Chemical Structure, Sources and Role of Bioactive Flavonoids in Cancer Prevention: A Review
Abstract
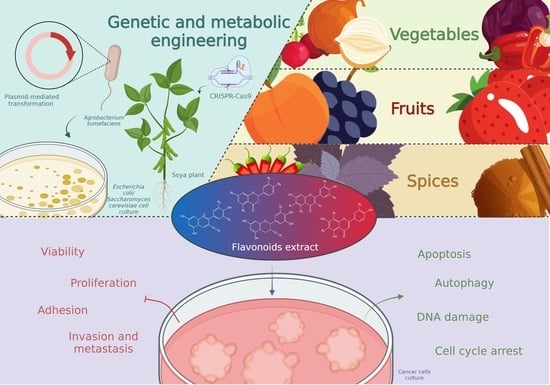
:1. Introduction
2. Methods
3. Chemical Structure and Classification of Flavonoids
4. Rich Sources of Flavonoids
4.1. Berries and Fruits
4.2. Vegetables
4.3. Spices
4.4. Genetically Modified Organisms
4.4.1. Plant Genetic Engineering and Editing
4.4.2. Bacterial DNA Recombination
5. Flavonoids and Human Health
5.1. Flavonoids in Cancer Prevention
5.1.1. Pro-Oxidant and Antioxidant Potential
5.1.2. DNA Protection and Depletion
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DW | Dry weight |
| RE | Rutin equivalent |
| FW | Fresh weight |
| CE | Catechin equivalents |
| PAP1/AtMYB75 | Production of anthocyanin product 1/A. Thaliana’s MYB transcription factor 75 |
| cDNA | Complementary DNA |
| ANS | Anthocyanidin synthase |
| MAS | Mannopine synthase |
| Pd35S | Cauliflower mosaic virus double 35S promoter |
| CHI | Chalcone isomerase |
| FNS | Flavone synthase |
| Tnos | Agrobacterium tumefaciens nos terminator |
| F3H | Flavanone-3-hydroxylase |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| Cas | CRISPR-associated proteins |
| IFS | Isoflavone synthase |
| SMV | Soya bean Mosaic virus |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| LDL | Low-density lipoprotein |
References
- Atik, M.; Danaci, H.M.; Erdoğan, R. Perception of Plants in Ancient Times and Their Use as Motifs Revealing Aspects of the Cultural Landscape in Side, Turkey. Landsc. Res. 2010, 35, 281–297. [Google Scholar] [CrossRef]
- Luca, F.; Perry, G.; Di Rienzo, A. Evolutionary Adaptations to Dietary Changes. Annu. Rev. Nutr. 2010, 30, 291–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogunmefun, O.T. Phytochemicals—God’s Endowment of Curative Power in Plants. Phytochemicals: Source of Antioxidants and Role in Disease Prevention; Asao, T., Asaduzzaman, M., Eds.; IntechOpen: London, UK, 2018; pp. 7–23. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 317S–325S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drețcanu, G.; Iuhas, C.I.; Diaconeasa, Z. The Involvement of Natural Polyphenols in the Chemoprevention of Cervical Cancer. Int. J. Mol. Sci. 2021, 22, 8812. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Kachlicki, P.; Piasecka, A.; Stobiecki, M.; Marczak, Ł. Structural Characterization of Flavonoid Glycoconjugates and Their Derivatives with Mass Spectrometric Techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.; Neves, B.J. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krga, I.; Monfoulet, L.-E.; Konic-Ristic, A.; Mercier, S.; Glibetic, M.; Morand, C.; Milenkovic, D. Anthocyanins and their gut metabolites reduce the adhesion of monocyte to TNFα-activated endothelial cells at physiologically relevant concentrations. Arch. Biochem. Biophys. 2016, 599, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Lin, H.-S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hefni, M.E.; Witthöft, C.M. Characterization of Flavonoid Compounds in Common Swedish Berry Species. Foods 2020, 9, 358. [Google Scholar] [CrossRef] [Green Version]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol Profiles of Vitis vinifera Red Grapes and Their Single-Cultivar Wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Nasser, M.; Cheikh-ali, H.; Hijazi, A.; Merah, O.; Awada, R.; Nasser, M.; Cheikh-ali, H.; Hijazi, A.; Merah, O.; Al-rekaby, A.E.N. Phytochemical Profile, Antioxidant and Antitumor Activities Activities of Green Grape Juice to Cite This Version: Activities of Green Grape Juice. Processes 2020, 1–11. [Google Scholar]
- Zhang, J.; Satterfield, M.B.; Brodbelt, J.S.; Britz, S.J.; Clevidence, B.; Novotny, J.A. Structural Characterization and Detection of Kale Flavonoids by Electrospray Ionization Mass Spectrometry Was Found to Reduce the Flavonols to ∼60% of the Levels. Metab. Clin. Exp. 2003, 75, 6401–6407. [Google Scholar]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.A.; Törrönen, A.R. Identification and Quantification of Phenolic Compounds in Berries of Fragaria and Rubus Species (Family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic Compounds and Antioxidant Capacity of Georgia-Grown Blueberries and Blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef] [PubMed]
- Ljevar, A.; Ćurko, N.; Tomašević, M.; Radošević, K.; Srček, V.G.; Ganić, K.K. Phenolic Composition, Antioxidant Capacity and in vitro Cytotoxicity Assessment of Fruit Wines. Food Technol. Biotechnol. 2016, 54, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Alemán, M.D.; Gironés-Vilaplana, A.; Perez-Garrido, A.; Caravaca, G.; Figueroa, F.; Mulero, J.; Zafrilla, P. Phenolic Composition, Antioxidant Activity, and in vitro Availability of Four Different Berries. J. Chem. 2016, 2016, 5194901. [Google Scholar] [CrossRef] [Green Version]
