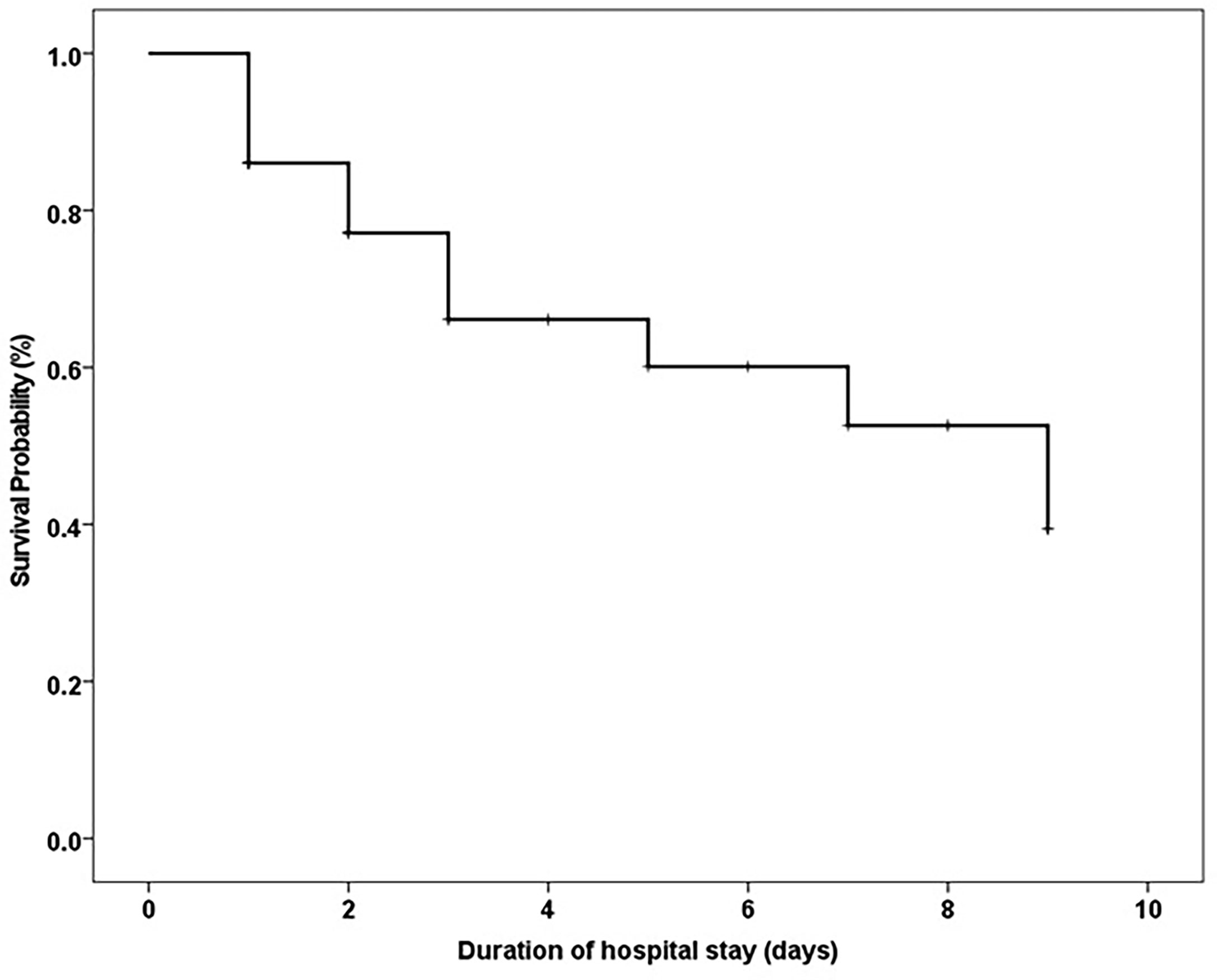
4 (9.3%) of 43 patients were diagnosed with cancer within the past year. 28 (65.1%) of 43 patients were diagnosed with cancer within the past year five years and 26 (60.4%) of 43 patients had an ECOG score higher than one before admission. 24 (55.9%) of 43 patients were in an advanced stage (III-IV). The case fatality rate in patients with solid tumors was 33.3% (13 of 39 patients) and that in hematological malignancies was 66.6% (2 of 3 patients), (Table 4). The median time of hospital stay was 2 days (range 1-9 days). The overall median survival time of hospital stay was 9 days (confidence interval 4-14 days) Figure 2.
