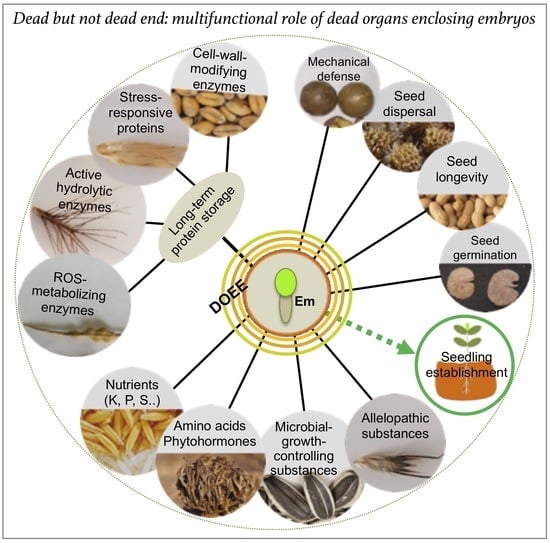
Dead but Not Dead End: Multifunctional Role of Dead Organs Enclosing Embryos in Seed Biology
Abstract
:1. Introduction
2. The Biological Significance of DOEEs
3. DOEEs Function as Long-Term Storage for Beneficial Proteins: Effect of Maternal Environment
4. Heat Shock Proteins (HSPs)
5. DOEEs Contain Plant Growth-Promoting Activity
6. DOEEs Accumulate Allelopathic Substances That Affect Germination of Neighboring Seeds
7. DOEEs and Microbial Growth
8. The Effect of Climate Change on DOEE Properties
9. Concluding Remarks
-
Does preservation of the whole dispersal unit has an advantage over naked seeds for longevity, germination and seedling establishment?
-
How do maternal growth conditions affect DU properties?
-
Can we develop markers for the assessment of seed viability based on the activity of enzymes or metabolites stored within DOEEs?
Funding
Conflicts of Interest
References
- Raviv, B.; Godwin, J.; Granot, G.; Grafi, G. The Dead Can Nurture: Novel Insights into the Function of Dead Organs Enclosing Embryos. Int. J. Mol. Sci. 2018, 19, 2455. [Google Scholar]
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Ann. Rev. Ecol. Evol. Syst. 1982, 13, 201–228. [Google Scholar]
- Eriksson, O. Evolution of seed size and biotic seed dispersal in angiosperms: Paleoecological and neoecological evidence. Int. J. Plant Sci. 2008, 169, 863–870. [Google Scholar]
- Booth, D.T. Plant diaspore functions. J. Seed Technol. 1990, 14, 61–73. [Google Scholar]
- Berenbaum, M.R.; Zangerl, A.R.; Nitao, J.K. Constraints on chemical coevolution: Wild parsnips and the parsnip webworm. Evolution 1986, 40, 1215–1228. [Google Scholar]
- Janzen, D.H. Seed predation by animals. Ann. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar]
- Sroelov, R. On germination inhibitors. IV. Germination inhibitors of Sinapis alba and other seeds when enclosed in their fruit. Palest. J. Bot. 1940, 2, 33–45. [Google Scholar]
- Miyamoto, T.; Tolbert, N.E.; Everson, E.H. Germination inhibitors related to dormancy in wheat seeds. Plant Physiol. 1961, 36, 739. [Google Scholar]
- Liu, Y.; Liu, G.; Li, Q.; Liu, Y.; Hou, L.; Li, G. Influence of pericarp, cotyledon, and inhibitory substances on sharp tooth oak (Quercus aliena var. acuteserrata) germination. PLoS ONE 2012, 7, e47682. [Google Scholar]
- Wurzburger, J.; Leshem, Y. Physiological action of the germination inhibitor in the husk of Aegilops kotschyi boiss. New Phytol. 1969, 68, 337–341. [Google Scholar]
- Gatford, K.T.; Eastwood, R.F.; Halloran, G.M. Germination inhibitors in bracts surrounding the grain of Triticum Tauschii. Funct. Plant Biol. 2002, 29, 881–890. [Google Scholar] [CrossRef]
- Adkins, S.W.; Bellairs, S.M.; Loch, D.S. Seed dormancy mechanisms in warm season grass species. Euphytica 2002, 126, 13–20. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar]
- Steinbrecher, T.; Leubner-Metzger, G. Tissue and cellular mechanics of seeds. Curr. Opin. Genet. Dev. 2018, 51, 1–10. [Google Scholar] [CrossRef]
- Booth, D.T. Seedbed ecology of winterfat: Cations in diaspore bracts and their effect on germination and early plant growth. J. Range Manag. 1989, 42, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Raviv, B.; Granot, G.; Chalifa-Caspi, V.; Grafi, G. The dead, hardened floral bracts of dispersal units of wild wheat function as storage for active hydrolases and in enhancing seedling vigor. PLoS ONE 2017, 12, e0177537. [Google Scholar] [CrossRef] [Green Version]