- Guenther, B.; Christensen, C.; Upatnieks, J. Coherent optical processing: Another approach. IEEE J. Quantum Electron. 1979, 15, 1348–1362. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Redondo, D.; Arias, E.; Oria, R.; Venturini, M.E. Thinned stone fruits are a source of polyphenols and antioxidant compounds. J. Sci. Food Agric. 2016, 97, 902–910. [Google Scholar] [CrossRef]
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A 1998, 799, 101–110. [Google Scholar] [CrossRef]
- Cao, J.; Chen, W.; Zhang, Y.; Zhang, Y.; Zhao, X. Content of Selected Flavonoids in 100 Edible Vegetables and Fruits. Food Sci. Technol. Res. 2010, 16, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [Green Version]
- Hallmann, E.; Rozpara, E.; Słowianek, M.; Leszczynska, J. The effect of organic and conventional farm management on the allergenic potency and bioactive compounds status of apricots (Prunus armeniaca L.). Food Chem. 2018, 279, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Yao, L.; Lu, C.; Li, C.; Zhou, Y.; Su, C.; Chen, B.; Shen, Y. Phenolic composition, antioxidant and antibacterial properties, and in vitro anti-HepG2 cell activities of wild apricot (Armeniaca Sibirica L. Lam) kernel skins. Food Chem. Toxicol. 2019, 129, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Cocconi, E.; Stingone, C.; Zanotti, A.; Trifirò, A. Characterization of polyphenols in apricot and peach purees by UHPLC coupled to HRMS Q-Exactive™mass spectrometer: An approach in the identification of adulterations. Biol. Mass Spectrom. 2016, 51, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Cheaib, D.; El Darra, N.; Rajha, H.N.; El-Ghazzawi, I.; Mouneimne, Y.; Jammoul, A.; Maroun, R.G.; Louka, N. Study of the Selectivity and Bioactivity of Polyphenols Using Infrared Assisted Extraction from Apricot Pomace Compared to Conventional Methods. Antioxidants 2018, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of Anthocyanins and Ellagitannins in Selected Foods Consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Jang, G.H.; Kim, H.W.; Lee, M.K.; Jeong, S.Y.; Bak, A.R.; Lee, D.J.; Kim, J.B. Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J. Biol. Sci. 2016, 25, 1622–1631. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Tomise, K.; Aburatani, M.; Onuki, H.; Hirorta, H.; Ishiharajima, A.E.; Ohta, T. Isolation of Cytochrome P450 Inhibitors from Strawberry Fruit, Fragaria ananassa. J. Nat. Prod. 2004, 67, 1839–1841. [Google Scholar] [CrossRef]
- Aaby, K.; Ekeberg, A.D.; Skrede§, G. Characterization of Phenolic Compounds in Strawberry (Fragaria × ananassa) Fruits by Different HPLC Detectors and Contribution of Individual Compounds to Total Antioxidant Capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef]
- Sandell, M.; Laaksonen, O.; Järvinen, R.; Rostiala, N.; Pohjanheimo, T.; Tiitinen, K.; Kallio, H. Orosensory Profiles and Chemical Composition of Black Currant (Ribes nigrum) Juice and Fractions of Press Residue. J. Agric. Food Chem. 2009, 57, 3718–3728. [Google Scholar] [CrossRef]
- Gudej, J. Kaempferol and quercetin glycosides from Rubus idaeus L. leaves. Acta Pol. Pharm. Drug Res. 2004, 60, 313–315. [Google Scholar]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef]
- Saeed, A.; Marwat, M.S.; Shah, A.H.; Naz, R.; Zain-Ul-Abidin, S.; Akbar, S.; Khan, R.; Navid, M.T.; Saeed, A.; Bhatti, M.Z. Assessment of Total Phenolic and Flavonoid Contents of Selected Fruits and Vegetables. Indian J. Tradit. Knowl. 2019, 18, 686–693. [Google Scholar]
- Paul, S.; Geng, C.-A.; Yang, T.-H.; Yang, Y.-P.; Chen, J.-J. Phytochemical and Health-Beneficial Progress of Turnip (Brassica rapa). J. Food Sci. 2018, 84, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive Compounds, Antioxidant Activity and Inhibition of Key Enzymes Relevant to Alzheimer’s Disease from Sweet Pepper (Capsicum annuum) Extracts. Prev. Nutr. Food Sci. 2019, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Pedersen, A.T.; Andersen, O.M. Flavonoids from red onion (Allium cepa). Phytochemistry 1998, 47, 281–285. [Google Scholar] [CrossRef]
- Tedesco, I.; Carbone, V.; Spagnuolo, C.; Minasi, P.; Russo, G.L. Identification and Quantification of Flavonoids from Two Southern Italian Cultivars of Allium cepa L., Tropea (Red Onion) and Montoro (Copper Onion), and Their Capacity to Protect Human Erythrocytes from Oxidative Stress. J. Agric. Food Chem. 2015, 63, 5229–5238. [Google Scholar] [CrossRef] [PubMed]
