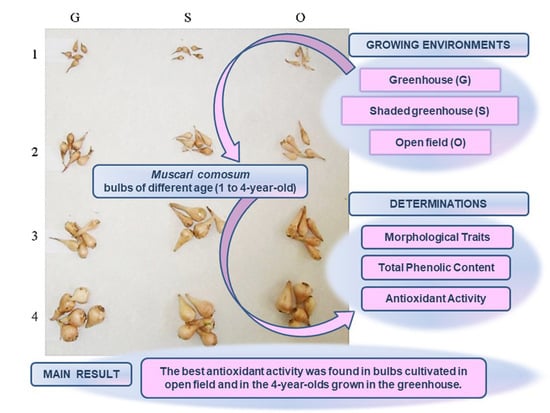
Antioxidant Activity, Total Phenolic Content and Morphological Traits in 1 to 4-Year-Old Muscari comosum Bulbs Cultivated in Three Growing Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Growth Conditions
2.3. Climate Condition Data
2.4. Morphological Parameters of “Lampascione” Bulbs
2.5. Samples Preparation and Extraction
2.6. Spectrophotometric Analysis
2.6.1. Spectrophotometric Analysis
2.6.2. Determination of Antioxidant Activity
2.7. Graphs and Data Analysis
3. Results
3.1. Monitoring of the Environmental Conditions
3.2. Morphological Parameters of Bulbs
3.3. Dry Matter Content
3.4. Total Phenolic Content
3.5. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonasia, A.; Conversa, G.; Lazzizera, C.; La Rotonda, P.; Elia, A. Weed Control in Lampascione—Muscari comosum (L.) Mill. Crop Prot. 2012, 36, 65–72. [Google Scholar] [CrossRef]
- Conte, A.; Lucera, A.; Costa, C.; Mastromatteo, M.; Del Nobile, M.A. Combined Strategies for Ready-to-Cook Lampascioni (Muscari comosum L.). J. Food Process. Preserv. 2011, 35, 861–868. [Google Scholar] [CrossRef]
- Doussi, M.; Thanos, C. Ecophysiology of Seed Germination in Mediterranean Geophytes. 1. Muscari spp. Seed Sci. Res. 2002, 12, 193–201. [Google Scholar] [CrossRef]
- Borgonovo, G.; Caimi, S.; Morini, G.; Scaglioni, L.; Bassoli, A. Taste-Active Compounds in a Traditional Italian Food: ‘Lampascioni’. Chem. Biodivers. 2008, 5, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Publications Office of the European Union. CELEX1, Commission Regulation (EC) No 1508/2001 of 24 July 2001 Laying Down the Marketing Standard for Onions and Amending Regulation (EEC) No 2213/83. Available online: https://op.europa.eu/en/publication-detail/-/publication/8eb76789-9e45-4bc1-bfec-2e56643b278a/language-en (accessed on 13 December 2023).
- Publications Office of the European Union. Commission Regulation (EC) No 1465/2003 of 19 August 2003 Amending Regulation (EC) No 1508/2001 Laying Down the Marketing Standard for Onions, CELEX1. Available online: https://op.europa.eu/en/publication-detail/-/publication/4ce2c556-7aa4-4630-9b80-f525647beedb/language-en/format-PDF (accessed on 13 December 2023).
- Kaur, C.; Kapoor, H.C. Antioxidants in Fruits and Vegetables—The Millennium’s Health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Sahraeian, S.; Rashidinejad, A.; Golmakani, M.-T. Recent Advances in the Conjugation Approaches for Enhancing the Bioavailability of Polyphenols. Food Hydrocoll. 2024, 146, 109221. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the Total Antioxidant Capacity and Total Polyphenol Content of 23 Commercially Available Vegetable Juices before and after in Vitro Digestion Measured by FRAP, DPPH, ABTS and Folin–Ciocalteu Methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Daayf, F.; Lattanzio, V.; Kroon, P.; Quideau, S.; Treutter, D.; Ralph, J.; Brunow, G.; Harris, P.; Dixon, R.; Boerjan, W. Recent Advances in Polyphenol Research, Volume 1; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Faller, A.L.K.; Fialho, E. Polyphenol Content and Antioxidant Capacity in Organic and Conventional Plant Foods. J. Food Compos. Anal. 2010, 23, 561–568. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cardinali, A.; Ruta, C.; Fortunato, I.; Lattanzio, V.; Linsalata, V.; Cicco, N. Relationship of Secondary Metabolism to Growth in Oregano (Origanum vulgare L.) Shoot Cultures under Nutritional Stress. Environ. Exp. Bot. 2009, 65, 54–62. [Google Scholar] [CrossRef]
- Lattanzio, V.; Carretto, S.; Linsalata, V.; Colella, G.; Mita, G. Signal Transduction in Artichoke [Cynara cardunculus L. subsp. scolymus (L.) Hayek] Callus and Cell Suspension Cultures under Nutritional Stress. Plant Physiol. Biochem. 2018, 127, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon Fluxes between Primary Metabolism and Phenolic Pathway in Plant Tissues under Stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef]