- Booth, D.T.; Schuman, G.E. Seedbed ecology of winterfat: Fruits versus threshed seeds. J. Range Manag. 1983, 36, 387–390. [Google Scholar] [CrossRef]
- Sankary, M.N.; Barbour, M.G. Autecology of Haloxylon articulatum in Syria. J. Ecol. 1972, 60, 697–711. [Google Scholar] [CrossRef]
- Raviv, B.; Khadka, J.; Swetha, B.; Singiri, J.R.; Grandhi, R.; Shapira, E.; Novoplansky, N.; Gutterman, Y.; Galis, I.; Sternberg, M.; et al. Extreme drought alters progeny dispersal unit properties of winter wild oat (Avena sterilis L.). Planta 2020, 252, 1–77. [Google Scholar] [CrossRef] [PubMed]
- El-Keblawy, A.; Elgabra, M.; Mosa, K.A.; Fakhry, A.; Soliman, S. Roles of hardened husks and membranes surrounding Brachypodium hybridum grains on germination and seedling growth. Plants 2019, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Varga, M. Germination- and growth-inhibiting substances in rice grains. Acta Biol. 1966, 10, 65–78. [Google Scholar]
- Ueno, K.; Miyoshi, K. Difference of optimum germination temperature of seeds of intact and dehusked japonica rice during seed development. Euphytica 2005, 143, 271–275. [Google Scholar] [CrossRef]
- Windsor, J.B.; Symonds, V.V.; Mendenhall, J.; Lloyd, A.M. Arabidopsis seed coat development: Morphological differentiation of the outer integument. Plant J. 2000, 22, 483–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haughn, G.; Chaudhury, A. Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci. 2005, 10, 472–477. [Google Scholar] [CrossRef]
- Lima, N.B.; Trindade, F.G.; da Cunha, M.; Oliveira, A.E.; Topping, J.; Lindsey, K.; Fernandes, K.V. Programmed cell death during development of cowpea (Vigna unguiculata (L.) Walp.) seed coat. Plant Cell Environ. 2015, 38, 718–728. [Google Scholar] [CrossRef]
- Van Durme, M.; Nowack, M.K. Mechanisms of developmentally controlled cell death in plants. Curr. Opin. Plant Biol. 2016, 29, 29–37. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Earl, S.; Harrison, E.; Mathas, E.; Navabpour, S.; Page, T.; Pink, D. The molecular analysis of leaf senescence—A genomics approach. Plant Biotechnol. J. 2003, 1, 3–22. [Google Scholar] [CrossRef]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, F.; Cejudo, F.J. Programmed cell death (PCD): An essential process of cereal seed development and germination. Front. Plant Sci. 2014, 5, 366. [Google Scholar] [CrossRef] [Green Version]
- Khadka, J.; Raviv, B.; Swetha, B.; Grandhi, R.; Singiri, J.R.; Novoplansky, N.; Gutterman, Y.; Galis, I.; Huang, Z.; Grafi, G. Maternal environment alters dead pericarp biochemical properties of the desert annual plant Anastatica hierochuntica L. PLoS ONE 2020, 15, e0237045. [Google Scholar] [CrossRef]
- Raviv, B.; Aghajanyan, L.; Granot, G.; Makover, V.; Frenkel, O.; Gutterman, Y.; Grafi, G. The dead seed coat functions as a long-term storage for active hydrolytic enzymes. PLoS ONE 2017, 12, e0181102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vriens, K.; Cammue, B.P.; Thevissen, K. Antifungal plant defensins: Mechanisms of action and production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Sharma, K.P.; Gaur, R.K.; Gupta, V.K. Role of chitinase in plant defense. Asian J. Biochem. 2011, 6, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Ceasar, S.A.; Ignacimuthu, S. Genetic engineering of crop plants for fungal resistance: Role of antifungal genes. Biotechnol. Lett. 2012, 34, 995–1002. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Ito, J.; Aoyagi, S.; Fukuda, H. Endonucleases. Plant Mol. Biol. 2000, 44, 387–397. [Google Scholar] [CrossRef]