- Fredotović, Ž.; Šprung, M.; Soldo, B.; Ljubenkov, I.; Budić-Leto, I.; Bilušić, T.; Čikeš-Čulić, V.; Puizina, J. Chemical Composition and Biological Activity of Allium cepa L. and Allium × cornutum (Clementi ex Visiani 1842) Methanolic Extracts. Molecules 2017, 22, 448. [Google Scholar] [CrossRef] [Green Version]
- Viña, S.Z.; Chaves, A.R. Respiratory activity and phenolic compounds in pre-cut celery. Food Chem. 2007, 100, 1654–1660. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Phenolic Composition and Antioxidant Activities of 11 Celery Cultivars. J. Food Sci. 2010, 75, C9–C13. [Google Scholar] [CrossRef]
- Hossain, M.B.; Patras, A.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N. Application of principal component and hierarchical cluster analysis to classify different spices based on in vitro antioxidant activity and individual polyphenolic antioxidant compounds. J. Funct. Foods 2011, 3, 179–189. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Bioactive Compounds, Antioxidant Activity and Nutritional Quality of Different Culinary Aromatic Herbs. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Marincaş, O.; Feher, I.; Magdas, D.A.; Puşcaş, R. Optimized and validated method for simultaneous extraction, identification and quantification of flavonoids and capsaicin, along with isotopic composition, in hot peppers from different regions. Food Chem. 2018, 267, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Oh, I.-N.; Kim, J.; Jung, D.; Cuong, N.P.; Kim, Y.; Lee, J.; Kwon, O.; Park, S.U.; Lim, Y.; et al. Phenolic compound profiles and their seasonal variations in new red-phenotype head-forming Chinese cabbages. LWT 2017, 90, 433–439. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Changes in the content and composition of anthocyanins in red cabbage and its antioxidant capacity during fermentation, storage and stewing. Food Chem. 2015, 167, 115–123. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Justesen, U.; Knuthsen, P. Composition of flavonoids in fresh herbs and calculation of flavonoid intake by use of herbs in traditional Danish dishes. Food Chem. 2001, 73, 245–250. [Google Scholar] [CrossRef]
- Mackelprang, R.; Lemaux, P.G. Genetic Engineering and Editing of Plants: An Analysis of New and Persisting Questions. Annu. Rev. Plant Biol. 2020, 71, 659–687. [Google Scholar] [CrossRef] [Green Version]
- Marsafari, M.; Samizadeh, H.; Rabiei, B.; Mehrabi, A.; Koffas, M.; Xu, P. Biotechnological Production of Flavonoids: An Update on Plant Metabolic Engineering, Microbial Host Selection, and Genetically Encoded Biosensors. Biotechnol. J. 2020, 15, e1900432. [Google Scholar] [CrossRef]
- Reddy, A.M.; Reddy, V.S.; Scheffler, B.E.; Wienand, U.; Reddy, A.R. Novel transgenic rice overexpressing anthocyanidin synthase accumulates a mixture of flavonoids leading to an increased antioxidant potential. Metab. Eng. 2007, 9, 95–111. [Google Scholar] [CrossRef]
- Schijlen, E.; Ric de Vos, C.H.; Jonker, H.; Van Den Broeck, H.; Molthoff, J.; van Tunen, A.; Martens, S.; Bovy, A. Pathway engineering for healthy phytochemicals leading to the production of novel flavonoids in tomato fruit. Plant Biotechnol. J. 2006, 4, 433–444. [Google Scholar] [CrossRef]
- Zhang, P.; Du, H.; Wang, J.; Pu, Y.; Yang, C.; Yan, R.; Yang, H.; Cheng, H.; Yu, D. Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnol. J. 2019, 18, 1384–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic Engineering of Isoflavones: An Updated Overview. Front. Plant Sci. 2021, 12, 670103. [Google Scholar] [CrossRef] [PubMed]
- Watts, K.T.; Lee, P.C.; Schmidt-Dannert, C. Exploring Recombinant Flavonoid Biosynthesis in Metabolically Engineered Escherichia coli. ChemBioChem 2004, 5, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Ng, K.R.; Lee, J.L.; Mark, R.; Chen, W.N. Enhancement of Naringenin Biosynthesis from Tyrosine by Metabolic Engineering of Saccharomyces cerevisiae. J. Agric. Food Chem. 2017, 65, 6638–6646. [Google Scholar] [CrossRef] [PubMed]
- Sarojini, N.S.; Manjari, A.; Kanti, C. Phytochemical Screening and Anthelmintic Activity Study of Saraca Indica Leaves Extracts. Int. Res. J. Pharm. 2011, 2, 194–197. [Google Scholar]
- Zeraik, M.L.; Serteyn, D.; Deby-Dupont, G.; Wauters, J.-N.; Tits, M.; Yariwake, J.H.; Angenot, L.; Franck, T. Evaluation of the antioxidant activity of passion fruit (Passiflora edulis and Passiflora alata) extracts on stimulated neutrophils and myeloperoxidase activity assays. Food Chem. 2011, 128, 259–265. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Antimicrobial Activities and Phytochemical Profiles of Endemic Medicinal Plants of Mauritius. Pharm. Biol. 2005, 43, 237–242. [Google Scholar] [CrossRef]
