Evaluation of the validated intraoperative bleeding scale in liver surgery: study protocol for a multicenter prospective study
- 1Department of Surgery, Hospital Universitario Miguel Servet, Zaragoza, Spain
- 2Department of Surgery, Hospital Universitario Gregorio Marañón, Madrid, Spain
- 3Department of Surgery, Hospital Universitario de Badajoz, Badajoz, Spain
- 4Department of Surgery, Hospital Universitario Mutua de Terrassa, Terrassa, Spain
- 5Department of Surgery, Hospital Universitario German Trials I Pujol, Barcelona, Spain
- 6Department of Surgery, Hospital Universitario Virgen del Rocío, Seville, Spain
- 7Department of Surgery, Hospital Universitario Dr. Josep Trueta, Girona, Spain
- 8Department of Surgery, Hospital Universitario La Princesa, Madrid, Spain
- 9Department of Surgery, Hospital Clínico Universitario, INCLIVA, Valencia, Spain
- 10Department of Surgery, Hospital General Universitario Dr. Balmis, ISABIAL, Universidad Miguel Hernández, Alicante, Spain
- 11Department of Surgery, Instituto de Investigación Sanitaria Aragón, Hospital Universitario Miguel Servet, Zaragoza, Spain
Background: Surgical hemostasis has become one of the key principles in the advancement of surgery. Hemostatic agents are commonly administered in many surgical specialties, although the lack of consensus on the definition of intraoperative bleeding or of a standardized system for its classification means that often the most suitable agent is not selected. The recommendations of international organizations highlight the need for a bleeding severity scale, validated in clinical studies, that would allow the selection of the best hemostatic agent in each case. The primary objective of this study is to evaluate the VIBe scale (Validated Intraoperative Bleeding Scale) in humans. Secondary objectives are to evaluate the scale's usefulness in liver surgery; to determine the relationship between the extent of bleeding and the hemostatic agent used; and to assess the relationship between the grade of bleeding and postoperative complications.
Methods: Prospective multicenter observational study including 259 liver resections that meet the inclusion criteria: patients scheduled for liver surgery at one of 10 medium-high volume Spanish HPB centers using an open or minimally invasive approach (robotic/laparoscopic/hybrid), regardless of diagnosis, ASA score <4, age ≥18, and who provide signed informed consent during the study period (September 2023 until the required sample size has been recruited). The participating researchers will be responsible for collecting the data and for reporting them to the study coordinators.
Discussion: This study will allow us to evaluate the VIBe scale for intraoperative bleeding in humans, with a view to its subsequent incorporation in daily clinical practice.
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT05369988?term = serradilla&draw = 2&rank = 3, [NCT0536998].
Introduction
Surgical hemostasis has become one of the key principles for the advancement of surgery (1). The use of hemostatic agents is standard in many surgical specialties (2–6), especially in major surgeries (7, 8). However, inadequate hemostasis significantly increases the risk of perioperative morbidity and mortality (9, 10), healthcare costs, and the use of resources (11–14). The need for hemostasis has led to the development of a range of topical hemostatic agents (15–17) to supplement hemostasis efforts.
Generally, bleeding from solid organs can be divided into two types: (a) First, bleeding which is due to inadequate control of surgical field and which requires either suture control or resection of the organ to stop the bleeding. The available hemostatic agents are essentially always ineffective with this degree of bleeding; (b) The second type of bleeding may be described as “medical”, which is due to coagulopathy or that which is controlled by packing.
Clinical studies that have analyzed the use of topical hemostats have not used standardized definitions or classifications to determine the severity of intraoperative bleeding (18–23), partly due to a lack of consensus on its definition. This has meant that many surgeons do not select the best hemostats for specific bleeding situations, and the results are unsatisfactory (8). Using standardized criteria, the results of clinical studies can be compared in order to determine the effectiveness of different hemostatic agents.
The Food and Drug Administration (FDA) (24) recommends the use of a bleeding severity scale validated in clinical studies of hemostatic agents. The VIBe scale (Validated Intraoperative Bleeding Scale) for intraoperative bleeding was developed in 2017 (25) and is approved by the FDA as a clinical report scale (Table 1). It has been validated by a large number of expert surgeons from all specialties, but only in animal models; it has not yet been clinically validated in humans. Depending on the degree of intraoperative bleeding, the scale recommends a specific type of hemostatic.