- Aleksandrushkina, N.I.; Vanyushin, B.F. Endonucleases and their involvement in plant apoptosis. Russ. J. Plant Physiol. 2009, 56, 291–305. [Google Scholar] [CrossRef]
- Granot, G.; Morgenstern, Y.; Khan, A.; Rapp, Y.G.; Pesok, A.; Nevo, E.; Grafi, G. Internucleosomal DNA fragmentation in wild emmer wheat is catalyzed by S1-type endonucleases translocated to the nucleus upon induction of cell death. Biochim. Biophys. Acta 2015, 1849, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Hugot, K.; Ponchet, M.; Marais, A.; Ricci, P.; Galiana, E. A tobacco S-like RNase inhibits hyphal elongation of plant pathogens. Mol. Plant-Microbe Interact. 2002, 15, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertini, L.; Caporale, C.; Testa, M.; Proietti, S.; Caruso, C. Structural basis of the antifungal activity of wheat PR4 proteins. FEBS Lett. 2009, 583, 2865–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. Int. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Passaia, G.; Margis-Pinheiro, M. Glutathione peroxidases as redox sensor proteins in plant cells. Plant Sci. 2015, 234, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheler, C.; Weitbrecht, K.; Pearce, S.P.; Hampstead, A.; Büttner-Mainik, A.; Lee, K.J.; Voegele, A.; Oracz, K.; Dekkers, B.J.; Wang, X.; et al. Promotion of testa rupture during garden cress germination involves seed compartment-specific expression and activity of pectin methylesterases. Plant Physiol. 2015, 167, 200–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sénéchal, F.; Wattier, C.; Rustérucci, C.; Pelloux, J. Homogalacturonan-modifying enzymes: Structure, expression, and roles in plants. J. Exp. Bot. 2014, 65, 5125–5160. [Google Scholar] [CrossRef] [Green Version]
- Kohli, P.; Kalia, M.; Gupta, R. Pectin Methylesterases: A Review. J. Bioprocess Biotech. 2015, 5, 5. [Google Scholar]
- Liu, N.; Sun, Y.; Pei, Y.; Zhang, X.; Wang, P.; Li, X.; Li, F.; Hou, Y. A Pectin Methylesterase Inhibitor Enhances Resistance to Verticillium Wilt. Plant Physiol. 2018, 176, 2202–2220. [Google Scholar] [CrossRef] [Green Version]
- An, S.H.; Sohn, K.H.; Choi, H.W.; Hwang, I.S.; Lee, S.C.; Hwang, B.K. Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 2008, 228, 61–78. [Google Scholar] [CrossRef] [Green Version]
- Waters, E.R.; Vierling, E. Plant small heat shock proteins—Evolutionary and functional diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Van Montagu, M.; Verbruggen, N. Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta 2002, 1577, 1–9. [Google Scholar] [CrossRef]
- Lee, G.J.; Roseman, A.M.; Saibil, H.R.; Vierling, E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997, 16, 659–671. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.R. Nutritive role of seed coats in developing legume seeds. Am. J. Bot. 1987, 74, 1122–1137. [Google Scholar] [CrossRef]
- Ribeiro, N.D.; Mazierio, S.M.; Prigol, M.; Nogueira, C.W.; Rosa, D.P.; Possobom, M.T.D.F. Mineral concentrations in the embryo and seed coat of common bean cultivars. J. Food Compos. Anal. 2012, 26, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Godwin, J.; Raviv, B.; Grafi, G. Dead Pericarps of Dry Fruits Function as Long-Term Storage for Active Hydrolytic Enzymes and Other Substances That Affect Germination and Microbial Growth. Plants 2017, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [Green Version]