- Enaru, B.; Socaci, S.; Farcas, A.; Socaciu, C.; Danciu, C.; Stanila, A.; Diaconeasa, Z. Novel Delivery Systems of Polyphenols and Their Potential Health Benefits. Pharmaceuticals 2021, 14, 946. [Google Scholar] [CrossRef]
- Enaru, B.; Dret, G.; Pop, T.D.; St, A. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Sayed, M.; Mahmoud, A.A.E. Cancer Chemoprevention by Dietary Polyphenols. In Carcinogenesis; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Pop, T.D.; Diaconeasa, Z. Recent Advances in Phenolic Metabolites and Skin Cancer. Int. J. Mol. Sci. 2021, 22, 9707. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Kukić, J.; Petrović, S.; Niketić, M. Antioxidant Activity of Four Endemic Stachys Taxa. Biol. Pharm. Bull. 2006, 29, 725–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, A.K. Imbalance in Antioxidant Defence and Human Diseases: Multiple Approach of Natural Antioxidants Therapy. Curr. Sci. 2001, 81, 1179–1187. [Google Scholar]
- Yokomizo, A.; Moriwaki, M. Effects of Uptake of Flavonoids on Oxidative Stress Induced by Hydrogen Peroxide in Human Intestinal Caco-2 Cells. Biosci. Biotechnol. Biochem. 2006, 70, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.L.; Krueger, C.G.; Wiebe, D.A.; Cunningham, D.G.; Reed, J.D. Cranberry proanthocyanidins associate with low-density lipoprotein and inhibitin vitro Cu2+-induced oxidation. J. Sci. Food Agric. 2001, 81, 1306–1313. [Google Scholar] [CrossRef]
- Neto, C.C.; Amoroso, J.W.; Liberty, A.M. Anticancer activities of cranberry phytochemicals: An update. Mol. Nutr. Food Res. 2008, 52, S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; McDonald, J.; Kalt, W.; Joseph, J.A. Potential role of dietary flavonoids in reducing microvascular endothelium vulnerability to oxidative and inflammatory insults. J. Nutr. Biochem. 2002, 13, 282–288. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, K.P. Flavonoids sensitize tumor cells to radiation: Molecular mechanisms and relevance to cancer radiotherapy. Int. J. Radiat. Biol. 2019, 96, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Deng, G.; Zhang, Y.-C. Multiple free radical scavenging reactions of flavonoids. Dye. Pigment. 2021, 198, 109877. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Lee, S.R.; Kwon, S.W.; Lee, Y.H.; Kaya, P.; Kim, J.M.; Ahn, C.; Jung, E.-M.; Lee, G.-S.; An, B.-S.; Jeung, E.-B.; et al. Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK-mediated apoptosis and anti-inflammation. BMC Cancer 2019, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Kar, S.; Sharma, G.; Das, P.K. Hesperetin Induces Apoptosis in Breast Carcinoma by Triggering Accumulation of ROS and Activation of ASK1/JNK Pathway. J. Cell. Physiol. 2014, 230, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Sharma, S.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin inhibits growth, induces G2/M arrest and apoptosis of human epidermoid carcinoma A431 cells: Role of mitochondrial membrane potential disruption and consequent caspases activation. Exp. Dermatol. 2013, 22, 470–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazhappilly, C.G.; Kumar, D.R.N.; Suresh, P.K.; Kumar, S.; Kumar, R.A. Comparative studies to evaluate relative in vitro potency of luteolin in inducing cell cycle arrest and apoptosis in HaCaT and A375 cells. Asian Pac. J. Cancer Prev. 2013, 14, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lan, Y.; Huang, Q.; Hua, Z. Galangin induces B16F10 melanoma cell apoptosis via mitochondrial pathway and sustained activation of p38 MAPK. Cytotechnology 2012, 65, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-H.; Huang, Z.; Kim, D.J.; Kim, S.-H.; Kim, M.O.; Lee, S.-Y.; Xie, H.; Park, S.J.; Kim, J.Y.; Kundu, J.K.; et al. Direct Targeting of MEK1/2 and RSK2 by Silybin Induces Cell-Cycle Arrest and Inhibits Melanoma Cell Growth. Cancer Prev. Res. 2013, 6, 455–465. [Google Scholar] [CrossRef] [Green Version]
- Pal, H.C.; Sharma, S.; Strickland, L.R.; Katiyar, S.K.; Ballestas, M.E.; Athar, M.; Elmets, C.A.; Afaq, F. Fisetin Inhibits Human Melanoma Cell Invasion through Promotion of Mesenchymal to Epithelial Transition and by Targeting MAPK and NFκB Signaling Pathways. PLoS ONE 2014, 9, e86338. [Google Scholar] [CrossRef] [Green Version]
