The Vulnerability of South African Estuaries to Climate Change: A Review and Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Key Climate Change Stressors and Associated Estuarine Responses
2.2. Delineation of Coastal Regions
3. Results and Discussion
3.1. Land Climatic and Hydrological Processes and Responses
3.1.1. Changes in Freshwater Inflows
3.1.2. Changes in Land-Sea and Alongshore Connectivity
3.1.3. Changes in Salinity Regimes
3.1.4. Changes in Biogeochemical Regimes
3.1.5. Changes in Sediment Dynamics
3.2. Ocean/Coastal Circulation and Temperature Regimes
3.3. Sea Level Rise
3.4. Increased Intensity and Frequency of Coastal Storms
3.5. Ocean Acidification
4. Conclusions
-
Land climatic/hydrological processes forcing changes in freshwater inflow and associated inputs; shifts in land-sea and longshore connectivity; modifications in salinity regimes; changes in biochemical inputs; changes in sediment deposition/erosion cycles.
-
Ocean circulation patterns resulting in shifts in temperature regimes and alongshore coastal connectivity;
-
Sea level rise and related impact on salinity regime, mouth state, and inundation of estuarine flood plain;
-
An increase in the frequency and intensity of coastal storms impacting salinity regimes and mouth state;
-
Ocean acidification amplifying existing pH fluctuations and impacting oceanic phases of estuarine species.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Environmental Affairs (DEA). South Africa’s Second National Communication under the United Nations Framework Conversion on Climate Change; Department of Environmental Affairs: Pretoria, South Africa, 2010.
- Department of Environmental Affairs (DEA). Long-Term Adaptation Scenarios Flagship Research Programme (LTAS) for South Africa. Climate Change Implications for Marine Fisheries in South Africa; Department of Environmental Affairs: Pretoria, South Africa, 2013; 60p.
- Intergovernmental Panel on Climate Change (IPCC). Special Report: Global Warming of 1.5C; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; IPCC: Geneva, Switzerland, 2019; Volume 1. [Google Scholar]
- International Panel on Climate Change (IPCC). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). 2022: Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Beckley, L.E. The ichthyofauna of the Sundays River estuary with particular reference to the juvenile marine component. Estuaries 1984, 7, 248–250. [Google Scholar] [CrossRef]
- Department of Environmental Affairs (DEA). South Africa’s Third National Communication under the United Nations Framework Convention on Climate Change; Draft Report; Department of Environmental Affairs (DEA): Pretoria, South Africa, 2017; 722p.
- Carter, T.R.; Jones, R.N.; Lu, X.; Bhadwal, S.; Conde, C.; Mearns, L.O.; O’Neill, B.C.; Rounsevell, M.D.A.; Zurek, M.B. New Assessment Methods and the Characterisation of Future Conditions. Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007; pp. 133–171. [Google Scholar]
- Jones, R.N.; Preston, B.L. Adaptation and risk management. Wiley Interdiscip. Rev. Clim. Change 2011, 2, 2. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2001: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change; McCarthy, J.J., Canziani, O.F., Leary, N.A., Dokken, D.J., White, K.S., Eds.; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report D336 of Working Groups I and II of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; 582p. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Day, J.W.; Christian, R.R.; Boesch, D.M.; Yáñez-Arancibia, A.; Morris, J.; Twilley, R.R.; Naylor, L.; Schaffner, L.; Stevenson, C. Consequences of climate change on the ecogeomorphology of coastal wetlands. Estuaries Coasts 2008, 31, 477–491. [Google Scholar] [CrossRef]
- Day, J.W.; Yáñez-Arancibia, A.; Rybczyk, J.M. Climate Change: Effects, Causes, Consequences: Physical, Hydromorphological, Ecophysiological, and Biogeographical Changes. Treatise on Estuarine and Coastal Science; Academic Press: London, UK, 2011; pp. 303–312. [Google Scholar]
- Gillanders, B.M.; Elsdon, T.S.; Halliday, I.A.; Jenkins, J.P.; Robins, J.B.; Valesini, F.J. Potential effects of climate change on Australian estuaries and fish utilising estuaries: A review. Mar. Freshw. Res. 2011, 62, 1115–1131. [Google Scholar] [CrossRef]
- Robins, P.E.; Skov, M.W.; Lewis, M.J.; Gimenez, L.; Davies, A.G.; Malham, S.K.; Neill, S.P.; McDonald, J.E.; Whitton, T.A.; Jackson, S.E.; et al. Impact of Climate Change on UK estuaries: A review of past trends and potential projections. Estuar. Coast. Shelf Sci. 2016, 169, 119–135. [Google Scholar] [CrossRef]
- Brito, A.C.; Newton, A.; Tett, P.; Fernandes, T.F. How will shallow coastal lagoons respond to climate change? A modelling investigation. Estuar. Coast. Shelf Sci. 2012, 112, 98–104. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Duong, T.M.; Uhlenbrook, S.; Roelvink, D.; Stive, M. Climate-change impact assessment for inlet-interrupted coastlines. Nat. Clim. Change 2013, 3, 83–87. [Google Scholar] [CrossRef]
- Prandle, D.; Lane, A. Sensitivity of estuaries to sea level rise: Vulnerability indices. Estuar. Coast. Shelf Sci. 2015, 160, 60–68. [Google Scholar] [CrossRef]
- Duong, T.; Ranasinghe, R.; Walstra, D.J.; Roelvink, D.J.A. Assessing Climate Change impacts on the stability of small tidal inlet systems: Why and how? Earth Sci. Rev. 2015, 154, 369–380. [Google Scholar] [CrossRef]
- Duong, T.; Ranasinghe, R.; Luijendijk, A.; Walstra, D.J.; Roelvink, D.J.A. Assessing Climate Change impacts on the stability of small tidal inlets: Part 1-Data poor environments. Mar. Geol. 2017, 390, 331–346. [Google Scholar] [CrossRef]
- Brown, S.; Nicholls, R.J.; Lowe, J.A.; Hinkel, J. Spatial variations of sea-level rise and impacts: An application of DIVA. Clim. Change 2016, 134, 403–416. [Google Scholar] [CrossRef]
- Brown, L.R.; Komoroske, L.M.; Wagner, R.W.; Morgan-King, T.; May, J.T.; Connon, R.E.; Fangue, N.A. Coupled Downscaled Climate Models and Ecophysiological Metrics Forecast Habitat Compression for an Endangered Estuarine Fish. PLoS ONE 2016, 11, e0146724. [Google Scholar] [CrossRef] [PubMed]
- House, A.R.; Thompson, J.R.; Roberts, C.; de Smeth, K.; Old, G.; Acreman, M.C. Projecting impacts of climate change on habitat availability in a macrophyte dominated chalk river. Ecohydrology 2017, 10, e1823. [Google Scholar] [CrossRef]
- Goodwin, P.; Haigh, I.D.; Rohling, E.J.; Slangen, A. A new approach to projecting 21st century sea-level changes and extremes. Earths Future 2017, 5, 240–253. [Google Scholar] [CrossRef]
- Bamunawala, J.; Dastgheib, A.; Ranasinghe, R.; van der Spek, A.; Maskey, S.; Murray, A.B.; Barnard, P.L.; Duong, T.M.; Sirisena, T.A.J.G. Probabilistic Application of an Integrated Catchment-Estuary-Coastal System Model to Assess the Evolution of Inlet-Interrupted Coasts Over the 21st Century. Front. Mar. Sci. 2020, 7, 579203. [Google Scholar] [CrossRef]
- Van Niekerk, L.; Adams, J.B.; Bate, G.C.; Forbes, A.T.; Forbes, N.T.; Huizinga, P.; Lamberth, S.J.; MacKay, C.F.; Petersen, C.; Taljaard, S.; et al. Country-wide assessment of estuary health: An approach for integrating pressures and ecosystem response in a data limited environment. Estuar. Coast. Shelf Sci. 2013, 130, 239–251. [Google Scholar] [CrossRef]
- Van Niekerk, L.; Adams, J.B.; James, N.C.; Lamberth, S.J.; Mackay, C.F.; Rajkaran, A.; Turpie, J.K.; Weerts, S.P.; Whitfield, A.K. An Estuary Ecosystem Classification that encompasses biogeography and a high diversity of types in support of protection and management. Afr. J. Aquat. Sci. 2020, 45, 199–216. [Google Scholar] [CrossRef]
- Cooper, J.A.G. Geomorphological variability among microtidal estuaries from the wave-dominated South African coast. Geomorphology 2001, 40, 99–122. [Google Scholar] [CrossRef]
- Whitfield, A.K. A characterization of southern African estuarine systems. Southern Afr. J. Aquat. Sci. 1992, 18, 89–103. [Google Scholar] [CrossRef]
- Day, J.H. Estuarine Currents, Salinities and Temperatures. In Estuarine Ecology with Particular Reference to Southern Africa; Day, J.H., Ed.; A.A. Balkema: Cape Town, South Africa, 1981; pp. 27–44. [Google Scholar]
- Raw, J.L.; Adams, J.B.; Bornman, T.G.; Riddin, T.; Vanderklift, M.A. Vulnerability to sea-level rise and the potential for restoration to enhance blue carbon sequestration in salt marshes of an urban estuary. Estuar. Coast. Shelf Sci. 2021, 260, 107495. [Google Scholar] [CrossRef]
- Reddering, J.S.V. Coastal and catchment basin controls on estuary morphology of the south-eastern Cape coast, South Africa. S. Afr. J. Sci. 1988, 84, 153–157. [Google Scholar]
- James, N.C.; Hermes, J. (Eds.) Insights into Impacts of Climate Change on the South African Marine and Coastal Environment; South African Environmental Observation Network (SAEON): Pretoria, South Africa, 2011. [Google Scholar]
- Van Niekerk, L.; Turpie, J.K. (Eds.) National Biodiversity Assessment 2011. Technical Report. Volume 3: Estuary Component; CSIR Report Number CSIR/NRE/ECOS/ER/2011/0045/B.; Council for Scientific and Industrial Research: Stellenbosch, South Africa, 2012. [Google Scholar]
- James, N.C.; van Niekerk, L.; Whitfield, A.K.; Potts, W.M.; Götz, A.; Paterson, A.W. Effects of climate change on South African estuaries and associated fish species. Clim. Res. 2013, 5, 233–248. [Google Scholar] [CrossRef]
- Driver, A.; Sink, K.J.; Nel, J.N.; Holness, S.; Van Niekerk, L.; Daniels, F.; Jonas, Z.; Majiedt, P.A.; Harris, L.; Maze, K. National Biodiversity Assessment 2011. An Assessment of South Africa’s Biodiversity and Ecosystems; Synthesis Report; Department of Environmental Affairs: Pretoria, South Africa, 2012; 180p.