- Worrall, D.; Holroyd, G.H.; Moore, J.P.; Glowacz, M.; Croft, P.; Taylor, J.E.; Paul, N.D.; Roberts, M.R. Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens. New Phytol. 2012, 193, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, R.R.; Gampala, S.S.; Rock, C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell 2002, 14, S15–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Gacio Mdel, C.; Matilla-Vázquez, M.A.; Matilla, A.J. Seed dormancy and ABA signaling: The breakthrough goes on. Plant Signal. Behav. 2009, 4, 1035–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A. Cytokinins: Permissive role in seed germination. Science 1971, 171, 853–859. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, T.; Zhao, S.; Liu, Z.; Feng, Y.Q.; Wu, Y. Cytokinin antagonizes ABA suppression to seed germination of Arabidopsis by downregulating ABI5 expression. Plant J. 2011, 68, 249–261. [Google Scholar] [CrossRef]
- Fan, J.; Hill, L.; Crooks, C.; Doerner, P.; Lamb, C. Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol. 2009, 150, 1750–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushima, M.; Kakuta, H.; Kosemura, S.; Yamamura, S.; Yamada, K.; Yokotani-Tomita, K.; Hasegawa, K. An allelopathic substance exuded from germinating watermelon seeds. Plant Growth Regul. 1998, 25, 1–4. [Google Scholar] [CrossRef]
- Ohno, S.; Tomita-Yokotani, K.; Kosemura, S.; Node, M.; Suzuki, T.; Amano, M.; Yasui, K.; Goto, T.; Yamamura, S.; Hasegawa, K. A species-selective allelopathic substance from germinating sunflower (Helianthus annuus L.) seeds. Phytochemistry 2001, 56, 577–581. [Google Scholar] [CrossRef]
- Evenari, M. Germination inhibitors. Bot. Rev. 1949, 15, 153–194. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.; Anthelme, F.; et al. Facilitation in plant communities: The past, the present and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Watson, T.G. Inhibition of microbial fermentations by Sorghum grain and malt. J. Appl. Microbiol. 1975, 38, 133–142. [Google Scholar] [CrossRef]
- Kremer, R.J. Antimicrobial activity of velvetleaf (Abutilon tbeophrasti) seeds. Weed Sci. 1986, 34, 617–622. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.P.; Ye, W.; Wang, M.; Yan, X.D. Climate change and drought: A risk assessment of crop-yield impacts. Clim. Res. 2009, 39, 31–46. [Google Scholar] [CrossRef]
- Lionello, P.; Abrantes, F.; Gacic, M.; Planton, S.; Trigo, R.; Ulbrich, U. The climate of the Mediterranean region: Research progress and climate change impacts. Reg. Environ. Chang. 2014, 14, 1679–1684. [Google Scholar] [CrossRef]
- Spinoni, J.; Naumann, G.; Carrao, H.; Barbosa, P.; Vogt, J. World drought frequency, duration, and severity for 1951–2010. Int. J. Climatol. 2014, 34, 2792–2804. [Google Scholar] [CrossRef] [Green Version]
- Ziv, B.; Saaroni, H.; Pargament, R.; Harpaz, T.; Alpert, P. Trends in rainfall regime over Israel, 1975-2010, and their relationship to large-scale variability. Reg. Environ. Chang. 2014, 14, 1751–1764. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, S.; Bradford, K.J.; Huxman, T.E.; Venable, D.L. The contribution of germination functional traits to population dynamics of a desert plant community. Ecology 2016, 97, 250–261. [Google Scholar] [CrossRef]
- Noodén, L.D.; Blakley, K.A.; Grzybowski, J.M. Control of seed coat thickness and permeability in soybean: A possible adaptation to stress. Plant Physiol. 1985, 79, 543–545. [Google Scholar] [CrossRef] [Green Version]
- Hill, H.J.; West, S.H.; Hinson, K. Effect of water stress during seedfill on impermeable expression in soybean. Crop Sci. 1986, 26, 807–812. [Google Scholar] [CrossRef]
- Dorne, A.J. Variation in seed germination inhibition of Chenopodium bonus-henricus in relation to altitude of plant growth. Can. J. Bot. 1981, 59, 1893–1901. [Google Scholar] [CrossRef]