- Diaconeasa, Z.; Ayvaz, H.; Ruginǎ, D.; Leopold, L.F.; Stǎnilǎ, A.; Socaciu, C.; Tăbăran, F.; Luput, L.; Mada, D.C.; Pintea, A.; et al. Melanoma Inhibition by Anthocyanins Is Associated with the Reduction of Oxidative Stress Biomarkers and Changes in Mitochondrial Membrane Potential. Mater. Veg. 2017, 72, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and proapoptotic activities of anthocyanin and anthocyanidin extracts from blueberry fruits on B16-F10 melanoma cells. Food Nutr. Res. 2017, 61, 1325308. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-C.; Wang, C.-J.; Huang, K.-H.; Lee, Y.-J.; Chan, W.-M.; Chang, Y.-C. Anthocyanins from Hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods 2018, 48, 614–631. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Kang, S.; Liu, C.; Hao, Y. Resveratrol enhances the effects of ALA-PDT on skin squamous cells A431 through p38/ MAPK signaling pathway. Cancer Biomark. 2018, 21, 797–803. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Zhang, W.; Ding, Y.; Zhao, T.; Zhang, M.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Bioaccessibility and biotransformation of anthocyanin monomers following in vitro simulated gastric-intestinal digestion and in vivo metabolism in rats. Food Funct. 2019, 10, 6052–6061. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-W.; Zhang, H.-D.; Mao, L.; Mao, C.-F.; Chen, W.; Cui, M.; Ma, R.; Cao, H.-X.; Jing, C.-W.; Wang, Z.; et al. Luteolin Inhibits Breast Cancer Development and Progression In Vitro and In Vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell. Physiol. Biochem. 2015, 37, 1693–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deb, G.; Thakur, V.S.; Limaye, A.M.; Gupta, S. Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol. Carcinog. 2014, 54, 485–499. [Google Scholar] [CrossRef]
- Seo, H.-S.; Jo, J.K.; Ku, J.M.; Choi, H.-S.; Choi, Y.K.; Woo, J.-K.; Kim, H.I.; Kang, S.-Y.; Lee, K.M.; Nam, K.W.; et al. Induction of caspase-dependent extrinsic apoptosis by apigenin through inhibition of signal transducer and activator of transcription 3 (STAT3) signalling in HER2-overexpressing BT-474 breast cancer cells. Biosci. Rep. 2015, 35, e00276. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, C.; Correia-Branco, A.; Araújo, J.R.; Guimarães, J.T.; Keating, E.; Martel, F. The Chemopreventive Effect of the Dietary Compound Kaempferol on the MCF-7 Human Breast Cancer Cell Line Is Dependent on Inhibition of Glucose Cellular Uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef]
- Li, C.; Yang, D.; Zhao, Y.; Qiu, Y.; Cao, X.; Yu, Y.; Guo, H.; Gu, X.; Yin, X. Inhibitory Effects of Isorhamnetin on the Invasion of Human Breast Carcinoma Cells by Downregulating the Expression and Activity of Matrix Metalloproteinase-2/9. Nutr. Cancer 2015, 67, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Thangavel, C.; Liu, Y.; Shoyele, S.; Den, R.B.; Selvakumar, P.; Lakshmikuttyamma, A. Quercetin regulates β-catenin signaling and reduces the migration of triple negative breast cancer. Mol. Carcinog. 2015, 55, 743–756. [Google Scholar] [CrossRef]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Hyder, S.M. Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. Breast Cancer: Targets Ther. 2016, 9, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Park, S.-H.; Lee, J.; Chun, H.; Choi, M.-K.; Yoon, J.-H.; Pham, T.-H.; Kim, K.H.; Kwon, T.; Ryu, H.-W.; et al. Differential effects of luteolin and its glycosides on invasion and apoptosis in MDA-MB-231 triple-negative breast cancer cells. Excli. J. 2019, 18, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Ge, R.; Li, Y.; Liu, S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3265–3271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Niazvand, F.; Orazizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of Quercetin-Loaded Nanoparticles on MCF-7 Human Breast Cancer Cells. Medicina 2019, 55, 114. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.; Jeong, W.; Song, G. Delphinidin suppresses proliferation and migration of human ovarian clear cell carcinoma cells through blocking AKT and ERK1/2 MAPK signaling pathways. Mol. Cell. Endocrinol. 2015, 422, 172–181. [Google Scholar] [CrossRef]
- Antosiak, A.; Milowska, K.; Maczynska, K.; Rozalska, S.; Gabryelak, T. Cytotoxic activity of genistein-8-C-glucoside form Lupinus luteus L. and genistein against human SK-OV-3 ovarian carcinoma cell line. Med. Chem. Res. 2016, 26, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tian, B.; Wang, Y.; Ding, H. Kaempferol Sensitizes Human Ovarian Cancer Cells-OVCAR-3 and SKOV-3 to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis via JNK/ERK-CHOP Pathway and Up-Regulation of Death Receptors 4 and 5. Med. Sci. Monit. 2017, 23, 5096–5105. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gong, W.; Yang, Z.Y.; Zhou, X.S.; Gong, C.; Zhang, T.R.; Wei, X.; Ma, D.; Ye, F.; Gao, Q.L. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis 2017, 22, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yin, J.; Rankin, G.O.; Chen, Y.C. Kaempferol Induces G2/M Cell Cycle Arrest via Checkpoint Kinase 2 and Promotes Apoptosis via Death Receptors in Human Ovarian Carcinoma A2780/CP70 Cells. Molecules 2018, 23, 1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teekaraman, D.; Elayapillai, S.P.; Viswanathan, M.P.; Jagadeesan, A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. Interact. 2019, 300, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, K.; Ghosh, S.; Mukherjee, A.; Sadhukhan, R.; Mondal, J.; Khuda-Bukhsh, A.R. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: Signal cascade and drug-DNA interaction. Cell Prolif. 2013, 46, 153–163. [Google Scholar] [CrossRef]