- Adinolfi, M.; Barone, G.; Belardw, M.; Lanzetta, R.; Laonigro, G.; Parrilli, M. Homoisoflavanones from Muscari comosum Bulbs. Phytochemistry 1985, 24, 2423–2426. [Google Scholar] [CrossRef]
- Pieroni, A.; Nebel, S.; Quave, C.; Münz, H.; Heinrich, M. Ethnopharmacology of Liakra: Traditional Weedy Vegetables of the Arbëreshë of the Vulture Area in Southern Italy. J. Ethnopharmacol. 2002, 81, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A Reproducible, Rapid and Inexpensive Folin–Ciocalteu Micro-Method in Determining Phenolics of Plant Methanol Extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Pugliese, A.; Bonesi, M.; Solimene, U.; Menichini, F. Chelating, Antioxidant and Hypoglycaemic Potential of Muscari comosum (L.) Mill. Bulb Extracts. Int. J. Food Sci. Nutr. 2010, 61, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Lee, J.-H. In-Vitro Evaluation for Antioxidant and Anti-Inflammatory Property of Flavanone Derivatives. Food Biosci. 2015, 11, 1–7. [Google Scholar] [CrossRef]
- Casacchia, T. Nutraceutical properties and health-promoting biological activities of fruits of watermelon cultivars with different origins. FARMACIA 2020, 68, 679–686. [Google Scholar] [CrossRef]
- Zhang, L.; Rocchetti, G.; Zengin, G.; AK, G.; Yildiztugay, E.; Mahomoodally, M.F.; Picot-Allain, M.K.N.; Lucini, L. Profiling of Polyphenols and Sesquiterpenoids Using Different Extraction Methods in Muscari turcicum, an Endemic Plant from Turkey. Ind. Crops Prod. 2020, 154, 112626. [Google Scholar] [CrossRef]
- Candido, V.; Castronuovo, D.; Fascetti, S.; Rosati, L.; Potenza, G. Seed-Propagated Muscari comosum (L.) Mill.: Effects of Sowing Date and Growing Conditions. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2017, 151, 484–492. [Google Scholar] [CrossRef]
- Enriquez, J.P.; Archila-Godinez, J.C. Social and Cultural Influences on Food Choices: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3698–3704. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, J. Effects of Cultural Background on Consumer Perception and Acceptability of Foods and Drinks: A Review of Latest Cross-Cultural Studies. Curr. Opin. Food Sci. 2021, 42, 248–256. [Google Scholar] [CrossRef]
- Lin, M.-P.; Marine-Roig, E.; Llonch-Molina, N. Gastronomy as a Sign of the Identity and Cultural Heritage of Tourist Destinations: A Bibliometric Analysis 2001–2020. Sustainability 2021, 13, 12531. [Google Scholar] [CrossRef]
- Lattanzio, V.; van Sumere, C.F. Changes in Phenolic Compounds during the Development and Cold Storage of Artichoke (Cynara scolymus L.) Heads. Food Chem. 1987, 24, 37–50. [Google Scholar] [CrossRef]
- Yu, L.; Perret, J.; Harris, M.; Wilson, J.; Haley, S. Antioxidant Properties of Bran Extracts from “Akron” Wheat Grown at Different Locations. J. Agric. Food Chem. 2003, 51, 1566–1570. [Google Scholar] [CrossRef]
- Mediani, A.; Abas, F.; Ping, T.C.; Khatib, A.; Lajis, N.H. Influence of Growth Stage and Season on the Antioxidant Constituents of Cosmos Caudatus. Plant Foods Hum. Nutr. 2012, 67, 344–350. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Savo, V. Perceived Health Properties of Wild and Cultivated Food Plants in Local and Popular Traditions of Italy: A Review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef]
- Wilczyńska, A. Effect of Filtration on Colour, Antioxidant Activity and Total Phenolics of Honey. LWT Food Sci. Technol. 2014, 57, 767–774. [Google Scholar] [CrossRef]