Discussion
Cancer patients are highly susceptible individuals in the COVID-19 pandemic (14, 19–21). It has been reported that the mortality rate is high in these patients (14). Diabetes is one of the leading causes of morbidity and mortality worldwide (22). This disorder is associated with several complications that eventually influence the overall survival of patients (23). It has been previously identified that there is an association between diabetes and infection (24). However, the data remains contentious regarding whether diabetes itself augments vulnerability and influences outcomes from infections (25). The scientific data on COVID-19-infected cancer patients with diabetes is scarce. This is the first retrospective study conducted in Pakistan to demonstrate the clinical features and management of COVID-19-infected cancer patients with diabetes.
COVID-19 disease characteristically showed two clinical phases (26). During the first phase (5 days after infection), patients undergo extensive viral replication (26). During this phase, the symptoms are mostly fever and dyspnea (26–29). In the current study, we observed that fever (74.4%) and dyspnea (58.1%) were the most common symptoms as well. After the onset of the symptoms (7-10 days), some patients may enter into a second phase (26). During this phase, patients develop pathological changes due to an explicit immune response caused by the release of cytokine storm (26). The patients eventually become seriously ill and may require ICU admission (27, 29).
In the current pandemic, some studies did not observe a well-defined relationship between diabetes and severe disease (22, 30). Nevertheless, few studies showed that elderly diabetic patients with comorbidities were at higher risk to develop severe COVID-19 and mortality (6, 7, 31). Although in our data, the overall mean age was 61.67 ± 11.80, we could not find a significant difference in ages between survivors and non-survivors COVID-19 infected cancer patients with diabetes. Furthermore, we observed that cardiovascular and renal comorbidities, including hypertension, were more frequent in our data set. We also observed abnormal pulse rate and blood oxygen saturation in the COVID-19-infected cancer patients with diabetes. Xie et al. reported a link between hypoxemia (SpO2 <90%) and mortality in COVID-19 patients (32). We found lower levels of blood oxygen saturation (SpO2) in non-survivors patients. Yang et al. also observed lower levels of blood oxygen saturation among non-survivors (14). Overweight and obesity are linked with poor prognosis in COVID-19 infected diabetic patients (33). We observed a statistically significant mean difference in body mass index (BMI) between survivors and non-survivors (P=0.001). Additionally, in non- survivor patients 4 (27%) had underweight BMI. Ye et al. reported that both underweight and overweight COVID-19 infected patients have a tendency to acquire acute lung injury as compared with normal-weight patients. Besides that, especially underweight patients were more prone to develop a secondary infection (34). In our data set, all the underweight patients were non-survivor and they acquired the secondary infection as well.
Pro-inflammatory neutrophils and C-reactive protein (CRP) can be used as prognostic indicators for critical illness and adverse clinical outcomes in the patients infected with COVID-19 (35). Zhang et al. reported higher levels of C-reactive protein and neutrophil counts in cancer patients infected with COVID-19 (35). We also observed higher levels of C-reactive protein and neutrophil counts in non-survivor vs survivor COVID-19 infected cancer patients with diabetes. There is a possibility that pro-inflammatory neutrophils and C-reactive protein provoke “cytokine storm” associated to endothelialitis in COVID-19 infected cancer patients (35, 36). Zhou et al. confirmed that SARS-CoV-2 utilizes angiotensin-converting enzyme 2 (ACE2) receptor to gain entry into cells (37). Hamming et al. observed that ACE2 receptors are not only expressed in alveolar epithelial cells but also in cells of bile duct (38). These studies propose that SARS-CoV-2 may infect the bile duct cells and cause abnormal liver function in these patients. Nevertheless, alkaline phosphatase (ALP) which is the bile duct injury marker, has been reported as not elevated in COVID-19 infected patients (1). Recently published data by Kumar et al. concluded that ALP is a better indicator of COVID-19 induced liver injury (39). We also found a statistically significant (P=0.03) difference of ALP in survivor and non-survivor COVID-19 infected cancer patients with diabetes. Hyperlactatemia may be considered as a predictor of death (40). Evaluation of serum lactate has clinical implication in the diagnosis and treatment of serious cases of severe sepsis (41). We observed a significant increase in the lactate levels of non-survivor COVID-19 infected cancer patients with diabetes. COVID-19 infected patients with hypoalbuminemia have a tendency to develop severe clinical manifestations (42). Huang et al. identified a strong association of systemic inflammation in COVID-19 with hypoalbuminemia (43). They also observed a considerable difference in albumin between survivors and non‐survivors COVID-19 infected patients (43). We found similar results in our study. Therefore, screening of neutrophil counts, CRP, ALP, serum lactate and albumin levels is advised in diabetic cancer patients infected with SARS-CoV-2.