- Kashafi, E.; Moradzadeh, M.; Mohamadkhani, A.; Erfanian, S. Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed. Pharmacother. 2017, 89, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.-Y.; Bai, H.-H.; Cai, J.-Y.; Deng, S.-P. The mechanism of kaempferol induced apoptosis and inhibited proliferation in human cervical cancer SiHa cell: From macro to nano. Scanning 2016, 38, 644–653. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Li, J.; Li, B.; Wang, Y.; Li, M.; Ma, D.; Wang, X. Genistein exhibits anti-cancer effects via down-regulating FoxM1 in H446 small-cell lung cancer cells. Tumor Biol. 2013, 35, 4137–4145. [Google Scholar] [CrossRef]
- Cho, H.-J.; Ahn, K.-C.; Choi, J.Y.; Hwang, S.-G.; Kim, W.-J.; Um, H.-D.; Park, J.K. Luteolin acts as a radiosensitizer in non-small cell lung cancer cells by enhancing apoptotic cell death through activation of a p38/ROS/caspase cascade. Int. J. Oncol. 2015, 46, 1149–1158. [Google Scholar] [CrossRef] [Green Version]
- Jo, E.; Park, S.J.; Choi, Y.S.; Jeon, W.-K.; Kim, B.-C. Kaempferol Suppresses Transforming Growth Factor-β1-Induced Epithelial-to-Mesenchymal Transition and Migration of A549 Lung Cancer Cells by Inhibiting Akt1-Mediated Phosphorylation of Smad3 at Threonine-179. Neoplasia 2015, 17, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-J.; Chung, T.-W.; Ha, K.-T. Luteolin inhibits recruitment of monocytes and migration of Lewis lung carcinoma cells by suppressing chemokine (C-C motif) ligand 2 expression in tumor-associated macrophage. Biochem. Biophys. Res. Commun. 2016, 470, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zang, A.; Jia, Y.; Shang, Y.; Zhang, Z.; Ge, K.; Zhang, J.; Fan, W.; Wang, B. Genistein inhibits A549 human lung cancer cell proliferation via miR-27a and MET signaling. Oncol. Lett. 2016, 12, 2189–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palozza, P.; Simone, R.; Catalano, A.; Boninsegna, A.; Böhm, V.; Fröhlich, K.; Mele, M.C.; Monego, G.; Ranelletti, F.O. Lycopene prevents 7-ketocholesterol-induced oxidative stress, cell cycle arrest and apoptosis in human macrophages. J. Nutr. Biochem. 2010, 21, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tang, M.; Liu, Y.; Zhang, Z.; Lu, R.; Lu, J. Apigenin inhibits cell proliferation, migration, and invasion by targeting Akt in the A549 human lung cancer cell line. Anti-Cancer Drugs 2017, 28, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Jeong, Y.-J.; Cho, H.-J.; Hoe, H.-S.; Park, K.-K.; Park, Y.-Y.; Choi, Y.H.; Kim, C.-H.; Chang, H.-W.; Park, Y.-J.; et al. Delphinidin inhibits angiogenesis through the suppression of HIF-1α and VEGF expression in A549 lung cancer cells. Oncol. Rep. 2017, 37, 777–784. [Google Scholar] [CrossRef]
- Han, X.; Liu, C.-F.; Gao, N.; Zhao, J.; Xu, J. RETRACTED: Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. Biomed. Pharmacother. 2018, 108, 809–816. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S. Fisetin inhibits the growth and migration in the A549 human lung cancer cell line via the ERK1/2 pathway. Exp. Ther. Med. 2017, 15, 2667–2673. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, M.A.; Białecka, A.; Pietruszka, B.; Hamułka, J. Vegetables and fruit, as a source of bioactive substances, and impact on memory and cognitive function of elderly. Postep. Hig. Med. Dosw. (Online) 2017, 71, 267–280. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Mandal, A.K.A. Next-Generation Sequencing Reveals the Role of Epigallocatechin-3-Gallate in Regulating Putative Novel and Known microRNAs Which Target the MAPK Pathway in Non-Small-Cell Lung Cancer A549 Cells. Molecules 2019, 24, 368. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Cho, H.J.; Yu, R.; Lee, K.W.; Chun, H.S.; Park, J.H.Y. Mechanisms Underlying Apoptosis-Inducing Effects of Kaempferol in HT-29 Human Colon Cancer Cells. Int. J. Mol. Sci. 2014, 15, 2722–2737. [Google Scholar] [CrossRef]