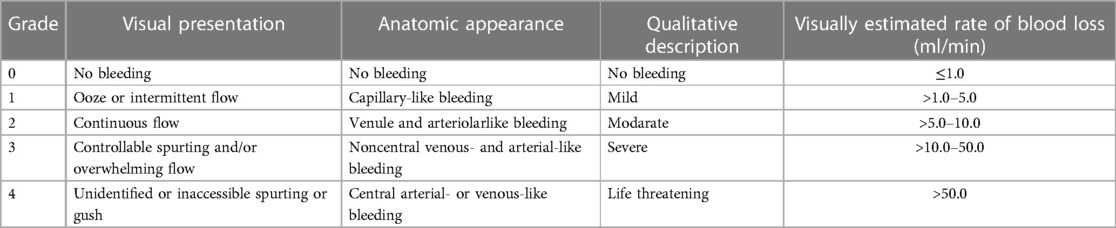
Table 1. Validated bleeding severity scale (25).
Liver surgery is one of the surgeries in which intraoperative bleeding is most important. In Spain, around 5,000 liver resections are performed annually, not including liver transplants. It is, therefore, a perfect setting in which to evaluate the applicability of the VIBe scale of intraoperative bleeding severity in humans.
To assess the feasibility of a prospective clinical study in liver surgery, the applicability of the VIBe scale was evaluated by 47 liver surgeons from 10 medium-high volume centers who viewed the 14 original video recordings used by Lewis et al. (25) for the validation of the original scale. The scale achieved a mean intraobserver agreement of 0.985 and an interobserver agreement of 0.929; neither score was influenced by surgeon experience or volume of surgeries per year.
With these preliminary results, based on the video classification exercise, we conclude that the VIBe scale is a useful instrument for liver surgeons assessing severity of bleeding. Therefore, it can be used to assess the severity of intraoperative bleeding in actual liver surgery.
This prospective multicenter study carried out at centers in Spain that perform liver surgery aims to evaluate a scale that allows the grading of intraoperative bleeding in surgery, and to determine whether there is a relationship between the maximum bleeding grade and complications.
Methods
Hypothesis and primary/secondary objectives
To date, the VIBe scale for the severity of intraoperative bleeding has only been validated in an animal model (25). Intraoperative bleeding occurs frequently in liver surgery and can have a major impact on the postoperative results. Liver surgery is thus an ideal setting for applying the VIBe scale in humans.
The primary objective is to evaluate the VIBe scale for intraoperative bleeding in liver surgery. The secondary objectives are to assess the usefulness of the scale in this type of surgery; to determine the relationship between bleeding grade and hemostatic method used; and to determine the relationship between the maximum bleeding grade reached on the VIBe scale and postoperative complications.
Variables analyzed
The key point of the study is to determine the grade of intraoperative bleeding evaluated with the VIBe scale. In the initial study by Lewis et al. (25), the only variable analyzed was the volume of intraoperative bleeding, in order to establish the grade of bleeding. In our study, we intend to evaluate the usefulness of this scale in daily clinical practice. For this reason, regardless of the volume of bleeding, we collect all those variables that may be related to intraoperative bleeding.
Thus, we will evaluate the VIBe bleeding scale by examining how well its recommended hemostatic interventions align with the actual usage of hemostatics in typical clinical scenarios. This evaluation will involve a calibration assessment using the Hosmer-Lemeshow test, which examines the agreement between predicted probabilities of hemostatic usage based on the scale and observed clinical outcomes. Additionally, we will utilize calibration plots to visually depict the consistency between predicted and observed outcomes. This analysis will provide valuable insights into the scale's accuracy and alignment with real-world practice, helping to determine its practical effectiveness in guiding hemostatic interventions (26).
Because there may be numerous bleeding episodes during a hepatectomy, it is impossible to measure each one individually. The collaborating researchers propose its assessment at two selected time points: the moment of greatest bleeding, and the end of the surgery. An intraoperative record sheet is prepared and completed according to the comments of the main or assistant surgeon and will be reviewed and finally signed by the main surgeon at the end of the procedure. Similarly, blood loss (the amount of fluid in the suction container minus the volume of liquid used for flushing), the units of blood transfused and the lowest hemoglobin value during admission (compared to the baseline rate) will be quantified. The rest of the data will be collected at the time of discharge and 90 days after surgery.