- Hewitson, B.C.; Crane, R.G. Consensus between GCM Climate Change projections with empirical downscaling: Precipitation downscaling over South Africa. Int. J. Climatol. 2006, 26, 1315–1337. [Google Scholar] [CrossRef]
- Engelbrecht, F.A.; McGregor, J.L.; Engelbrecht, C. Dynamics of the conformal-cubic atmospheric model projected climate-change signal over southern Africa. Int. J. Climatol. 2009, 29, 1013–1033. [Google Scholar] [CrossRef]
- Engelbrecht, F.A.; Landman, W.A.; Engelbrecht, C.J.; Landman, S.; Bopape, M.M.; Roux, B.; McGregor, J.L.; Thatcher, M. Multi-scale climate modeling over Southern Africa using a variable-resolution global model. Water SA 2011, 37, 647–658. [Google Scholar] [CrossRef]
- Engelbrecht, C.J.; Engelbrecht, F.A.; Dyson, L. High-resolution model-projected changes in mid-tropospheric closed-lows and extreme rainfall events over southern Africa. Int. J. Climatol. 2013, 33, 173–187. [Google Scholar] [CrossRef]
- Engelbrecht, F.; Adegoke, J.; Bopape, M.-J.; Naidoo, M.; Garland, R.; Thatcher, M.; McGregor, J.; Katzfey, J.; Werner, M.; Ichoku, C.; et al. Projections of rapidly rising surface temperatures over Africa under low mitigation. Environ. Res. Lett. 2015, 10, 8. [Google Scholar] [CrossRef]
- Lumsden, T.G.; Schulze, R.E.; Hewitson, B.C. Evaluation of potential changes in hydrologically relevant statistics of rainfall in Southern Africa under conditions of Climate Change. Water SA 2009, 35, 5. [Google Scholar] [CrossRef]
- Newton, A.; Icely, J.; Cristina, S.; Brito, A.; Cardoso, A.C.; Colijn, F.; Riva, S.D.; Gertz, F.; Hansen, J.W.; Holmer, M.; et al. An overview of ecological status, vulnerability and future perspectives of European large shallow, semi-enclosed coastal systems, lagoons and transitional waters. Estuar. Coast. Shelf Sci. 2014, 140, 95–122. [Google Scholar] [CrossRef]
- Duarte, C.M.; Hendriks, I.E.; Moore, T.S.; Olsen, Y.S.; Steckbauer, A.; Ramajo, L.; Carstensen, J.; Trotter, J.A.; McCullochIs, M. Ocean Acidification an Open-Ocean Syndrome? Understanding Anthropogenic Impacts on Seawater pH. Estuaries Coasts 2013, 36, 221–236. [Google Scholar] [CrossRef]
- Vizzini, S.; Di Leonardo, R.; Costa, V.; Tramati, C.D.; Luzzu, F.; Mazzola, A. Trace element bias in the use of CO2 vents as analogues for low pH environments: Implications for contamination levels in acidified oceans. Estuar. Coast. Shelf Sci. 2013, 134, 19–30. [Google Scholar] [CrossRef]
- Kerfahi, D.; Hall-Spencer, J.M.; Tripathi, B.M.; Milazzo, M.; Lee, J.; Adams, J.M. Shallow water marine sediment bacterial community shifts along a natural CO2 gradient in the Mediterranean Sea off Vulcano, Italy. Microb. Ecol. 2014, 67, 819–828. [Google Scholar] [CrossRef]
- Milazzo, M.; Rodolfo-Metalpa, R.; San Chan, V.B.; Fine, M.; Alessi, C.; Thiyagarajan, V.; Hall-Spencer, J.M.; Chemello, R. Ocean acidification impairs vermetid reef recruitment. Sci. Rep. 2014, 4, 4189. [Google Scholar] [CrossRef]
- Ge, C.; Chai, Y.; Wang, H.; Kan, M. Ocean acidification: One potential driver of phosphorus eutrophication. Mar. Pollut. Bull. 2017, 115, 149–153. [Google Scholar] [CrossRef]
- Rees, A.P.; Turk-Kubo, K.A.; Al-Moosawi, L.; Alliouane, S.; Gazeau, F.; Hogan, M.E.; Zehr, J.P. Ocean acidification impacts on nitrogen fixation in the coastal western Mediterranean Sea. Estuar. Coast. Shelf Sci. 2017, 186, 45–57. [Google Scholar] [CrossRef]
- Laurent, A.; Fennel, K.; Cai, W.C.; Huang, W.H.; Barbero, L.; Wanninkhof, A.R. Eutrophication induced acidification of coastal waters in the northern Gulf of Mexico: Insights into origin and processes from a coupled physical-biogeochemical model. Geophys. Res. Lett. 2017, 44, 946–956. [Google Scholar] [CrossRef]
- Mvungi, E.F.; Pillay, D. Eutrophication overrides warming as a stressor for a temperate African seagrass (Zostera capensis). PLoS ONE 2019, 14, 0215129. [Google Scholar] [CrossRef]
- Van der Walt, K.A.; Porri, F.; Potts, W.M.; Duncan, M.I.; James, N.C. Thermal tolerance, safety margins and vulnerability of coastal species: Projected impact of climate change induced cold water variability in a temperate African region. Mar. Environ. Res. 2021, 169, 105346. [Google Scholar] [CrossRef]
- Jones, P.D.; Lister, D.H.; Osborne, T.J.; Harphan, C.; Salmon, M.; Morice, C.P. Hemispheric and large-scale land-surface air temperature variations: An extensive revision and an update to 2010. J. Geophys. Res. 2012, 117, D05127. [Google Scholar] [CrossRef]
- Kruger, A.C.; Sekele, S. Trends in extreme temperature indices in South Africa: 1962–2009. Int. J. Climatol. 2012, 33, 661–676. [Google Scholar] [CrossRef]
- MacKellar, N.; New, M.; Jack, C. Observed and modelled trends in rainfall and temperature for South Africa: 1960–2010. S. Afr. J. Sci. 2014, 10, 13. [Google Scholar] [CrossRef]
- Kruger, A.C.; Nxumalo, M. Surface Temperature Trends from Homogenised Time Series in South Africa: 1931–2015. Int. J. Climatol. 2017, 37, 2364–2377. [Google Scholar] [CrossRef]
- Hewitson, B.; Tadross, M.; Jack, C. Scenarios from the University of Cape Town. In Climate Change and Water Resources in Southern Africa: Studies on Scenarios, Impacts, Vulnerabilities and Adaptation; WRC Report, 1430/1/05; Schulze, R.E., Ed.; Water Research Commission: Pretoria, South Africa, 2005; pp. 39–56. [Google Scholar]
- Cai, W. Antarctic ozone depletion causes an intensification of the Southern Ocean super-gyre circulation. Geophys. Res. Lett. 2006, 33, L03712. [Google Scholar] [CrossRef]
- Saenko, O.A.; Fyfe, J.C.; England, M.H. On the response of the oceanic wind-driven circulation to atmospheric CO2 increase. Clim. Dyn 2005, 25, 415–426. [Google Scholar] [CrossRef]
- Roemich, D. Physical Oceanography: Super spin in the southern seas. Nature 2007, 449, 34–35. [Google Scholar] [CrossRef]
- Hagen, B.; Feistel, R.; Agenbag, J.J.; Ohde, T. Seasonal and interannual changes in Intense Benguela Upwelling (1982–1999). Oceanol. Acta 2001, 24, 557–568. [Google Scholar] [CrossRef]
- Lutjeharms, J.R.; Monteiro, P.M.S.; Tyson, P.D.; Obura, D. The oceans around southern Africa and regional effects of global change. S. Afr. J. Sci. 2001, 97, 119–130. [Google Scholar]
- Bakun, A.; Field, D.B.; Rodriguez, A.R.; Weeks, S.J. Greenhouse gas, upwelling-favorable winds, and the future of coastal ocean upwelling ecosystems. Glob. Chang. Biol. 2010, 16, 1213–1228. [Google Scholar] [CrossRef]
- Rouault, M.; Pohl, B.; Penven, P. Coastal oceanic Climate Change and variability from 1982 to 2009 around South Africa. Afr. J. Mar. Sci. 2010, 32, 237–246. [Google Scholar] [CrossRef]
- Dufois, F.; Rouault, M. Sea surface temperature in False Bay (South Africa): Towards a better understanding of its seasonal and inter-annual variability. Cont. Shelf Res. 2012, 43, 24–35. [Google Scholar] [CrossRef]
- Beal, L.M.; De Ruijter, W.P.M.; Biastoch, A.; Zahn, R. On the role of the Agulhas system in ocean circulation and climate. Nature 2011, 472, 429–436. [Google Scholar] [CrossRef]