Diabetic patients are at an increased risk of developing the severe form of COVID-19 (44). Salehi et al. reported that the finding of consolidation was the initial presentation among COVID-19 infected patients (45). In the current study, 67.4% patients showed abnormal features in the radiological findings and 46.5% patients presented with consolidation. The involvement of the lungs indicates that cancer patients with diabetes are more prone to serious lung disease after COVID-19-infection (11).
Furthermore, we observed that complications such as ARDS, septic shock, coagulopathy, abnormal liver function and secondary infection were common in these patients. Our findings are in keeping with the data published previously (14). Yang et al. described that COVID-19 infected cancer patients receiving chemotherapy within four weeks before the onset of symptoms were vulnerable to death (14). In contrast, another study on COVID-19 infected cancer patients demonstrated that chemotherapy had no effect on patient death (42). We observed a similar finding in COVID-19 infected cancer patients with diabetes.
We acknowledge some limitations. Our current study is a retrospective cohort. We enrolled only those diabetic cancer patients who were infected with COVID-19 infection and admitted to our hospital from March 2020 to May 18, 2021. The sample size of our study was limited because of a small population of diabetic cancer patients who tested positive for COVID-19 infection. Additionally, we have not assessed the variation in pathological factors during hospitalization. To characterize the effect of the disease, a long-term follow up of the COVID-19 infected survivor diabetic cancer patients is required. Another limitation of our study is that since our hospital is a dedicated cancer care hospital, we could not include non-cancer patients.
Despite these limitations, the study had substantial strengths. Scarce data exist regarding clinical characteristics of survivors and non-survivors COVID-19-infected diabetic cancer patients. It is an attempt to identify the risk factors associated with severe disease in this highly susceptible group of patients in a pandemic. Besides that, it is the first study conducted in Pakistan for this specific cohort of patients.
In conclusion, we presented comprehensive data of the clinical features and outcomes of COVID-19-infected cancer patients with diabetes from Pakistan. Although diabetes is linked with severe outcomes in COVID-19 patients, the diabetic patients may not be highly susceptible to SARS-CoV-2 infection (16, 46–50). Since severity of COVID-19 changes promptly, it is favorable to identify the contributors of severity at the initial stage. Diabetes along with other comorbidities are substantial predictors of mortality in COVID-19 patients. We need to be more alert to the levels of CRP, ALP, serum lactate, albumin, neutrophils and regular monitoring of blood glucose, once diabetic cancer patients are infected with COVID-19. Future studies are warranted to understand the pathophysiological mechanisms of the association between COVID-19 and diabetes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by institutional review board (IRB) of SKMCH & RC. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
KA had the idea for and designed the study. KA, MA, SA, AA, AF, and RR were involved in the acquisition of the data. KA, MA, AF, and RR summarized the data. KA, MA, WS, UA, AS, and KS were involved in data interpretation. KA and MA drafted the manuscript. MA, KS, UA, AS, and WS critically revised the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to appreciate all hospital management and healthcare professionals who worked extensively during the pandemic.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.922579/full#supplementary-material
References
1. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the united states. New Engl J Med (2020) 382(10):929–36. doi: 10.1056/NEJMoa2001191
2. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol (2020) 92(6):568–76. doi: 10.1002/jmv.25748
3. Sun J, He WT, Wang L, Lai A, Ji X, Zhai X, et al. COVID-19: Epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol Med (2020) 26(5):483–95. doi: 10.1016/j.molmed.2020.02.008
4. Yusuf A. Cancer care in the time of COVID-19-a perspective from Pakistan. Ecancermedicalscience (2020) 14:1026. doi: 10.3332/ecancer.2020.1026
5. Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: Challenges for fighting the storm. Eur J Clin Invest (2020) 50(3):e13209. doi: 10.1111/eci.13209
6. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in china. New Engl J Med (2020) 382(18):1708–20. doi: 10.1056/NEJMoa2002032
7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (covid-19) outbreak in china: Summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA (2020) 323(13):1239–42. doi: 10.1001/jama.2020.2648
8. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in wuhan, China: A retrospective cohort study. Lancet (London England) (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3
9. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int J Infect Dis: IJID (2020) 94:91–5. doi: 10.1016/j.ijid.2020.03.017
10. International Agency for Research on Cancer Globocan. Pakistan Fact sheet (2020). Available at: https://gco.iarc.fr/today/data/factsheets/populations/586-pakistan-fact-sheets.pdf.