Patient comorbidities will be classified using the Charlson Comorbidity Index (27). Likewise, the patient's coagulation status (PT/PTT/INR/TEG) and treatments that may alter it (anticoagulants and antiaggregants) will be collected. Postoperative complications will be classified using the Clavien-Dindo Classification of Surgical Complications (28) and the Comprehensive Complication Index (CCI) (29). Major complications are those defined as Clavien-Dindo grade IIIa or higher. To record complications, the medical and nursing notes from each electronic or physical record of each patient included in the project will be compared. Intraoperative complications will be measured according to the Satava classification (30). For the specific complications of liver surgery, the definitions of the International Study Group of Liver Surgery (ISGLS) for liver failure (31) and postoperative bleeding (32) will be used. Complications, readmissions and mortality will be measured at postoperative day 90, through the medical history and, if necessary, by telephone communication with the patient or relatives (Supplementary Files S1, S2).
We plan to examine the relationship between the maximum bleeding grade and the occurrence of complications through logistic models, calculating odds ratios to quantify the risk of experiencing complications in relation to different bleeding grades, particularly for dichotomous variables. For variables that evolve over time, we will employ Cox proportional hazard models to estimate hazard ratios, allowing us to understand the impact of bleeding grades on the timing of complications.
Study design
This study is a prospective observational multicenter study lasting 12 months (the period can be lengthened if the stipulated number of patients has not been reached) involving 10 Spanish HPB Surgery units with medium-high volume of liver surgery (Supplementary File S3). Medium-volume centers will be considered those that perform 40–60 liver surgeries per year, and high-volume centers those with more than 60 per year (33). Surgeons from participating centers will receive training prior to the start of the study on how to properly categorize the degree of intraoperative bleeding from the videos in the Lewis article (25).
Patients operated on either via open surgery or via a minimally invasive approach will be included; thus, the VIBe scale will also be evaluated for minimally invasive surgery. In this sense, an analysis by subgroups of “open surgery” vs. “minimally invasive surgery” will be performed.
Inclusion and exclusion criteria and rationale for the study population
Participants meeting all the following inclusion criteria are eligible for the study:
- Patients scheduled for liver surgery
- Open or minimally invasive approach (laparoscopic/robotic/hybrid)
- Any diagnosis
- ASA score <4
- Age ≥ 18
- Provision of signed informed consent
Patients with any of the following exclusion criteria will not take part:
- Contraindications for liver surgery
- Emergency surgical interventions
- Age <18 years
- Failure to provide signed informed consent
- Loss to follow-up and impossibility of completing the data record.
Recruitment, screening and informed consent procedure
This registry includes all the patients undergoing liver surgery at the participating Spanish centers in the study period who meet the inclusion criteria. All ten are reference centers which participated in the first phase of the study with the validation based on the videos. The registry will remain open for a further three months after the required sample size has been recruited to record postoperative morbidity and mortality at 90 days.
When confirming their participation, the participating centers will appoint a contact person.
The study will be presented to the patients during the first preoperative consultation by the investigators at each participating hospital. The investigators will explain to each participant the nature of the study, its purpose, the procedures involved, the expected duration, the potential risks and benefits, and any discomfort it may entail. Participants will be informed that their participation in the study is voluntary and that they may withdraw at any time, and that withdrawal of consent will not affect their subsequent medical assistance and treatment. They will be notified that their medical records may be examined by authorized individuals other than their treating physician. They will be given a participant information sheet and a consent form describing the study and providing sufficient information for them to make an informed decision about whether to take part. Patients will have the opportunity to ask any questions they may have.
Formal consent will be obtained from participants using the approved consent form, before they undergo any of the study procedures. The consent form will be signed and dated by the investigator or designee at the same time as the participant signs. A copy of the signed informed consent will be given to the participant. The consent form will be retained as part of the study records. The informed consent process will be documented in the patient file and any discrepancies with regard to the process described in this protocol will be explained (Supplementary File S4).
Planning
Data collection will begin in September 2023.