- Gründlingh, M.L. On the Course of the Agulhas Current. S. Afr. Geogr. J. 1983, 65, 49–57. [Google Scholar] [CrossRef]
- Bryden, H.L.; Beal, L.M.; Duncan, L.M. Structure and transport of the Agulhas Current and its temporal variability. J. Oceanogr. 2005, 61, 479–492. [Google Scholar] [CrossRef]
- Goschen, W.S.; Schumann, E.H. Agulhas current variability and inshore structures off the Cape Province, South Africa. J. Geophys. Res. 1990, 95, 667–678. [Google Scholar] [CrossRef]
- Rouault, M.J.; Penven, P. New perspectives on Natal Pulses from satellite observations. J. Geophys. Res. 2011, 116, C07013. [Google Scholar]
- Krug, M.; Tournadre, J.; Dufois, F. Interactions between the Agulhas Current and the eastern margin of the Agulhas Bank. Cont. Shelf Res. 2014, 81, 67–79. [Google Scholar] [CrossRef]
- Beal, L.M.; Elipot, S.; Houk, A.; Leber, G.M. Capturing the Transport Variability of a Western Boundary Jet: Results from the Agulhas Current Time-Series Experiment (ACT). J. Phys. Oceanogr. 2015, 45, 1302–1324. [Google Scholar] [CrossRef]
- Lutjeharms, J.R.E.; van Ballegooyen, R.C. Anomalous Upstream Retroflection in the Agulhas Current. Science 1988, 240, 1770. [Google Scholar] [CrossRef]
- Van Leeuwen, P.J.; de Ruijter, W.P.M.; Lutjeharms, J.R.E. Natal pulses and the formation of Agulhas rings. J. Geophys. Res. 2000, 105, 6425–6436. [Google Scholar] [CrossRef]
- Beal, L.M.; Elipot, S. Broadening not strengthening of the Agulhas Current since the early 1990s. Nature 2016, 540, 570–573. [Google Scholar] [CrossRef]
- Backeberg, B.C.; Penven, P.; Rouault, M. Impact of intensified Indian Ocean winds on mesoscale variability in the Agulhas system. Nat. Clim. Chang. 2012, 2, 608–612. [Google Scholar] [CrossRef]
- Cai, W.; Cowan, T.; Dix, M.; Rotstayn, L.; Ribbe, J.; Shi, G.; Wijffels, S. Anthropogenic aerosol forcing and the structure of temperature trends in the southern Indian Ocean. Geophys. Res. Lett. 2007, 34, L14611. [Google Scholar] [CrossRef]
- Rouault, M.; Penven, P.; Pohl, B. Warming in the Agulhas Current system since the 1980′s. Geophys. Res. Lett. 2009, 36, L12602. [Google Scholar] [CrossRef]
- Gordon, A.L.; Lutjeharms, J.R.E.; Gründlingh, M.L. Stratification and circulation at the Agulhas Retroflection. Deep Sea Res. 1987, 34, 565–599. [Google Scholar] [CrossRef]
- Matano, R.P.; Beier, E.J. A kinematic analysis of the Indian/Atlantic interocean exchange. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 229–249. [Google Scholar] [CrossRef]
- Duncombe Rae, C.M.; Boyd, A.J.; Crawford, R.J.M. “Predation” on anchovy by an Agulhas Ring: A possible contributory cause of the poor year-class of 1989. S. Afr. J. Mar. Sci. 1992, 12, 167–174. [Google Scholar] [CrossRef]
- Mather, A.A.; Garland, G.G.; Stretch, D.D. Southern African sea levels: Corrections, influences and trends. Afr. J. Mar. Sci. 2009, 31, 2. [Google Scholar] [CrossRef]
- Mather, A.A.; Stretch, D.D. A perspective on sea level rise and coastal storm surge from southern and eastern Africa: A case study near Durban, South Africa. Water 2012, 4, 237–259. [Google Scholar] [CrossRef]
- DeConto, R.M.; Pollard, D.; Alley, R.B.; Velicogna, I.; Gasson, E.; Gomez, N.; Sadai, S.; Condron, A.; Gilford, D.M.; Ashe, E.L.; et al. The Paris Climate Agreement and future sea-level rise. Nature 2021, 593, 83–89. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). AR5 SPM. Approved Summary for Policymakers. IPClimate Change WGI AR5 SPM 27 September 2013. Available online: http://www.climatechange2013.org/images/uploads/WGIAR5-SPM_Approved27Sep2013.pdf (accessed on 8 October 2013).
- Church, J.A.; Clark, P.U.; Cazenave, A.; Gregory, J.M.; Jevrejeva, S.; Levermann, A.; Merrifield, M.A.; Milne, G.A.; Nerem, R.S.; Nunn, P.D.; et al. Sea Level Change. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Rahmstorf, S.; Cazenave, A.; Church, J.A.; Hansen, J.E.; Keeling, R.F.; Parker, D.E.; Somerville, R.C.J. Recent Climate Observations Compared to Projections. Science 2007, 316, 709. [Google Scholar] [CrossRef]
- Pfeffer, W.T.; Harper, J.T.; O’Neel, S. Kinematic constraints on glacier contributions to 21st-century sea-level rise. Science 2008, 321, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.A.; Gehrels, R.W.; Hughes, C.W.; Tamisiea, M.E. Identifying the Causes of Sea-level Change-Sea Level Review Postscript. In Nature Geoscience Letters; Macmillan Publishers Limited: New York, NY, USA, 2009. [Google Scholar]
- Rossouw, M.; Theron, A.K. Aspects of Potential Climate Change Impacts on Ports & Maritime Operations around the Southern African Coast. In Proceedings of the Proceedings-UNCTAD Intergovernmental Expert Meeting on “Maritime Transport and the Climate Change Challenge”, First Expert Meeting on Climate Change and Maritime Transport Issues, Geneve, Switzerland, 16–18 February 2009. [Google Scholar]
- Nicholls, R.J.; Cazenave, A. Sea-Level Rise and Its Impact on Coastal Zones. Science 2010, 328, 1517. [Google Scholar] [CrossRef] [PubMed]
- SWIPA. Snow, Water, Ice and Permafrost in the Arctic (SWIPA). In Proceedings of the Assessment-Coordinated by AMAP and Produced in Collaboration with IASC, WMO/Clic and IASSA-Executive Summary, Nuuk, Greenland, 12 May 2011. [Google Scholar]
- Theron, A.K.; Barwell, L.B.; Rossouw, M.; Maherry, A. Coastal Planning and Adaptation to Mitigate Climate Change Impacts-Responding to Climate Change in Mozambique (Phase II, Theme 2); Report prepared for National Institute for Disaster Management (INGC) by CSIR; CSIR: Stellenbosch, South Africa, 2012; p. 266. [Google Scholar]
- Kopp, R.E.; DeConto, R.M.; Bader, D.A.; Hay, C.C.; Horton, R.M.; Kulp, S.; Oppenheimer, M.; Pollard, D.; Strauss, B.H. Evolving Understanding of Antarctic Ice-Sheet Physics and Ambiguity in Probabilistic Sea-Level Projections. Earths Future 2017, 5, 1217–1233. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S.D., Qin, M., Manning, Z., Chen, M., Marquis, K.B., Averyt, M., Miller, T., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Wang, X.L.; Zwiers, F.W.; Swail, V.R. North Atlantic Ocean Wave Climate Change Scenarios for the Twenty-First Century. J. Clim. 2004, 17, 2368–2383. [Google Scholar] [CrossRef]
- Komar, P.D.; Allan, J.C. Increasing Hurricane-Generated Wave Heights along the U.S. East Coast and Their Climate Controls. J. Coast. Res. 2008, 24, 2. [Google Scholar] [CrossRef]
- Ruggiero, P.; Komar, P.D.; Allan, J.C. Increasing Wave Heights and Extreme Value Projections: The Wave Climate of the U.S. Pacific Northwest. Coast. Eng. 2010, 57, 539–552. [Google Scholar] [CrossRef]
- Guastella, L.A.; Rossouw, M. What will be the impact of increasing frequency and intensity of coastal storms along the South African coast? Reef J. 2012, 2, 129–139. [Google Scholar]
- Harris, L.R. The Ecological Implications of Sea-Level Rise and Storms for Sandy Beaches in KwaZulu-Natal. Master’s Thesis, University of KwaZulu-Natal, Durban, South Africa, 2008. Available online: http://hdl.handle.net/10413/460 (accessed on 1 September 2019).