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martín-Serrano, M.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem. Toxicol. 2015, 84, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, B.; Hermant, M.; Castrogiovanni, C.; Staudt, C.; Dumont, P. Resveratrol induces DNA damage in colon cancer cells by poisoning topoisomerase II and activates the ATM kinase to trigger p53-dependent apoptosis. Toxicol. Vitr. 2015, 29, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Song, H.M.; Park, G.H.; Eo, H.J.; Jeong, J.B. Naringenin-Mediated ATF3 Expression Contributes to Apoptosis in Human Colon Cancer. Biomol. Ther. 2016, 24, 140–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.; Teng, J.; Zhu, Z.; Chen, J.; Huang, W.-J. Genistein induces activation of the mitochondrial apoptosis pathway by inhibiting phosphorylation of Akt in colorectal cancer cells. Pharm. Biol. 2015, 54, 74–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, H.-R.; Liu, C.-J.; Yeh, C.-C. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem. Interact. 2015, 235, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kunnimalaiyaan, S.; Sokolowski, K.M.; Balamurugan, M.; Gamblin, T.C.; Kunnimalaiyaan, M. Xanthohumol Inhibits Notch Signaling and Induces Apoptosis in Hepatocellular Carcinoma. PLoS ONE 2015, 10, e0127464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, K.; Liang, B.; Huang, X. Anticancer effect of xanthohumol induces growth inhibition and apoptosis of human liver cancer through NF-κB/p53-apoptosis signaling pathway. Oncol. Rep. 2015, 35, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Kavoosi, F.; Sanaei, M.; Valiani, A.; Ghobadifar, M.A. Effect of genistein on apoptosis and proliferation of hepatocellular Carcinoma Hepa1-6 Cell Line. Int. J. Prev. Med. 2018, 9, 12. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, X.; Li, H.; Yan, Y.; Hong, X.; Lin, Z. RETRACTED: Kaempferol inhibits proliferation, migration, and invasion of liver cancer HepG2 cells by down-regulation of microRNA-21. Int. J. Immunopathol. Pharmacol. 2018, 32, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sambantham, S.; Radha, M.; Paramasivam, A.; Anandan, B.; Malathi, R.; Chandra, S.R.; Jayaraman, G. Molecular Mechanism Underlying Hesperetin-induced Apoptosis by in silico Analysis and in Prostate Cancer PC-3 Cells. Asian Pac. J. Cancer Prev. 2013, 14, 4347–4352. [Google Scholar] [CrossRef] [Green Version]
- Halimah, E.; Diantini, A.; Destiani, D.P.; Pradipta, I.S.; Sastramihardja, H.S.; Lestari, K.; Subarnas, A.; Abdulah, R.; Koyama, H. Induction of caspase cascade pathway by kaempferol-3-O-rhamnoside in LNCaP prostate cancer cell lines. Biomed. Rep. 2014, 3, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Sun, Y.; Sukumaran, P.; Singh, B.B. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol. Carcinog. 2015, 55, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Li, Z.; Liu, C.; Yin, L. The apoptotic effect of apigenin on human gastric carcinoma cells through mitochondrial signal pathway. Tumor Biol. 2014, 35, 7719–7726. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Vikash; Song, J.; Wang, J.; Yi, J.; Dong, W. Hesperetin Induces the Apoptosis of Gastric Cancer Cells via Activating Mitochondrial Pathway by Increasing Reactive Oxygen Species. Am. J. Dig. Dis. 2015, 60, 2985–2995. [Google Scholar] [CrossRef]
- Feng, J.; Chen, X.; Wang, Y.; Du, Y.; Sun, Q.; Zang, W.; Zhao, G. Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Mol. Cell. Biochem. 2015, 408, 163–170. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2019, 69, 200–211. [Google Scholar] [CrossRef]
- Vazhappilly, C.G.; Amararathna, M.; Cyril, A.C.; Linger, R.; Matar, R.; Merheb, M.; Ramadan, W.S.; Radhakrishnan, R.; Rupasinghe, H.V. Current methodologies to refine bioavailability, delivery, and therapeutic efficacy of plant flavonoids in cancer treatment. J. Nutr. Biochem. 2021, 94, 108623. [Google Scholar] [CrossRef]



| Source | Subclass | Major Compounds | Conc. mg/100 g FW | Conc. mg/100 g DW | Conc. mg/100 mL | Refs. |
|---|---|---|---|---|---|---|
| Blackberry (Rubus spp.) |
Flavan-3-ols | (+)-Catechin | 0.166–1.029 | [24] | ||
| (−)-Epicatechin | 0.012–6.14 | |||||
| Flavonols | Quercetin | 0.39–1.794 | [24] | |||
| Anthocyanins | Cyanidin-3-glucoside | 57.2 ± 2.5 | 0.039–2.551 | [24,25] | ||
| Cyanidin-3-rutinoside | 25.0 ± 2.8 | 0.192–11.933 | ||||
| Cyanidin-3-xyloside | 48.3 ± 5.6 | [25] | ||||
| Delphinidin-3-glucoside | 516.5 ± 9.3 | |||||
| Chokeberry (Aronia melanocarpa) |
Flavonols | Kaempferol | 0.00–0.69 | [26] | ||
| Quercetin | 8.90–37.46 | |||||
| Anthocyanins | Cyanidin | 26.95–947.52 | [26] | |||
| Delphinidin | 0.65 | |||||
| Malvidin | 1.22 | |||||
| Pelargonidin | 0.51–1.44 | |||||
| Peonidin | 0.08 | |||||