Withdrawal and discontinuation
As it is a prospective registry that does not involve any intervention in the patient, consecutive cases that meet the inclusion criteria operated on at the participating centers will be included in the study. Only those patients who, having signed the informed consent, refuse to participate in the study prior to the intervention will be excluded; in this case they will be replaced by a new patient until the required sample size is obtained.
Follow-up
In liver surgery, complications may occur beyond 30 postoperative days. Therefore, the international scientific community has agreed to record complications at 90 days.
Statistical analysis and sample size calculation
Depending on the number of cases contributed by each collaborating hospital, a retrospective analysis will be carried out to detect the power of the differences observed in the data. The variables of interest will be displayed in univariate and bivariate tables according to study group. For comparisons between groups, parametric (t-student, ANOVA) and non-parametric (Mann-Whitney, Kruskal-Wallis) tests will be used in continuous variables depending on their distribution, and Fisher or chi-square tests for categorical variables.
To investigate the relationship between different variables, correlation analysis and/or linear regression and bi- or multivariate logistic analysis will be used. In addition, the longitudinal variation of certain variables of interest will be studied with Kaplan-Meier estimators and bi- or multivariate analysis using Cox models. The analyses will be performed with the R statistical software package. Statistical significance will be considered for values of p < 0.05 (34–36).
Based on an estimated population size of 5,000 liver surgeries per year in Spain, a sample size of 259 cases will be selected to achieve a margin of error of 5% with a confidence level of 90%. Based on the normal distribution, the sample size n and the margin of error E are given by:
Where N is the size of the population, r is the fraction of responses (set at 50% as the most conservative assumption) and Z (c/100) is the critical value for the confidence level c (34–36).
Data record
Each participating center will appoint a contact person responsible for data collection and for all communication with the study coordinators. Subsequently, each contact person will receive a login code and passwords for the online electronic case report form environment (REDCap®, University of Vanderbilt, Nashville, Tennessee, United States). Each data collector will receive a separate login account from which the lead study coordinators can monitor all activity. All data will be recorded in accordance with the Good Clinical Practice (GCP) guidelines.
The variables collected in this form are shown in Supplementary Files S1, S2.
Authorship and publication policy
Authorship will be based on the guide of the “International Committee of Medical Journal Editors” (37). The study coordinators will occupy the first and last positions of authorship, followed by the principal investigators of each center. A collaborative group will be created that will include the rest of the participating surgeons from each center in the study. The data obtained will be communicated to the local research ethics committee in accordance with Spanish legislation and then published.
The data generated by the study will be sent to journals with a high impact factor indexed in the Journal Citation Reports (JCR). The results will also be presented as communications at international HPB surgery conferences (IHPBA/ E-AHPBA congress).
Discussion
Anticipated results
Despite the extensive literature published on surgery, there is no evidence at present to help create validated scales that quantify the severity of intraoperative bleeding and thus select the best hemostatic procedure for each case. Likewise, the Spanish Health System (where this study is to be carried out) does not impose limitations on the use of intraoperative surgical material (i.e., local hemostatics, types of suture, sealants, mechanical staplers, etc.). The choice of the method for each situation is left the discretion of the liver surgeon. All this has repercussions for patient safety; it may lead to the inappropriate use of healthcare resources and increased costs, and it may increase postoperative morbidity and mortality. Against this background, liver surgery appears to be an ideal setting for applying the VIBe scale for grading intraoperative bleeding in humans.
The authors expect to find an adequate clinical correlation of the VIBe scale with the clinical results of intraoperative bleeding in liver surgery at two time points: the moment of maximum bleeding and the end of the surgery. This will allow its evaluation and its later incorporation in daily clinical practice. In addition, intraoperative bleeding is expected to correlate with postoperative complications.
Overall ethical considerations
As there are no previous references in the literature to the existence of a validated scale that categorizes intraoperative bleeding in liver surgery, the study design (a ospective multicenter registry lasting 12 months) should have good internal validity. Medium/high volume HBP surgery centers in Spain will participate in this multicenter study, thus obtaining a large sample size that will allow us to collect highly detailed, representative, and reliable information and will increase the generalizability of the results (external validity).