- Canadell, J.G.; Le Quere, C.; Raupach, M.R.; Field, C.B.; Buitenhuis, E.T.; Ciais, P.; Conway, T.J.; Gillett, N.P.; Houghton, R.A.; Marland, G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc. Natl. Acad. Sci. USA 2007, 104, 18866–18870. [Google Scholar] [CrossRef]
- Le Quéré, C.; Raupach, M.R.; Canadell, J.G.; Marland, G.; Bopp, L.; Ciais, P.; Conway, T.J.; Doney, S.C.; Feely, R.A.; Foster, P.; et al. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009, 2, 831–836. [Google Scholar] [CrossRef]
- Feely, R.A.; Alin, S.A.; Newton, J.; Sabine, C.L.; Warner, M.; Devol, M.; Krembs, C.; Maloy, C. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar. Coast. Shelf Sci. 2010, 88, 442–449. [Google Scholar] [CrossRef]
- Feely, R.A.; Sabine, C.L.; Lee, K.; Berelson, W.; Kleypas, J.; Fabry, V.J.; Millero, F.J. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Feely, R.A.; Doney, S.C.; Cooley, S.R. Ocean acidification: Present conditions and future changes in a high-CO2 world. Oceanography 2009, 22, 36–47. [Google Scholar] [CrossRef]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidification: The other CO2 problem. Ann. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef]
- Hauri, C.; Gruber, N.; Vogt, M.; Doney, S.C.; Feely, R.A.; Lachkar, Z.; Leinweber, A.; McDonnell, A.M.P.; Munnich, M.; Plattner, G.K. Spatiotemporal variability and long-term trends of ocean acidification in the California Current System. Biogeosciences 2013, 10, 193–216. [Google Scholar] [CrossRef]
- Van Niekerk, L.; Taljaard, S.; Lamberth, S.J.; Adams, J.B.; Weerts, S.P.; MacKay, F. Disaggregation and assessment of estuarine pressures at the country-level to better inform management and resource protection-the South African experience. Afr. J. Aquat. Sci. 2022, 47, 127–148. [Google Scholar] [CrossRef]
- Emanuel, B.P.; Bustamante, R.H.; Branch, G.M.; Eekhout, S.; Odendal, F.J. A zoogeographic and functional approach to the selection of marine reserves on the west coast of South Africa. South Afr. J. Mar. Sci. 1992, 12, 341–354. [Google Scholar] [CrossRef]
- Harrison, T.D. Preliminary assessment of the biogeography of fishes in South African estuaries. Mar. Freshw. Res. 2002, 53, 479–490. [Google Scholar] [CrossRef]
- Turpie, J.K.; Beckley, L.E.; Kauta, S.M. Biogeography and the selection of priority areas for conservation of South African coastal fishes. Biol. Conserv. 2000, 92, 59–72. [Google Scholar] [CrossRef]
- Reddering, J.S.V.; Rust, I.C. Historical changes and sedimentary characteristics of southern African estuaries. S. Afr. J. Sci. 1990, 86, 425–428. [Google Scholar]
- Jezewski, W.A.; Pyke, P.D.; Roberts, C.P.R. Estuarine and Lake Freshwater Requirements; Technical Report No TR123; Department of Water Affairs and Forestry: Pretoria, South Africa, 1984.
- Davies, B.; Day, J. Vanishing Waters; University of Cape Town Press: Cape Town, South Africa, 1998. [Google Scholar]
- Schulze, R.E.; Lynch, S.D. Annual Precipitation. In South African Atlas of Climatology and Agrohydrology; WRC Report 1489/1/06, Section 6.2.; Schulze, R.E., Ed.; Water Research Commission: Pretoria, South Africa, 2007. [Google Scholar]
- Schulze, R.E.; Lumsden, T.G.; Horan, M.J.C.; Warburton, M.; Maharaj, M. An Assessment of Impacts of Climate Change on Agrohydrological Responses Over Southern Africa. In Climate Change and Water Resources in Southern Africa: Studies on Scenarios, Impacts, Vulnerabilities and Adaptation; WRC Report 1430/1/05; Schulze, R.E., Ed.; Water Research Commission: Pretoria, South Africa, 2005; pp. 141–189. [Google Scholar]
- Dieppois, B.; Rouault, M.; New, M. The impact of ENSO on Southern African rainfall in CMIP5 ocean atmosphere coupled climate models. Clim. Dyn. 2015, 45, 2425–2442. [Google Scholar] [CrossRef]
- Kruger, A.C. Observed trends in daily precipitation indices in South Africa: 1910–2004. Int. J. Climatol. 2006, 26, 2275–2286. [Google Scholar] [CrossRef]
- Christensen, J.H.; Hewitson, B.; Busuioc, A.; Chen, A.; Gao, X.; Held, I.; Jones, R.; Kolli, R.K.; Kwon, W.-T.; Laprise, R.; et al. Regional Climate Projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Engelbrecht, F.A. The Physical Basis for Climate Change over Southern Africa. In Climate Change and Trade: The Challenges for Southern Africa; Taylor & Francis: Oxfordshire, UK, 2010; ISBN 978-1-920196-28-8. [Google Scholar]
- Malherbe, J.; Landman, W.A.; Engelbrecht, F.A. The bi-decadal rainfall cycle, Southern Annular. Mode and tropical cyclones over the Limpopo River Basin, southern Africa. Clim. Dyn. 2013, 43, 3121–3138. [Google Scholar] [CrossRef]
- Alber, M. A conceptual model of estuarine freshwater inflow management. Estuaries 2002, 25, 1246–1261. [Google Scholar] [CrossRef]
- USEPA. Synthesis of Adaptation Options for Coastal Areas; Climate Ready Estuaries Program. EPA 430-F-08-024; Environmental Protection Agency: Washington, DC, USA, 2009.
- Bunn, S.E. Grand challenge for the future of freshwater ecosystems. Front. Environ. Sci. 2016, 4, 21. [Google Scholar] [CrossRef]
- South African Weather Services (SAWS). A Climate Change Reference Atlas based on CMIP5–CORDEX Downscaling; Water Research Commission Report; SAWS: Pretoria, South Africa, 2017. [Google Scholar]
- Van Niekerk, L.; Taljaard, S.; Adams, J.B.; Lamberth, S.J.; Huizinga, P.; Turpie, J.K.; Wooldridge, T.H. An environmental flow determination method for integrating multiple-scale ecohydrological and complex ecosystem processes in estuaries. Sci. Total Environ. 2019, 656, 482–494. [Google Scholar] [CrossRef]
- Froneman, P.W.; Vorwerk, P.D. Response of the plankton to a freshwater pulse in a fresh water deprived, permanently open South African estuary. J. Water Resour. Prot. 2013, 5, 405–413. [Google Scholar] [CrossRef]
- Nodo, P.; James, N.C.; Childs, A.-R.; Naikin, M. Response of demersal fish assemblages to an extreme flood event in a freshwater deprived estuary, South Africa. Mar. Freshw. Res. 2018, 69, 253–266. [Google Scholar] [CrossRef]
- Vorwerk, P.D.; Froneman, P.W.; Paterson, A.W.; Whitfield, A.K. Fish community response to increased river flow in the Kariega Estuary, a freshwater-deprived, permanently open southern African system. Afr. J. Aquat. Sci. 2008, 33, 189–200. [Google Scholar] [CrossRef]
- James, N.C.; Lamberth, S.J.; Midgley, C.; Whitfield, A.K. Resilience of fish assemblages in the Breede Estuary, South Africa, to environmental perturbations. Environ. Biol. Fishes 2018, 101, 109–126. [Google Scholar] [CrossRef]
- James, N.C.; Adams, J.B.; Connell, A.D.; Lamberth, S.J.; MacKay, C.F.; Snow, G.; Van Niekerk, L.; Whitfield, A.K. High flow variability and storm events shape the ecology of the Mbhashe Estuary, South Africa. Afr. J. Aquat. Sci. 2020, 45, 131–151. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Adams, J.B.; Bate, G.C.; Bezuidenhout, K.; Bornman, T.G.; Cowley, P.D.; Froneman, P.W.; Gama, P.T.; James, N.; Mackenzie, B.; et al. A multidisciplinary study of a small, temporarily open/closed South African estuary, with particular emphasis of the influence of mouth state on the ecology of the system. Afr. J. Mar. Sci. 2008, 30, 453–473. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Morgenthal, T.L.; Malherbe, J.; Pretorius, D.J.; Sumner, P.D. Water erosion prediction at a national scale in South Africa. Water SA 2008, 34, 305–314. [Google Scholar] [CrossRef]
- Nozaisa, C.; Perissinottoa, R.; Titac, G. Seasonal dynamics of meiofauna in a South African temporarily open/closed estuary (Mdloti Estuary, Indian Ocean). Estuar. Coast. Shelf Sci. 2005, 62, 325–338. [Google Scholar] [CrossRef]
- Thomas, C.M.; Perissinotto, R.; Kibirige, I. Phytoplankton biomass and size structure in two South African eutrophic, temporarily open/closed estuaries. Estuar. Coast. Shelf Sci. 2005, 65, 223–238. [Google Scholar] [CrossRef]
- Riddin, T.; Adams, J.B. Influence of mouth status and water level on the macrophytes in a small temporarily open/closed estuary. Estuar. Coast. Shelf Sci. 2008, 79, 86–92. [Google Scholar] [CrossRef]
- Riddin, T.; Adams, J.B. The effect of a storm surge event on the macrophytes of a temporarily open/closed estuary, South Africa. Estuar. Coast. Shelf Sci. 2010, 89, 119–123. [Google Scholar] [CrossRef]