- Gutterman, Y. Maternal effects on plants during development. In Seeds: The Ecology of Regeneration in Plant Communities, 2nd ed.; Fenner, M., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 59–84. [Google Scholar]
- Raikhel, N.V.; Lee, H.I.; Broekaert, W.F. Structure and Function of Chitin-Binding Proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 591–615. [Google Scholar] [CrossRef]
- Chu, C.C.; Lee, W.C.; Guo, W.Y.; Pan, S.M.; Chen, L.J.; Li, H.M.; Jinn, T.L. A copper chaperone for superoxide dismutase that confers three types of copper/zinc superoxide dismutase activity in Arabidopsis. Plant Physiol. 2005, 139, 425–436. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Bandehagh, A.; Taylor, N.L. Can alternative metabolic pathways and shunts overcome salinity induced inhibition of central carbon metabolism in crops? Front. Plant Sci. 2020, 11, 1072. [Google Scholar] [CrossRef]
- Vandvik, V.; Klanderud, K.; Meineri, E.; Måren, I.E.; Töpper, J. Seed banks are biodiversity reservoirs: Species-area relationships above versus below ground. Oikos 2015, 125, 218–228. [Google Scholar] [CrossRef] [Green Version]
- Doungous, O.; Minyaka, E.; Longue, E.A.M.; Nkengafac, N.J. Potentials of cocoa pod husk-based compost on Phytophthora pod rot disease suppression, soil fertility, and Theobroma cacao L. growth. Environ. Sci. Pollut. Res. 2018, 25, 25327–25335. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Mei, X.; Wu, J. Effects of an environmentally friendly seed coating agent on combating head smut of corn caused by Sphacelotheca reiliana and corn growth. J. Agric. Biotechnol. Sustain. Dev. 2010, 2, 108–112. [Google Scholar]
- Budge, G.E.; Garthwaite, D.; Crowe, A.; Boatman, N.D.; Delaplane, K.S.; Brown, M.A.; Thygesen, H.H.; Pietravalle, S. Evidence for pollinator cost and farming benefits of neonicotinoid seed coatings on oilseed rape. Sci. Rep. 2015, 5, 12574. [Google Scholar] [CrossRef] [Green Version]
- Tembo, Y.; Mkindi, A.G.; Mkenda, P.A.; Mpumi, N.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.A.; Belmain, S.R. Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Front. Plant Sci. 2018, 9, 1425. [Google Scholar] [CrossRef]
- Amirkhani, M.; Mayton, H.S.; Netravali, A.N.; Taylor, A.G. A seed coating delivery system for bio-based biostimulants to enhance plant growth. Sustainability 2019, 11, 5304. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Amirkhani, M.; Mayton, H.; Chen, Z.; Taylor, A.G. Biostimulant seed coating treatments to improve cover crop germination and seedling growth. Agronomy 2020, 10, 154. [Google Scholar] [CrossRef] [Green Version]
- Rao, N.K.; Dulloo, M.E.; Engels, J.M.M. A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genet. Resour. Crop Evol. 2017, 64, 1061–1074. [Google Scholar]


|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
|
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grafi, G. Dead but Not Dead End: Multifunctional Role of Dead Organs Enclosing Embryos in Seed Biology. Int. J. Mol. Sci. 2020, 21, 8024. https://doi.org/10.3390/ijms21218024
Grafi G. Dead but Not Dead End: Multifunctional Role of Dead Organs Enclosing Embryos in Seed Biology. International Journal of Molecular Sciences. 2020; 21(21):8024. https://doi.org/10.3390/ijms21218024
Chicago/Turabian StyleGrafi, Gideon. 2020. "Dead but Not Dead End: Multifunctional Role of Dead Organs Enclosing Embryos in Seed Biology" International Journal of Molecular Sciences 21, no. 21: 8024. https://doi.org/10.3390/ijms21218024