If the results are favorable, the VIBe scale will be considered validated as a severity scale for intraoperative bleeding in clinical studies that allows the selection of the best hemostatic method in each case, in compliance with the recommendations of international organizations. The results of the study can be implemented and promptly transferred into daily clinical practice.
The need for research in this field is clear, since the issue of intraoperative bleeding, more specifically in liver surgery, has not been resolved and there is no consensus among surgeons. This situation leads to a notable heterogeneity in clinical practice. The results of this study would go beyond their mere scientific interest, since they will have a direct impact on the outcomes of patients undergoing liver surgery. Given that the indications for liver resections are rising, the importance of the results will increase correlatively.
Risk-benefit assessment
This is a prospective registry of patients undergoing liver surgery. Liver surgery is a common procedure that does not require the performance of any unusual measures.
Limitations
The study has a multicenter design, and so there may be selection or information biases if centers apply different criteria and surgical indications. The quality of the outcome measurement is limited by the possible heterogeneity between centers in their assessment of intraoperative bleeding.
Conclusion
It is hoped that this study will provide new insights into the impact of intraoperative bleeding in liver surgery. It is designed to validate a tool that allows the categorization of bleeding and the selection of the best individualized hemostatic method available for each patient.
Ethics and dissemination
This study has the approval of the Research Ethics Committee of Aragon (CEICA). Session of 01/26/2022, Acta No. 02/2022. C.P.—I.C. PI21/510. All included patients will provide written consent to participate in this study.
The dissemination of the study will be carried out through the Division of HPB Surgery of the Spanish Association of Surgeons (AEC), and by the Spanish Chapter of the International Association of Hepato-Pancreato-Biliary Surgery (CE-IHPBA).
Data availability statement
The original data will be included in the articles and Supplementary Materials. Further requests for data may be addressed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethical Committee for Clinical Research of Aragon. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors meet the ICMJE authorship criteria. Contributions: (I) Conception and design: MS-M and JR; (II) Administrative support: DA-L; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: MS-M, JR and DA-L; (V) Data analysis and interpretation: MS-M, JR and DA-L; (VI) Manuscript writing: DA-L, MS-M, JR; (VII) Final approval of manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study received an Investigator Initiated Research Grant from Baxter Healthcare Corporation, Deerfield, IL, USA.
Acknowledgments
This study is designed by an independent, interdisciplinary academic non-profit research organization in Spain. We have received an Investigator Initiated Research Grant from Baxter Healthcare Corporation to increase study funding. The present work shows partial results of the doctoral thesis by DA-L at the University of Zaragoza, Spain.
Conflict of interest
MS-M and JR receive fees from Baxter International Inc. (Deerfield, Illinois, United States) as consultants.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2023.1223225/full#supplementary-material
Supplementary File S1
Study variables.
Supplementary File S2
Schedule of assessments.
Supplementary File S3
Participating centers.
Supplementary File S4
Patient information document and individual informed consent.
References
2. American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American society of anesthesiologists task force on perioperative blood management. Anesthesiology. (2015) 122:241–75. doi: 10.1097/ALN.0000000000000463
3. Shander A, Van Aken H, Colomina MJ, Gombotz H, Hofmann A, Krauspe R, et al. Patient blood management in Europe. Br J Anaesth. (2012) 109:55–68. doi: 10.1093/bja/aes139