- Gorinstein, S.; Martin-Belloso, O.; Katrich, E.; Lojek, A.; Cíz, M.; Gligelmo-Miguel, N.; Haruenkit, R.; Park, Y.-S.; Jung, S.-T.; Trakhtenberg, S. Comparison of the Contents of the Main Biochemical Compounds and the Antioxidant Activity of Some Spanish Olive Oils as Determined by Four Different Radical Scavenging Tests. J. Nutr. Biochem. 2003, 14, 154–159. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic Compounds and Antioxidant Capacity of Georgia-Grown Blueberries and Blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant Genotype Affects Total Antioxidant Capacity and Phenolic Contents in Fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Gao, N.; Zanga, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Bhuyan, U.; Handique, J.G. Plant Polyphenols as Potent Antioxidants: Highlighting the Mechanism of Antioxidant Activity and Synthesis/Development of Some Polyphenol Conjugates. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 75, pp. 243–266. [Google Scholar]
- Jagtap, U.B.; Panaskar, S.N.; Bapat, V.A. Evaluation of Antioxidant Capacity and Phenol Content in Jackfruit (Artocarpus heterophyllus Lam.) Fruit Pulp. Plant Foods Hum. Nutr. 2010, 65, 99–104. [Google Scholar] [CrossRef]
- Pieroni, A.; Nebel, S.; Santoro, R.F.; Heinrich, M. Food for Two Seasons: Culinary Uses of Non-Cultivated Local Vegetables and Mushrooms in a South Italian Village. Int. J. Food Sci. Nutr. 2005, 56, 245–272. [Google Scholar] [CrossRef]





| Age (Years) |
Mean Weight (mg) | Diameter (mm) | Height (mm) | Shape Index | |
|---|---|---|---|---|---|
| Environments (E) 1 | |||||
| Open field | 2965 b | 13.6 b | 29.0 b | 2.19 c | |
| Greenhouse | 3511 a | 14.5 a | 32.9 a | 2.45 a | |
| Shaded greenhouse | 3396 a | 14.6 a | 33.5 a | 2.33 b | |
| Significance 2 | * | * | * | ** | |
| Propagation material (P) 1 | |||||
| Seed | 1 | 463 d | 7.6 d | 18.8 d | 2.48 b |
| 1-year-old “seedling bulbs” | 2 | 1808 c | 12.0 c | 32.1 c | 2.67 a |
| 2-year-old “seedling bulbs” | 3 | 4232 b | 16.7 b | 38.7 b | 2.31 c |
| 3-year-old “seedling bulbs” | 4 | 6660 a | 20.7 a | 37.5 a | 1.84 d |
| Significance 2 | ** | ** | ** | ** | |
| “E x P” Interaction | |||||
| Open field | 1 | 435 | 7.6 | 16.7 | 2.20 |
| Greenhouse | 1 | 520 | 7.5 | 22.7 | 3.03 |
| Shaded greenhouse | 1 | 433 | 7.8 | 17.0 | 2.20 |
| Open field | 2 | 1500 | 11.2 | 28.6 | 2.57 |
| Greenhouse | 2 | 2032 | 12.2 | 33.4 | 2.73 |
| Shaded greenhouse | 2 | 1893 | 12.7 | 34.4 | 2.70 |
| Open field | 3 | 3738 | 16.1 | 33.7 | 2.07 |
| Greenhouse | 3 | 4308 | 16.7 | 40.0 | 2.40 |
| Shaded greenhouse | 3 | 4650 | 17.3 | 42.3 | 2.47 |
| Open field | 4 | 6186 | 19.4 | 36.8 | 1.93 |
| Greenhouse | 4 | 7185 | 21.8 | 35.0 | 1.63 |
| Shaded greenhouse | 4 | 6608 | 20.8 | 40.3 | 1.98 |
| Significance 2 | * | ** | ** | ** | |
| LSD 3 | 41.2 | 0.60 | 1.02 | 0.13 |
| TPC (µg Chlorogenic Acid Equivalents mL−1 of Extract) | |||||
|---|---|---|---|---|---|
| Age of Bulbs (A) | Growing Environments (E) | ||||
| Greenhouse | Shaded Greenhouse | Open Field | Means 1 | Significance 2 | |
| Seed bulbs | 489 | 461 | 459 | 470 a | ** |
| 2-year-old bulbs | 298 | 356 | 282 | 312 bc | ** |
| 3-year-old bulbs | 182 | 313 | 305 | 267 c | ** |
| 4-year-old bulbs | 259 | 393 | 367 | 340 b | ** |
| Significance 2 “A x E” Interaction ** LSD 3 = 43.7 | |||||
| Means 1 | 307 b | 381 a | 353 ab | ||
| Significance 2 | ** | ** | ** | ||
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
|
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicco, N.; Castronuovo, D.; Candido, V. Antioxidant Activity, Total Phenolic Content and Morphological Traits in 1 to 4-Year-Old Muscari comosum Bulbs Cultivated in Three Growing Environments. Agriculture 2024, 14, 178. https://doi.org/10.3390/agriculture14020178
Cicco N, Castronuovo D, Candido V. Antioxidant Activity, Total Phenolic Content and Morphological Traits in 1 to 4-Year-Old Muscari comosum Bulbs Cultivated in Three Growing Environments. Agriculture. 2024; 14(2):178. https://doi.org/10.3390/agriculture14020178
Chicago/Turabian StyleCicco, Nunzia, Donato Castronuovo, and Vincenzo Candido. 2024. "Antioxidant Activity, Total Phenolic Content and Morphological Traits in 1 to 4-Year-Old Muscari comosum Bulbs Cultivated in Three Growing Environments" Agriculture 14, no. 2: 178. https://doi.org/10.3390/agriculture14020178