- Riddin, T.; Adams, J.B. Predicting macrophyte states in a small temporarily open/closed estuary. Mar. Freshw. Res. 2012, 63, 616–623. [Google Scholar] [CrossRef]
- James, N.C.; Cowley, P.D.; Whitfield, A.K. The marine fish assemblage of the East Kleinemonde Estuary over 20 years: Declining abundance and nursery function? Estuar. Coast. Shelf Sci. 2018, 214, 64–71. [Google Scholar] [CrossRef]
- Wooldridge, T.H.; Loubser, H. Larval release rhythms and tidal exchange in the estuarine mudprawn, Upogebia africana. Hydrobiologia 1996, 337, 113–121. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Wooldridge, T.H. Small-scale distribution and variability of demersal zooplankton in a shallow, temperate estuary: Tidal and depth effects on species-specific heterogeneity. Cah. Biol. Mar. 1996, 36, 211–227. [Google Scholar]
- Vorwerk, P.D.; Whitfield, A.K.; Cowley, P.D.; Paterson, A.W. The influence of selected environmental variables on fish assemblage structure in a range of southeast African estuaries. Environ. Biol. Fishes. 2003, 66, 237–247. [Google Scholar] [CrossRef]
- Department of Environment, Forestry and Fisheries South (DEFF). Status of the South African Marine Fishery Resources; DEFF: Cape Town, South Africa, 2020. [Google Scholar]
- Blaber, S.J.M. Population size and mortality of juveniles of the marine teleost Rhabdosargus holubi (Pisces: Sparidae) in a closed estuary. Mar. Biol. 1973, 21, 219–225. [Google Scholar] [CrossRef]
- Cowley, P.D.; Whitfield, A.K. Biomass production estimates of a fish community in a small South African estuary. J. Fish Biol. 2002, 61, 74–89. [Google Scholar] [CrossRef]
- Von der Heyden, S.; Toms, J.; Teske, P.; Lamberth, S.; Holleman, W. Contrasting signals of genetic diversity and historical demography between two recently diverged marine and estuarine fish species. Mar. Ecol. Prog. Ser. 2015, 526, 157–167. [Google Scholar] [CrossRef]
- Bate, G.C.; Adams, J.B. The effects of a single freshwater release into the Kromme Estuary. 5. Overview and interpretation for the future. Water SA 2000, 26, 329–332. [Google Scholar]
- Bate, G.C.; Whitfield, A.K.; Adams, J.B.; Huizinga, P.; Wooldridge, T.H. The importance of the river-estuary interface zone in estuaries. Water SA 2002, 28, 271–279. [Google Scholar] [CrossRef]
- Whitfield, A.K. Distribution patterns of fishes in a freshwater deprived Eastern Cape estuary, with particular emphasis on the geographical headwater region. Water SA 2003, 29, 61–67. [Google Scholar] [CrossRef]
- Whitfield, A.K. Fishes and freshwater in South African estuaries–A review. Aquat. Living Resour. 2005, 18, 275–289. [Google Scholar] [CrossRef]
- Bennett, B.A. A mass mortality of fish associated with low salinity conditions in the Bot River estuary. Trans. R. Soc. S. Afr. 1985, 45, 437–447. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Taylor, R.H.; Fox, C.; Cyrus, D.P. Fishes and salinities in the St Lucia estuarine system—A Review. Rev. Fish. Biol. Fisheries 2006, 16, 1–20. [Google Scholar] [CrossRef]
- Taljaard, S.; Snow, G.; Gama, P.; Van Niekerk, L. Verification of a conceptual model of water quality for small temporarily open/closed estuaries: East Kleinemonde Estuary, South Africa. Mar. Freshw. Res. 2009, 60, 234–245. [Google Scholar] [CrossRef]
- Taljaard, S.; Van Niekerk, L.; Joubert, W. Extension of a qualitative model on nutrient cycling and transformation to include microtidal estuaries on wave-dominated coasts: Southern Hemisphere perspective. Estuar. Coast. Shelf Sci. 2009, 85, 407–421. [Google Scholar] [CrossRef]
- McCallister, S.; Bauer, L.; Ducklow, J.E.; Canuel, E.A. Sources of estuarine dissolved and particulate organic matter: A multi-tracer approach. Org. Geochem. 2006, 37, 454–468. [Google Scholar] [CrossRef]
- De Villiers, S.; Thiart, C. The nutrient status of South African rivers: Trends and fluxes from the 1970s to 2005. S. Afr. J. Sci. 2007, 103, 343–349. [Google Scholar]
- He, D.; Mead, R.N.; Belicka, L.; Pisani, O.; Jaffe, R. Assessing source contributions to particulate organic matter in a subtropical estuary: A biomarker approach. Org. Geochem. 2014, 75, 129–139. [Google Scholar] [CrossRef]
- Lemley, D.A.; Adams, J.B.; Strydom, N.A. Testing the efficacy of an estuarine eutrophic condition index: Does it account for shifts in flow conditions? Ecol. Indic. 2017, 74, 357–370. [Google Scholar] [CrossRef]
- Omarjee, A.; Taljaard, S.; Ramjukadh, C.-L.; van Niekerk, L. pH variability in catchment flows to estuaries–A South African perspective. Estuar. Coast. Shelf Sci. 2021, 262, 107605. [Google Scholar] [CrossRef]
- Midgley, J.; Shafer, G. Correlates of water colour in streams rising in the southern Cape catchments vegetated by fynbos and/or forest. Water SA 1992, 18, 93–100. [Google Scholar]
- Adams, J.B.; Taljaard, S.; Van Niekerk, L.; Lemley, D.A. Nutrient enrichment as a threat to the ecological resilience and health of microtidal estuaries. Afr. J. Aquat. Sci. 2020, 45, 23–40. [Google Scholar] [CrossRef]
- Strydom, N.A.; Whitfield, A.K. The effects of a single freshwater release into the Kromme Estuary. 4: Larval fish response. Water SA 2000, 26, 319–328. [Google Scholar]
- Snow, G.C.; Adams, J.B. Response of micro-algae in the Kromme Estuary to managed freshwater inputs. Water SA 2006, 32, 71–80. [Google Scholar] [CrossRef]
- Strydom, N.A. Patterns in Larval Fish Diversity, Abundance, and Distribution in Temperate South African Estuaries. Estuaries Coasts 2015, 38, 268–284. [Google Scholar] [CrossRef]
- Lemley, D.A.; Adams, J.B.; Taljaard, S.; Strydom, N.A. Towards the classification of eutrophic condition in estuaries. Estuar. Coast. Shelf Sci. 2015, 164, 221–232. [Google Scholar] [CrossRef]
- Justic, D.; Bierman, V.J.; Scavia, D.; Hetland, R.D. Forecasting Gulf’s Hypoxia: The Next 50 Years? Estuaries Coasts 2007, 30, 791–801. [Google Scholar] [CrossRef]
- O’Boyle, S.; McDermott, G.; Noklegaard, T.; Wilkes, R. A Simple Index of Trophic Status in Estuaries and Coastal Bays Based on Measurements of pH and Dissolved Oxygen. Estuaries Coasts 2013, 36, 158–173. [Google Scholar] [CrossRef]
- Wallace, R.B.; Baumann, H.; Grear, J.S.; Aller, R.C.; Gobler, C.J. Coastal ocean acidification: The other eutrophication problem. Estuar. Coast. Shelf Sci. 2014, 148, 1–13. [Google Scholar] [CrossRef]
- Kennedy, V.S.; Twilley, R.R.; Kleypas, J.A.; Cowan, J.H., Jr.; Hare, S.R. Coastal and Marine Ecosystems and Global Climate Change: Potential Effects on U.S. Resources; Pew Center on Global Climate Change: Arlington, VA, USA, 2002; 52p. [Google Scholar]
- Schiedek, D.; Sundelin, B.; Readman, J.W.; Macdonald, R.W. Interactions between climate change and contaminants. Mar. Pollut. Bull. 2007, 54, 1845–1856. [Google Scholar] [CrossRef]
- Whitfield, A.K. Mass mortalities of fish in South African estuaries. S. Afr. J. Aquat. Sci. 1995, 21, 29–34. [Google Scholar] [CrossRef]
- Harrison, T.D.; Whitfield, A.K. A multi-metric fish index to assess the environmental condition of estuaries. J. Fish Biol. 2004, 65, 683–710. [Google Scholar] [CrossRef]
- Whitfield, A.K. Biology and ecology of fishes in Southern African estuaries. Ichthyol. Monogr. J.L.B. Smith Inst. Ichthyol. 1998, 2, 1–223. [Google Scholar]
- Human, L.R.D.; Snow, G.C.; Adams, J.B.; Bate, G.C.; Yang, S.-C. The role of submerged macrophytes and macroalgae in nutrient cycling: A budget approach. Estuar. Coast. Shelf Sci. 2015, 154, 169–178. [Google Scholar] [CrossRef]
- Morant, P.; Quinn, N.W. Influence of man and management of South African estuaries. In Estuaries of South Africa; Allanson, B.R., Baird, D., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 289–321. [Google Scholar]
- Skowno, A.L.; Poole, C.J.; Raimondo, D.C.; Sink, K.J.; Van Deventer, H.; Van Niekerk, L.; Harris, L.R.; Smith-Adao, L.B.; Tolley, K.A.; Zengeya, T.A.; et al. National Biodiversity Assessment 2018: The Status of South Africa’s Ecosystems and Biodiversity; Synthesis Report; South African National Biodiversity Institute, Department of Environment, Forestry and Fisheries: Pretoria, South Africa, 2019.