4. Society of Thoracic Surgeons Blood Conservation Guideline Task Force, Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, et al. 2011 Update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. (2011) 91(3):944–82. doi: 10.1016/j.athoracsur.2010.11.078
5. Menkis AH, Martin J, Cheng DC, Fitzgerald DC, Freedman JJ, Gao C, et al. Drug, devices, technologies, and techniques for blood management in minimally invasive and conventional cardiothoracic surgery: a consensus statement from the international society for minimally invasive cardiothoracic surgery (ISMICS) 2011. Innovations (Phila). (2012) 7:229–41. doi: 10.1097/imi.0b013e3182747699
6. Kommu SS, McArthur R, Emara AM, Reddy UD, Anderson CJ, Barber NJ, et al. Current status of hemostatic agents and sealants in urologic surgical practice. Rev Urol. (2015) 17:150–9.26543429
7. Wright JD, Ananth CV, Lewin SN, Burke WM, Siddiq Z, Neugut AI, et al. Patterns of use of hemostatic agents in patients undergoing major surgery. J Surg Res. (2014) 186:458–66. doi: 10.1016/j.jss.2013.07.042
8. Tollec SM, Trossaert M, Duveau D, Grimandi G, Sellal KO. A proposed model for evaluating surgical hemostatic agents. Le Pharmacien Hospitalier et Clinicien. (2014) 49: 262–76. doi: 10.1016/j.phclin.2013.07.020
9. Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. (2007) 116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977
10. Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, et al. Association between intraoperative blood transfusion and mortality and morbidity in patients under- going noncardiac surgery. Anesthesiology. (2011) 114:283–92. doi: 10.1097/ALN.0b013e3182054d06
11. Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA Jr. Hospital costs associated with surgical complications: a report from the private-sector national surgical quality improvement program. J Am Coll Surg 2004; 199:531–7. doi: 10.1016/j.jamcollsurg.2004.05.276
12. Shander A. Financial and clinical outcomes associated with surgical bleeding complications. Surgery. (2007) 142(Suppl 4):S20–5. doi: 10.1016/j.surg.2007.06.025
13. Christensen MC, Krapf S, Kempel A, von Heymann C. Costs of excessive postoperative hemorrhage in cardiac surgery. J Thorac Cardiovasc Surg. (2009) 138:687–93. doi: 10.1016/j.jtcvs.2009.02.021
14. Stokes ME, Ye X, Shah M, Mercaldi K, Reynolds MW, Rupnow MF, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. (2011) 11(135). doi: 10.1186/1472-6963-11-135
15. Levy JH, Dutton RP, Hemphill JC 3rd, Shander A, Cooper D, Paidas MJ, et al. Multidisciplinary approach to the challenge of hemostasis. Anesth Analg 2010;110:354–64. doi: 10.1213/ANE.0b013e3181c84ba5
16. Yamamoto K, Wada H, Sakakura K, Ikeda N, Yamada Y, Katayama T, et al. Cardiovascular and bleeding risk of non-cardiac surgery in patients on antiplatelet therapy. J Cardiol. (2014) 64:334–8. doi: 10.1016/j.jjcc.2014.02.027
17. Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso- Coello P, Kurz A, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. (2014) 370:1494–503. doi: 10.1056/NEJMoa1401105
18. Schuhmacher C, Pratschke J, Weiss S, Schneeberger S, Mihaljevic AL, Schirren R, et al. Safety and effectiveness of a synthetic hemostatic patch for intraoperative soft tissue bleeding. Med Devices (Auckl). (2015) 8:167–74. doi: 10.1016/j.avsg.2015.09.007
19. Fischer CP, Bochicchio G, Shen J, Patel B, Batiller J, Hart JC. A prospective, randomized, controlled trial of the efficacy and safety of fibrin pad as an adjunct to control soft tissue bleeding during abdominal, retroperitoneal, pelvic, and thoracic surgery. J Am Coll Surg. (2013) 217:385–93. doi: 10.1016/j.jamcollsurg.2013.02.036
20. Siegel JM, Cummings JF, Clymer JW. Reproducible, repeat- able and clinically-relevant hemostasis scoring. J Adv Med Pharm Sci. (2014) 1:30–9. doi: 10.9734/JAMPS/2014/11653
21. Ng CS, Pickens A, Siegel JM, Clymer JW, Cummings JF. A novel narrow profile articulating powered vascular stapler provides superior access and haemostasis equivalent to conventional devices. Eur J Cardiothorac Surg. (2016) 49(Suppl 1):i73–8. doi: 10.1093/ejcts/ezv352
22. Jackman SV, Cadeddu JA, Chen RN, Micali S, Bishoff JT, Lee BR, et al. Utility of the harmonic scalpel for laparoscopic partial nephrectomy. J Endourol. (1998) 12:441–4. doi: 10.1089/end.1998.12.441