11. Asghar K, Loya A, Rana IA, Tahseen M, Ishaq M, Farooq A, et al. Indoleamine 2,3-dioxygenase expression and overall survival in patients diagnosed with breast cancer in Pakistan. Cancer Manage Res (2019) 11:475–81. doi: 10.2147/CMAR.S184221
12. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol (2020) 21(3):335–7. doi: 10.1016/S1470-2045(20)30096-6
13. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within wuhan, China. Ann Oncol (2020) 31(7):894–901. doi: 10.1016/j.annonc.2020.03.296
14. Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in hubei, China: A multicentre, retrospective, cohort study. Lancet Oncol (2020) 21(7):904–13. doi: 10.1016/S1470-2045(20)30310-7
15. Asghar K, Abu Bakar M, Akram MJ, Farooq A, Siddique K, Rana IA, et al. Clinical characteristics of covid-19-infected cancer patients in pakistan: Differences between survivors and non-survivors. Front Oncol (2021) 11:655634. doi: 10.3389/fonc.2021.655634
16. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract (2020) 162:108142. doi: 10.1016/j.diabres.2020.108142
17. Wojciechowska J, Krajewski W, Bolanowski M, Kręcicki T, Zatoński T. Diabetes and cancer: A review of current knowledge. Exp Clin Endocrinol Diabetes: Off J German Soc Endocrinol [and] German Diabetes Assoc (2016) 124(5):263–75. doi: 10.1055/s-0042-100910
18. WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: Interim guidance (2020). Available at: https://www.who.int/internalpublicationsdetail/clinical-management-ofsevere-acute.
19. Tsatsakis A, Calina D, Falzone L, Petrakis D, Mitrut R, Siokas V, et al. SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem Toxicol (2020) 146:111769. doi: 10.1016/j.fct.2020.111769
20. Vivarelli S, Falzone L, Grillo CM, Scandurra G, Torino F, Libra M. Cancer management during covid-19 pandemic: Is immune checkpoint inhibitors-based immunotherapy harmful or beneficial? Cancers (2020) 12(8):2237. doi: 10.3390/cancers12082237
21. Al-Quteimat OM, Amer AM. The impact of the covid-19 pandemic on cancer patients. Am J Clin Oncol (2020) 43(6):452–5. doi: 10.1097/COC.0000000000000712
22. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med (2020) 58(7):1131–4. doi: 10.1515/cclm-2020-0198
23. Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract (2020) 162:108072. doi: 10.1016/j.diabres.2020.108072
24. Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol (2016) 4(2):148–58. doi: 10.1016/S2213-8587(15)00379-4
25. Knapp S. Diabetes and infection: Is there a link?–A mini-review. Gerontology (2013) 59(2):99–104. doi: 10.1159/000345107
26. Sherren PB, Ostermann M, Agarwal S, Meadows C, Ioannou N, Camporota L. COVID-19-related organ dysfunction and management strategies on the intensive care unit: A narrative review. Br J Anaesthesia (2020) 125(6):912–25. doi: 10.1016/j.bja.2020.08.050
27. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis (2020) 34:101623. doi: 10.1016/j.tmaid.2020.101623
28. Wong C, Wong J, Tang E, Au CH, Wai A. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: A systematic review and meta-analysis. Sci Rep (2020) 10(1):19765. doi: 10.1038/s41598-020-74988-9
29. da Rosa Mesquita R, Francelino Silva Junior LC, Santos Santana FM, Farias de Oliveira T, Campos Alcântara R, Monteiro Arnozo G, et al. Clinical manifestations of COVID-19 in the general population: Systematic review. Wiener Klinische Wochenschrift (2021) 133(7-8):377–82. doi: 10.1007/s00508-020-01760-4
30. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in wuhan, China. Allergy (2020) 75(7):1730–41. doi: 10.1111/all.14238
31. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA (2020) 323(18):1775–6. doi: 10.1001/jama.2020.4683
32. Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G, et al. Association between hypoxemia and mortality in patients with covid-19. Mayo Clinic Proc (2020) 95(6):1138–47. doi: 10.1016/j.mayocp.2020.04.006
33. Smati S, Tramunt B, Wargny M, Caussy C, Gaborit B, Vatier C, et al. Relationship between obesity and severe COVID-19 outcomes in patients with type 2 diabetes: Results from the CORONADO study. Diabetes Obes Metab (2021) 23(2):391–403. doi: 10.1111/dom.14228