- Adams, J.B.; Rajkaran, A. Changes in mangroves at their southernmost African distribution limit. Estuar. Coast. Shelf Sci. 2021, 248, 107158. [Google Scholar] [CrossRef]
- Friess, D.A.; Adame, F.M.; Adams, J.B.; Lovelock, C.E. 2022. Mangrove forests under climate change in a 2 °C world. Wiley Interdiscip. Rev. Clim. Change 2022, 13, e792. [Google Scholar] [CrossRef]
- Schlegel, R.W.; Smit, A.J. Climate Change in Coastal Waters: Time Series Properties Affecting Trend Estimation. J. Clim. 2016, 29, 9113–9124. [Google Scholar] [CrossRef]
- Lima, F.; Wethey, D. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat. Commun. 2012, 3, 704. [Google Scholar] [CrossRef]
- Wooldridge, T.H.; Deyzel, S.H.P. Variability in estuarine water temperature gradients and influence on the distribution of zooplankton: A biogeographical perspective. Afr. J. Mar. Sci. 2012, 34, 465–477. [Google Scholar] [CrossRef]
- Veitch, J.; Penven, P.; Shillington, F. Modeling equilibrium dynamics of the Benguela Current System. J. Phys. Oceanogr. 2010, 40, 1942–1964. [Google Scholar] [CrossRef]
- Veitch, J.; Hermes, J.; Lamont, T.; Penven, P.; Dufois, F. Shelf-edge jet currents in the southern Benguela: A modelling approach. J. Mar. Syst. 2018, 188, 27–38. [Google Scholar] [CrossRef]
- Lamont, T.; Garcia-Reyes, M.; Bograd, S.J.; van der Lingen, C.D.; Sydeman, W.J. Upwelling indices for comparative ecosystem studies: Variability in the Benguela Upwelling System. J. Mar. Syst. 2018, 118, 3–16. [Google Scholar] [CrossRef]
- Duncan, M.I.; James, N.C.; Bates, A.E.; Goschen, W.S.; Potts, W.M. Localised intermittent upwelling intensity has increased along South Africa’s south coast due to el Niño-Southern Oscillation phase state. Afr. J. Mar. Sci. 2019, 41, 325–330. [Google Scholar] [CrossRef]
- Tomety, F.S. Coastal Climate Change and Variability in the Benguela Current System. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2021. Available online: http://sillig.free.fr/Publis/Tomety_2022.pdf (accessed on 20 July 2022).
- Lima, D.C.A.; Soares, P.M.M.; Semedo, A.; Cardoso, R.M.; Cabos, W.; Sein, D.V. How will a warming climate affect the Benguela coastal low-level wind jet? J. Geophys. Res. Atmos. 2019, 124, 5010–5028. [Google Scholar] [CrossRef]
- Wu, L.; Cai, W.; Zhang, L.; Nakamura, H.; Timmermann, A.; Joyce, T.; McPhaden, M.J.; Alexander, M.; Qiu, B.; Visbeck, M.; et al. Enhanced warming over the global subtropical western boundary currents. Nat. Clim. Change 2012, 2, 161–166. [Google Scholar] [CrossRef]
- Yang, H.; Lohmann, G.; Wei, W.; Dima, M.; Ionita, M.; Liu, J. Intensification and poleward shift of subtropical western boundary currents in a warming climate. J. Geophys. Res. Oceans. 2016, 121, 4928–4945. [Google Scholar] [CrossRef]
- Malan, N.; Backeberg, B.; Biastoch, A.; Durgadoo, J.V.; Samuelsen, A.; Reason, C.; Hermes, J. Agulhas Current Meanders facilitate shelf-slope exchange on the Eastern Agulhas Bank. J. Geophys. Res. Oceans 2018, 123, 4762–4778. [Google Scholar] [CrossRef]
- Yang, C.; Leonelli, F.E.; Marullo, S.; Artale, V.; Beggs, H.; Bunogiorno Nardelli, B.; Chin, T.M.; De Toma, V.; Good, S.; Huang, B.; et al. Sea surface temperature intercomparison in the framework of the Copernicus Climate Change Service (C3S). J. Clim. 2021, 34, 5257–5283. [Google Scholar] [CrossRef]
- Rijnsdorp, A.D.; Peck, M.A.; Engelhard, G.H.; Möllmann, C.; Pinnegar, J.K. Resolving the effect of Climate Change on fish populations. ICES J. Mar. Sci. 2009, 66, 1–14. [Google Scholar] [CrossRef]
- Blamey, L.K.; Shannon, L.J.; Bolton, J.J.; Crawford, R.J.M.; Dufois, F.; Evers-King, H.; Griffith, C.L.; Hutchings, L.; Jarre, A.; Rouault, M.; et al. Ecosystem change in the southern Benguela and the underlying processes. J. Mar. Syst. 2015, 144, 9–29. [Google Scholar] [CrossRef]
- Whitfield, A.K.; James, N.C.; Lamberth, S.J.; Adams, J.B.; Perissinotto, R.; Rajkaran, A.; Bornman, T.G. The role of pioneers as indicators of biogeographic range expansion caused by global change in southern African coastal waters. Estuar. Coast. Shelf Sci. 2016, 172, 138–153. [Google Scholar] [CrossRef]
- Maree, R.C.; Whitfield, A.K.; Booth, A.J. Effect of water temperature on the biogeography of South African estuarine fish species associated with the subtropical/warm temperate subtraction zone. S. Afr. J. Sci. 2002, 96, 184–188. [Google Scholar]
- Elliott, M. An overview of the status, study and management of fishes in estuaries. In Fishes in estuaries; Elliott, M., Hemingway, K.L., Eds.; Blackwell Science Ltd.: Oxford, UK, 2002; pp. 553–575. [Google Scholar]
- Harrison, T.D.; Whitfield, A.K. Temperature and salinity as primary determinants influencing the biogeography of fishes in South African estuaries. Estuar. Coast. Shelf Sci. 2006, 66, 335–345. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Hofmann, G.E. Changes in latitudes, changes in aptitudes: Nucella canaliculata (Mollusca: Gastropoda) is more stressed at its range edge. Mar. Ecol. Prog. Ser. 2004, 274, 263–268. [Google Scholar] [CrossRef]
- Murawski, S.A. Climate Change and marine fish distributions: Forecasting from historical analogy. Trans. Am. Fish. Soc. 1993, 122, 647–657. [Google Scholar] [CrossRef]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate Change and distribution shifts in marine fishes. Science 2005, 308, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.M. Climate Change: A looming challenge for fisheries management in southern Africa. Mar. Policy 2006, 30, 84–95. [Google Scholar] [CrossRef]
- Harley, C.D.G.; Hughes, A.R.; Hultgren, K.M.; Miner, B.G.; Sorte, C.J.B.; Thornber, C.S.; Rodriguez, L.F.; Tomanek, L.; Williams, S.L. The impacts of Climate Change in coastal marine systems. Ecol. Lett. 2006, 9, 228–241. [Google Scholar] [CrossRef] [PubMed]
- James, N.C.; Whitfield, A.K.; Harrison, T.D. Grey mullet (Mugilidae) as possible indicators of global warming in South African estuaries and coastal waters. Mar. Environ. Res. 2016, 122, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Knust, R. Climate Change affects marine fishes through the oxygen limitation of thermal tolerance. Science 2007, 315, 95–97. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Peck, M.A. Climate Change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef]
- Lamberth, S.J.; Da Silva, C.; Erasmus, C.; Kerwath, S.E.; McCord, M.E.; Wilke, C.G. Range Expansion and Behavioural Shifts in an Estuary Associated Fish Spotted Grunter Pomadasys Commersonnii, Hypothesis Testing with Acoustic Telemetry. In Proceedings of the 2nd International Conference on Fish Telemetry, Grahamstown, South Africa, 14–19 July 2013. [Google Scholar]
- Steinke, T.D. Mangroves in South African estuaries. In Estuaries of South Africa; Allanson, B.R., Baird, D., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 119–140. [Google Scholar]
- Adam, P. Saltmarshes in a time of change. Environ. Conserv. 2002, 29, 39–61. [Google Scholar] [CrossRef]
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to mangroves from Climate Change and adaptations options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Hoppe-Speer, S.C.L.; Adams, J.B.; Rajkaran, A. Mangrove expansion and population structure at a planted site, East London, South Africa. South. For. A J. For. Sci. 2015, 77, 131–139. [Google Scholar] [CrossRef]
- Raw, J.L.; Godbold, J.A.; van Niekerk, L.; Adams, J.B. Drivers of mangrove cover and species richness at a high-energy, wave-dominated, southern distribution limit. Estuar. Coast. Shelf Sci. 2019, 226, 106296. [Google Scholar] [CrossRef]