23. Adams GL, Manson RJ, Hasselblad V, Shaw LK, Lawson JH. Acute in-vivo evaluation of bleeding with gelfoam plus saline and gelfoam plus human thrombin using a liver square lesion model in swine. J Thromb Thrombolysis. (2009) 28:1–5. doi: 10.1007/s11239-008-0249-3
24. United States Food and Drug Administration. Clinical outcome assessment qualification program. Silver Spring (MD): US food and drug administration; (2016). Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm284077.htm
25. Lewis KM, Li Q, Jones DS, Corrales JD, Du H, Spiess PE, Lo Menzo E, DeAnda A Jr. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. (2017) 161(3):771–81. doi: 10.1016/j.surg.2016.09.022
26. Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. (2016) 25(4):1692–706. doi: 10.1177/0962280213497434
27. Roffman C, Buchanan J, Allison GT. Charlson comorbidities index. J Physiother. (2016) 62(3):171. doi: 10.1016/j.jphys.2016.05.008
28. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205. doi: 10.1097/01.sla.0000133083.54934.ae
29. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. (2013) 258(1):1–7. doi: 10.1097/SLA.0b013e318296c732
30. Satava RM. Identification and reduction of surgical error using simulation. Minim Invasive Ther Allied Technol. (2005) 14(4):257–61. doi: 10.1080/13645700500274112
31. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Post hepatectomy liver failure: a definition and grading by the international study group of liver surgery (ISGLS). Surgery. (2011) 149(5):713–24. doi: 10.1016/j.surg.2010.10.001
32. Rahbari N, Garden J, Padbury R, Maddern G, Koch M, Hugh T, et al. Post-hepatectomy haemorrhage: a definition and grading by the international study group of liver surgery (ISGLS). HPB (Oxford). (2011) 13(8):528–3. doi: 10.1111/j.1477-2574.2011.00319.x
33. Egeland C, Rostved AA, Schultz NA, Pommergaard HC, Daugaard TR, Thøfner LB, et al. Morbidity and mortality after liver surgery for colorectal liver metastases: a cohort study in a high-volume fast-track programme. BMC Surg. (2021) 21(1):312. doi: 10.1186/s12893-021-01301-4
34. Swindle MM, Makin A, Herron AJ, Clubb FJ Jr, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol. (2012) 49:344–56. doi: 10.1177/0300985811402846
35. Lesaffre AR. Statistical and methodological aspects of oral health research. West Sussex (United Kingdom): John Wiley & Sons Ltd. (2009).
36. Schmidt RC. Managing delphi surveys using nonparametric statistical techniques. Decis Sci. (1997) 28:763–74. doi: 10.1111/j.1540-5915.1997.tb01330.x
Keywords: liver surgery, predictive score, intraoperative bleeding, surgical hemostasis, hemostatic agent
Citation: Aparicio-López D, Asencio-Pascual JM, Blanco-Fernández G, Cugat-Andorrá E, Gómez-Bravo MÁ, López-Ben S, Martín-Pérez E, Sabater L, Ramia JM and Serradilla-Martín M (2023) Evaluation of the validated intraoperative bleeding scale in liver surgery: study protocol for a multicenter prospective study. Front. Surg. 10:1223225. doi: 10.3389/fsurg.2023.1223225
Received: 15 May 2023; Accepted: 18 September 2023;
Published: 2 October 2023.
Edited by:
Abraham Fingerhut, Shanghai Jiao Tong University, ChinaReviewed by:
Vincenzo Lizzi, Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia, ItalyIvan Romic, University Hospital Centre Zagreb, Croatia
Chadli Dziri, Tunis El Manar University, Tunisia
Andrew Peitzman, University of Pittsburgh, United States
© 2023 Aparicio-López, Asencio-Pascual, Blanco-Fernández, Cugat-Andorrá, Gómez-Bravo, López-Ben, Martín-Pérez, Sabater, Ramia and Serradilla-Martín. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Aparicio-López dapariciol@salud.aragon.es
†These authors have contributed equally to this work and share senior authorship
 Daniel Aparicio-López
Daniel Aparicio-López José Manuel Asencio-Pascual
José Manuel Asencio-Pascual Gerardo Blanco-Fernández
Gerardo Blanco-Fernández Esteban Cugat-Andorrá4,5
Esteban Cugat-Andorrá4,5  Miguel Ángel Gómez-Bravo
Miguel Ángel Gómez-Bravo Santiago López-Ben
Santiago López-Ben Elena Martín-Pérez
Elena Martín-Pérez Luis Sabater
Luis Sabater José Manuel Ramia
José Manuel Ramia Mario Serradilla-Martín
Mario Serradilla-Martín