34. Ye P, Pang R, Li L, Li HR, Liu SL, Zhao L. Both underweight and obesity are associated with an increased risk of coronavirus disease 2019 (covid-19) severity. Front Nutr (2021) 8:649422. doi: 10.3389/fnut.2021.649422
35. Zhang B, Yu Y, Hubert SM, Zhang Y, Lu J, Liu S, et al. Prognostic value of pro-inflammatory neutrophils and c-reactive protein in cancer patient with coronavirus disease 2019: A multi-center, retrospective study. Front Pharmacol (2020) 11:576994. doi: 10.3389/fphar.2020.576994
36. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London England) (2020) 395(10234):1417–8. doi: 10.1016/S0140-6736(20)30937-5
37. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature (2020) 579(7798):270–3. doi: 10.1038/s41586-020-2012-7
38. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. a first step in understanding SARS pathogenesis. J Pathol (2004) 203(2):631–7. doi: 10.1002/path.1570
39. Kumar A, Kumar P, Dungdung A, Kumar Gupta A, Anurag A, Kumar A. Pattern of liver function and clinical profile in COVID-19: A cross-sectional study of 91 patients. Diabetes Metab Syndrome (2020) 14(6):1951–4. doi: 10.1016/j.dsx.2020.10.001
40. Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest (2013) 123(9):3685–92. doi: 10.1172/JCI69741
41. Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med (2004) 32(8):1637–42. doi: 10.1097/01.ccm.0000132904.35713.a7 (44 doi: 10.1097/01.CCM.0000132904.35713.A7
42. Chen C, Zhang Y, Zhao X, Tao M, Yan W, Fu Y. Hypoalbuminemia - an indicator of the severity and prognosis of covid-19 patients: A multicentre retrospective analysis. Infect Drug Resistance (2021) 14:3699–710. doi: 10.2147/IDR.S327090
43. Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol (2020) 92(10):2152–8. doi: 10.1002/jmv.26003(46
44. Rangankar V, Koganti DV, Lamghare P, Prabhu A, Dhulipala S, Patil P, et al. Correlation between ct severity scoring and diabetes mellitus in patients with covid-19 infection. Cureus (2021) 13(12):e20199. doi: 10.7759/cureus.20199
45. Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): A systematic review of imaging findings in 919 patients. AJR Am J Roentgenol (2020) 215(1):87–93. doi: 10.2214/AJR.20.23034
46. Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet (London England) (2020) 395(10241):1919–26. doi: 10.1016/S0140-6736(20)31173-9
47. Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol (2020) 109(5):531–8. doi: 10.1007/s00392-020-01626-9
48. Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinological Invest (2020) 43(6):867–9. doi: 10.1007/s40618-020-01236-2
49. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA (2017) 317(24):2515–23. doi: 10.1001/jama.2017.7596
50. Longato E, Di Camillo B, Sparacino G, Saccavini C, Avogaro A, Fadini GP. Diabetes diagnosis from administrative claims and estimation of the true prevalence of diabetes among 4.2 million individuals of the veneto region (North East Italy). Nutrition Metabolism Cardiovasc Dis: NMCD (2020) 30(1):84–91. doi: 10.1016/j.numecd.2019.08.017
Keywords: cancer, diabetes, survivors, non-survivors, Pakistan, COVID-19
Citation: Asghar K, Abu Bakar M, Ashfaq S, Alvi AM, Shafiq W, Azmat U, Siddiqi AI, Farooq A, Raza R and Siddique K (2022) COVID-19 in cancer patients with diabetes in Pakistan: Clinical features and management. Front. Oncol. 12:922579. doi: 10.3389/fonc.2022.922579
Received: 18 April 2022; Accepted: 27 July 2022;
Published: 18 August 2022.
Edited by:
Philip Robert Debruyne, AZ groeninge, BelgiumReviewed by:
Antonio Giordano, Temple University, United StatesSmreti Vasudevan, Rajiv Gandhi Cancer Institute and Research Centre, India
Copyright © 2022 Asghar, Abu Bakar, Ashfaq, Alvi, Shafiq, Azmat, Siddiqi, Farooq, Raza and Siddique. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kashif Asghar, kashifasghar@skm.org.pk
 Kashif Asghar
Kashif Asghar Muhammad Abu Bakar2
Muhammad Abu Bakar2