- De Lange, W.P.; de Lange, P.J. An appraisal of factors controlling the latitudinal distribution of mangrove (Avicannia marina var. resinifera) in New Zealand. J. Coast. Res. 1994, 10, 539–548. [Google Scholar]
- Hodgson, A.N.; Dickens, J. Activity of the mangrove snail Cerithidea decollate (Gastropoda: Potamididae) in a warm temperate South African Estuary. Estuar. Coast. Shelf Sci. 2012, 109, 98–106. [Google Scholar] [CrossRef]
- Peer, N.; Miranda, N.A.F.; Perissinotto, R. Fiddler crabs (genus Uca Leach 1814) in South Africa: A review and case study of the St Lucia Estuary community. Afr. Zool. 2015, 50, 187–284. [Google Scholar] [CrossRef]
- Allison, L.C.; Palmer, M.D.; Haigh, I.D. Projections of 21st century sea level rise for the coast of South Africa. Environ. Res. Commun. 2022, 4, 025001. [Google Scholar] [CrossRef]
- Clark, B.M.; Turpie, J.K.; Cullis, J.D.S.; Dawson, J.; Dobinson, L.; Kunneke, M.M.; Horn, A. The impacts of long-term flow reductions and an extreme drought on a large, permanently open estuary, and implications for setting the ecological reserve. Water SA 2022, 48, 134–150. [Google Scholar] [CrossRef]
- Saintilan, N.; Kovalenko, K.E.; Guntenspergen, G.; Rogers, K.; Lynch, J.C.; Cahoon, D.; Lovelock, C.; Friess, D.A.; Ashe, E.; Krauss, K.; et al. Global patterns and drivers of tidal marsh response to accelerating sea-level rise. Science 2022. (in Literature). [Google Scholar] [CrossRef]
- Whitfield, A.K.; Harrison, T.D. Gilchristella aestuaria (Pisces: Clupeidae) biomass and consumption of zooplankton in the Sundays Estuary. S. Afr. J. Mar. Sci. 1996, 17, 49–53. [Google Scholar] [CrossRef]
- Nashima, F.P.; Strydom, N.A.; Lamberth, S.J.; Connan, M.; Butler, M. Stable isotopes reveal trophic linkages among fish species utilising the Orange River Estuary Continuum. FoodWebs 2021, 24, e00145. [Google Scholar]
- Snow, G.C.; Adams, J.B.; Bate, G.C. Effect of river flow on estuarine microalgal biomass and distribution. Estuar. Coast. Shelf Sci. 2000, 51, 255–266. [Google Scholar] [CrossRef]
- Adams, J.B. Salt marsh at the tip of Africa: Patterns, processes and changes in response to climate change. Estuar. Coast. Shelf Sci. 2020, 237, 06650. [Google Scholar] [CrossRef]
- Theron, A.K.; Rossouw, M.; Barwell, L.; Maherry, A.; Diedericks, G.; De Wet, P. Quantification of Risks to Coastal Areas and Development: Wave Run-Up and Erosion; In Proceedings of the CSIR 3rd Biennial Conference 2010. Science Real and Relevant, CSIR International Convention Centre, Pretoria, South Africa, 30 August–1 September 2010; Available online: http://hdl.handle.net/10204/4261 (accessed on 17 August 2022).
- Theron, A.K. Analysis of Potential Coastal Zone Climate Change Impacts and Possible Response Options in the Southern African Region. In Proceedings of the IPCC TGICA Conference: Integrating Analysis of Regional Climate Change and Response Options, Nadi, Fiji, 20–22 June 2007; pp. 205–216. Available online: https://www.ipcc.ch/site/assets/uploads/2018/08/tgica_reg-meet-fiji-2007.pdf (accessed on 17 August 2022).
- Nel, J.L.; Le Maitre, D.C.; Nel, D.C.; Reyers, B.; Archibald, S.; van Wilgen, B.W.; Forsyth, G.G.; Theron, A.K.; O’Farrell, P.J.; Kahinda, J.-M.M.; et al. Natural Hazards in a Changing World: A Case for Ecosystem-Based Management. PLoS ONE 2014, 9, e95942. [Google Scholar] [CrossRef]
- Dasgupta, S.; Laplante, B.; Murray, S.; Wheeler, D. Sea-level Rise and Storm Surges: A Comparative Analysis of Impacts in Developing Countries. World Bank Policy Res. Work. Pap. 2009, 1–43. [Google Scholar] [CrossRef]
- Kamranzad, B.; Lavidas, G. Change of nearshore extreme wind and wave climate in Southeast Africa. Ital. J. Eng. Geol. Environ. 2020, 26, 115–119. [Google Scholar]
- Booysen, Z.; Theron, A.K. Methods for predicting berm height at Temporarily Open / Closed Estuaries. Estuar. Coast. Shelf Sci. 2020, 245, 15. [Google Scholar] [CrossRef]
- Cowley, P.D.; Whitfield, A.K.; Bell, K.N.I. The surf zone ichthyoplankton adjacent to an intermittently open estuary, with evidence of recruitment during marine overwash events. Estuar. Coast. Shelf Sci. 2001, 52, 339–348. [Google Scholar] [CrossRef]
- Augustyn, J.; Cockcroft, A.; Kerwath, S.; Lamberth, S.; Githaiga-Mwicigi, J.; Pitcher, G.; Roberts, M.; van der Lingen, C.; Auerswald, L. Chapter 15 South Africa. In The Impacts of Climate Change on Marine Fisheries and Aquaculture; Phillips, B., Perez-Ramirez, M., Eds.; John Wiley & Sons: Oxford, UK, 2017; 1048p. [Google Scholar]
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef]
- Gruber, N.; Hauri, C.; Lachkar, Z.; Loher, D.; Frölicher, T.L.; Plattner, G.K. Rapid Progression of Ocean Acidification in the California Current System. Science 2012, 337, 220–223. [Google Scholar] [CrossRef]
- Gregor, L. Seasonality of the Marine Carbonate System in the Southern Benguela: Nutrient Stoichiometry, Alkalinity Production, and CO2 Flux. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2012. [Google Scholar]
- Chavez, F.P.; Sevadjian, J.; Wahl, C.; Friederich, J.; Friederich, G.E. Measurements of pCO2 and pH from an autonomous surface vehicle in a coastal upwelling system. Deep-Sea Res. Part II 2018, 151, 137–146. [Google Scholar] [CrossRef]
- Flynn, R.F.; Granger, J.; Veitch, J.A.; Siedlecki, S.; Burger, J.M.; Pillay, K.; Fawcett, S.E. On-shelf nutrient trapping enhances the fertility of the southern Benguela upwelling system. J. Geophys. Res. Oceans. 2020, 125, e2019JC015948. [Google Scholar] [CrossRef]
- Rixen, T.; Lahajnar, N.; Lamont, T.; Koppelmann, R.; Martin, B.; van Beusekom, J.E.E.; Siddiqui, C.; Pillay, K.; Meiritz, L. Oxygen and nutrient trapping in the Southern Benguela Upwelling System. Front. Mar. Sci. 2021, 8, 730591. [Google Scholar] [CrossRef]
- Lamberth, S.J.; Branch, G.M.; Clark, B.M. Estuarine refugia and fish responses to a large anoxic, hydrogen sulphide‚ black tide‘ event in the adjacent marine environment. Estuar. Coast. Shelf Sci. 2010, 86, 203–215. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, X.; Zhuang, J. The positive relationship between ocean acidification and pollution. Mar. Pollut. Bull. 2015, 91, 14–21. [Google Scholar] [CrossRef]
- Howland, R.J.M.; Tappin, A.D.; Uncles, R.J.; Plummer, D.H.; Bloomer, N.J. Distributions and seasonal variability of pH and alkalinity in the Tweed Estuary, UK. Sci. Total Environ. 2000, 251, 125–138. [Google Scholar] [CrossRef]
- Aufdenkampe, A.K.; Mayorga, E.; Raymond, P.A.; Melack, J.M.; Doney, S.C.; Alin, S.R.; Aalto, R.E.; Yoo, K. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front Ecol Environ. 2011, 9, 53–60. [Google Scholar] [CrossRef]
- Cai, W.-J.; Hu, X.; Huang, W.-J.; Murrell, M.C.; Lehrter, J.C.; Lohrenz, S.E.; Chou, W.C.; Zhai, W.; Hollibaugh, J.T.; Wang, Y.; et al. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 2011, 4, 766–770. [Google Scholar] [CrossRef]
- Salisbury, J.; Green, M.; Hunt, C.; Campbell, J. Coastal Acidification by Rivers: A Threat to Shellfish? Eos Trans. Am. Geophys. Union 2008, 89, 513. [Google Scholar] [CrossRef]
- Lemley, D.A.; Lamberth, S.J.; Manuel, W.; Nunes, M.; Rishworth, G.M.; van Niekerk, L.; Adams, J.B. Effective management of closed hypereutrophic estuaries requires catchment-scale interventions. Front. Mar. Sci. 2021, 8, 688933. [Google Scholar] [CrossRef]
- Spilling, K. Dense sub-ice bloom of dinoflagellates in the Baltic Sea, potentially limited by high pH. J. Plankton Res. 2007, 29, 895–901. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Smith, J.E.; Johnson, K.S.; Send, U.; Levin, L.A.; Micheli, F.; Paytan, A.; Price, N.N.; Peterson, B.; Takeshita, Y.; et al. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS ONE 2011, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, I.E.; Olsen, Y.S.; Ramajo, L.; Basso, L.; Steckbauer, A.; Moore, T.S.; Howard, J.; Duarte, C.M. Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 2014, 11, 333–346. [Google Scholar] [CrossRef]
- Baumann, H.; Wallace, R.B.; Tagliaferri, T.; Gobler, C.J. Large Natural pH, CO2 and O2 fluctuations in a Temperate Tidal Salt Marsh on Diel, Seasonal, and Interannual Time Scales. Estuaries Coasts 2015, 38, 220–231. [Google Scholar] [CrossRef]
- Azevedo, L.B.; De Schryver, A.M.; Hendriks, A.J.; Huijbregts, M.A.J. Calcifying Species Sensitivity Distributions for Ocean Acidification. Environ. Sci. Technol. 2015, 49, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Fabry, V.J.; Seibel, B.A.; Feely, R.A.; Orr, J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008, 65, 414–432. [Google Scholar] [CrossRef]
- Anderson, A.; Karar, E.; Farolfi, S. Synthesis: IWRM lessons for implementation. Water S.A. 2008, 34, 665–669. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Langenbuch, M.; Reipschläger, A. Biological impact of elevated ocean CO2 concentrations: Lessons from animal physiology and earth history. J. Oceanogr. 2004, 60, 705–718. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Kikkawa, T.; Hayashi, M.; Lee, K.; Kita, J. Effects of CO2 on marine fish: Larvae and adults. J. Oceanogr. 2004, 60, 731–741. [Google Scholar] [CrossRef]
- Potts, W.M.; Gotz, A.; James, N.C. Review of the projected impacts of climate change on coastal fishes in southern Africa. Rev. Fish Biol. Fish. 2015, 25, 603–630. [Google Scholar] [CrossRef]
- Browning, Z.S.; Wilker, A.A.; Moore, E.J.; Lancon, T.W.; Clubb, F.J. The effect of otolith malformation on behavior and cortisol levels in juvenile red drum fish (Sciaenops ocellatus). Comp. Med. 2012, 62, 251–256. [Google Scholar]
- Huchzermeyer, K.D.A.; van der Waal, B.C.W. Epizootic ulcerative syndrome: Exotic fish disease threatens Africa’s aquatic ecosystems. J. S. Afr. Vet. Assoc. 2012, 83, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Conn, D.B. Aquatic invasive species and emerging infectious disease threats: A One Health perspective. Aquat. Invasions 2014, 9, 383–390. [Google Scholar] [CrossRef]
- Keightley, J.; von der Heyden, S.; Jackson, S. Introduced Pacific oysters Crassostrea gigas in South Africa: Demographic change, genetic diversity and body condition. Afr. J. Mar. Sci. 2015, 37, 89–98. [Google Scholar] [CrossRef]
- Strong, A.L.; Kroeker, K.J.; Teneva, L.T.; Mease, L.A.; Kelly, R.P. Ocean Acidification 2.0: Managing our Changing Coastal Ocean Chemistry. BioScience 2014, 64, 581–592. [Google Scholar] [CrossRef]
- Kelly, R.P.; Foley, M.M.; Fisher, W.S.; Feely, R.A.; Halpern, B.S.; Waldbusser, G.G.; Caldwell, M.R. Mitigating Local Causes of Ocean Acidification with Existing Laws. Science 2011, 332, 1036–1037. [Google Scholar] [CrossRef]
- Meyer, J.L.; Sale, M.J.; Mulholland, P.J.; Poff, N.L. Impacts of climate change on aquatic ecosystem functioning and health. J. Am. Water Resour. Assoc. 1999, 35, 1373–1386. [Google Scholar] [CrossRef]
- Thompson, J.R.; Laizé, C.; Acreman, M.C. Climate Change uncertainty in environmental flows for the Mekong River. Hydrol Sci. J. 2014, 59, 935–954. [Google Scholar] [CrossRef]
- Theron, A.K. Methods for Determination of Coastal Development Setback Lines in South Africa. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2016. [Google Scholar]






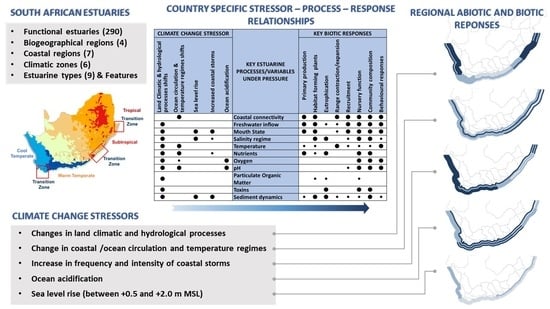
| Climate Change Stressor | Key Estuarine Processes/Variables under Pressure |
Key Biotic Responses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Land Climatic & hydrological processes | Ocean circulation & temperature regimes | Sea level rise | Increased coastal storms | Ocean acidification | Primary production | Habitat-forming plants | Eutrophication | Range contraction/expansion | Recruitment | Nursery function | Community composition | Behavioural responses | |
| ⬤ | Alongshore coastal connectivity | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | |||||
| ⬤ | Freshwater inflow | ⬤ | ⬤ | ● | ● | ⬤ | ⬤ | ⬤ | ⬤ | ||||
| ⬤ | ⬤ | ⬤ | Mouth state | ⬤ | ⬤ | ● | ⬤ | ⬤ | ⬤ | ⬤ | |||
| ⬤ | ⬤ | ● | Salinity regime | ⬤ | ⬤ | ● | ⬤ | ⬤ | ● | ||||
| ⬤ | ⬤ | Temperature | ● | ● | ⬤ | ● | ● | ● | ⬤ | ||||
| ⬤ | ⬤ | ● | Nutrients | ⬤ | ● | ⬤ | ⬤ | ⬤ | |||||
| ⬤ | ● | ⬤ | Oxygen | ⬤ | ⬤ | ⬤ | |||||||
| ⬤ | ⬤ | ⬤ | pH | ● | ⬤ | ⬤ | ⬤ | ||||||
| ⬤ | Particulate organic matter | ● | ● | ● | |||||||||
| ⬤ | Toxins | ⬤ | ⬤ | ⬤ | |||||||||
| ⬤ | ⬤ | ⬤ | Sediment dynamics | ● | ⬤ | ⬤ | ● | ● | ● | ⬤ | ● | ||
 |
|||||||||||||
| Biogeographical Zone | Cool Temperate | Warm Temperate | Subtropical | ||||
|---|---|---|---|---|---|---|---|
| Cool Temperate | Warm and Cool Temperate Transition Zone | Warm Temperate | Subtropical-Warm Temperate Transition Zone | Subtropical | Tropical- Subtropical Transition Zone |
||
| Coastal region | West Coast | Western Cape | Southern Cape | Eastern Cape | Wild Coast | KwaZulu Natal | Delagoa |
| Orange to Krom (n = 18) |
Buffels (Wes) to Breede (n = 18) | Duiwenhoks to Papenkuils (n = 39) |
Swartkops to Cwili (n = 55) | Great Kei to Umtamvuna (n= 85) |
Zolwane to St Lucia (n = 73) |
uMgobezeleni to Kosi (n = 2) | |
| Rainfall seasonality | Winter (Note: Orange catchment mid/late summer) |
Predominantly winter | All year, peaks in spring and autumn. Very late summer in large catchments | Late summer to all year | Late summer | Mid to late summer, early summer in larger catchments | Late summer |
| Mean annual precipitation (mm) | <100–200 | 200–600 (mountains > 1000) | 200–800 | 200–800 | 400–800 | 600–1200 | 900 |
| Dominant catchment size | Three very large catchments, rest small catchments | Small to large catchments | Small to large catchments | Small catchments interspersed with large catchments | Numerous small catchments | Numerous small catchments | Ground water fed, with little surface runoff |
| Coastal topography | Coastal plain | Varies from steeply incised to coastal plain | Varies from steeply incised to coastal plain | Varies from steeply incised to coastal plain | Steeply incised | Steeply incised, coastal plain in northern parts | Coastal plain |
| Dominant mouth position | Mostly perched | Mostly not perched | Mostly not perched | Mostly not perched | Mostly not perched | Mostly perched | Mostly not perched |
% Estuaries
|
33% | 22% | 13% | 4% | 0% | 7% | 100% |
| 17% | 17% | 21% | 20% | 9% | 10% | 0% | |
| 17% | 0% | 3% | 16% | 7% | 7% | 0% | |
| 17% | 11% | 13% | 25% | 26% | 37% | 0% | |
| 17% | 50% | 51% | 35% | 58% | 40% | 0% | |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
|
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Niekerk, L.; Lamberth, S.J.; James, N.C.; Taljaard, S.; Adams, J.B.; Theron, A.K.; Krug, M. The Vulnerability of South African Estuaries to Climate Change: A Review and Synthesis. Diversity 2022, 14, 697. https://doi.org/10.3390/d14090697
van Niekerk L, Lamberth SJ, James NC, Taljaard S, Adams JB, Theron AK, Krug M. The Vulnerability of South African Estuaries to Climate Change: A Review and Synthesis. Diversity. 2022; 14(9):697. https://doi.org/10.3390/d14090697
Chicago/Turabian Stylevan Niekerk, Lara, Stephen J. Lamberth, Nicola C. James, Susan Taljaard, Janine B. Adams, Andre K. Theron, and Marjolaine Krug. 2022. "The Vulnerability of South African Estuaries to Climate Change: A Review and Synthesis" Diversity 14, no. 9: 697. https://doi.org/10.3390/d14090697