| Petunidin | 2.79 | |||||
| Blueberry (Vaccinium angustifolium) |
Anthocyanins | Cyanidin-3-glucoside | 5.1 ± 0.9 | 7.7 ± 0.7 | [15,25] | |
| Delphinidin-3-glucoside | 27.3 ± 3.1 | 47 ± 2.4 | ||||
| Malvidin-3-glucoside | 1.9 ± 0.8 | 94.3 ± 4.5 | ||||
| Peonidin-3-glucoside | 15.1 ± 2.4 | |||||
| Petunidin-3-glucoside | 28.1 ± 4.1 | 37.7 ± 1.9 | ||||
| Flavan-3-ols | (+)-Catechin | 81.8 ± 9.17 | 43.1 ± 1.9 | [15,27] | ||
| (+)-Epicatechin | 9.25 ± 0.15 | |||||
| Epicatechin gallate | 0.48 ± 0.52 | |||||
| Proanthocyanins | 35 ± 1.3 | [15] | ||||
| Flavonols | Kaempferol | 5.17 ± 0.04 | [15,27] | |||
| Kaempferol-3-glucoside | 5.45 ± 0.24 | |||||
| Quercetin-3-galactoside | 0.19 ± 0.09 | 78.6 ± 1.1 | ||||
| Quercetin-3-glucoside | 2.38 ± 0.35 | |||||
| Quercetin-3-glucuronide | 1.76 ± 0.12 | |||||
| Cherry (Prunus spp.) |
Flavan-3-ols | (+)-Catechin | 0.036–1.117 | [24] | ||
| (−)-Epicatechin | 0.051–2.406 | |||||
| Proanthocyanidins | 10.54 ± 0.19 | [28] | ||||
| Flavonols | Kaempherol-3-rutinoside | 0.03 ± 0.00 | [28] | |||
| Quercetin | 0.026–0.391 | [24] | ||||
| Quercetin-3-rutinoside | 0.09 ± 0.01 | [28] | ||||
| Anthocyanins | Cyanidin-3-glucoside | 0.078–1.207 | [24] | |||
| Cyanidin-3- glucosylrutinoside |
0.737–36.128 | |||||
| Cyanidin-3-rutinoside | 0.321–9.144 | |||||
| Raspberry (Rubus idaeus) |
Flavan-3-ols | (+)-Catechin | 7.4 ± 0.1 | 0.544–1.540 | [15,24] | |
| (−)-Epicatechin | 2.94 ± 1.25 | 102.4 ± 4 | 2.165–4.359 | [15,24,27] | ||
| Flavonols | Kaempferol-3-glucoside | 0.30 ± 0.45 | [27] | |||
| Quercetin | 0.5 | 0.196–0.392 | [24,29] | |||
| Quercetin-3-glucoside | 0.10 ± 0.32 | [27] | ||||
| Quercetin-3-glucuronide | 0.54 ± 0.75 | |||||
| Anthocyanins | Cyanidin 3-glucoside | 57.5 ± 3.4 | 74.4 ± 0.8 | [15,25] | ||
| Cyanidin-3-glucosylrutinoside | 56.4 ± 3.8 | 0.489–2.529 | [24,25] | |||
| Cyanidin-3-rutinoside | 19.6 ± 1.2 | 0.594–1.072 | ||||
| Cyanidin-3-sophoroside | 0.4 ± 0.1 | 5.783–12.469 | ||||
| Petunidin 3-glucoside | 57.5 ± 3.4 | [25] | ||||
| Blackcurrant (Ribes nigrum) |
Flavonols | Quercetin | 3.7 ± 0.1 | 0.2–0.385 | [24,29] | |
| Kaempferol | 0.1 ± 0.1 | [29] | ||||
| Anthocyanins | Cyanidin-3-rutinoside | 1.616–8.877 | [24] | |||
| Delphinidin-3-glucoside | 0.22–2.674 | |||||
| Delphinidin-3-rutinoside | 2.404–17.921 | |||||
| Strawberry (Fragaria × ananassa) |
Flavan-3-ols | (+)-Catechin | 2.51 ± 0.05 | 45.8 ± 0.5 | 0.704–0.813 | [15,24,27] |
| (−)-Epicatechin | 6.80 ± 2.20 | 0.153–0.201 | ||||
| Epicatechin gallate | 0.45 ± 0.32 | [27] | ||||
| Flavonols | Isorhamnetin | 0.57 ± 0.01 | [30] | |||
| Kaempferol | 0.28 ± 0.01 0.5 ± 0.3 6.13 ± 0.52 |
[27,29,30] | ||||
| Kaempferol-3-glucoside | 1.04 ± 0.28 | 8 ± 0.1 | [15,27] | |||
| Quercetin | 0.6 ± 0.5 19.0 ± 2.20 |
0.031–0.168 | [24,27,29] | |||
| Quercetin-3-galactoside | 0.35 ± 0.49 | [27] | ||||
| Quercetin-3-glucoside | 0.20 ± 0.48 | |||||
| Quercetin-3-glucuronide | 3.35 ± 1.58 | |||||
| Flavones | Apigenin | 0.24 ± 0.01 | [30] | |||
| Anthocyanins | Cyanidin 3-rutinoside | 0.7 ± 0.1 | [25] | |||
| Pelargonidin 3-glucoside | 347.8 ± 10.5 | |||||
| Pelargonidin 3-rutinoside | 52.4 ± 4.8 | |||||
| Peonidin 3-rutinoside | 7.6 ± 1.4 | |||||
| Apple (Malus domestica) |
Flavan-3-ols | (+)-Catechin | 0.152–1.523 | [24] | ||
| (−)-Epicatechin | 0.414–2.591 | |||||
| Flavonols | Isorhamnetin | 14.42 ± 0.97 | [30] | |||
| Kaempferol | 5.07 ± 0.71 | |||||
| Quercetin | 2.0 ± 0.4 5.16 ± 0.32 |
0.04–0.092 | [24,29,30] | |||
| Flavones | Luteolin | 1495 ± 45 | [30] | |||
| Plum (Prunus spp.) |
Flavonols | Isorhamnetin | 5.23 ± 0.3 | [30] | ||
| Kaempferol | 3.17 ± 0.12 | [30] | ||||
| Quercetin | 1.5 0.34 ± 0.6 |
[29,30] | ||||
| Quercetin 3-rutinoside | 15 ± 2 | [28] | ||||
| Flavones | Luteolin | 3.98 ± 0.04 | [30] | |||
| Flavan-3-ols | Proanthocyanidins | 969 ± 187 | [28] | |||
| Peach (Prunus persica) |
Flavonols | Kaempferol | 1.43 ± 0.17 | [30] | ||
| Kaempherol-3-hexoside | 4 ± 1 | [28] | ||||
| Kaempherol-3-rutinoside | 5 ± 1 | |||||
| Quercetin 3-rutinoside | 6 ± 1 | |||||
| Flavones | Luteolin | 3.39 ± 0.42 | [30] | |||
| Flavan-3-ols | Proanthocyanidins | 1379 ± 62 | [28] | |||
| Grapes (Vitis vinifera) |
Flavonols | Kaempferol | 8.91 ± 0.4 5.35 ± 0.59 |
[27,30] | ||
| Kaempferol-3-glucoside | 0.68 ± 1.2 | [27] | ||||
| Quercetin | 1.19 ± 0.03 0.2 |
[29,30] | ||||
| Quercetin-3-glucoside | 0.36 ± 0.48 | [27] | ||||
| Quercetin-3-glucuronide | 3.11 ± 1.54 | |||||
| Flavan-3-ols | Catechin | 1.44 ± 0.09 | [27] | |||
| Epicatechin | 2.02 ± 1.17 | |||||
| Epicatechin gallate | 0.29 ± 0.30 | |||||
| Orange (Citrus × sinensis) |
Flavonols | Isorhamnetin | 0.87 ± 0.08 | [30] | ||
| Kaempferol | 0.51 ± 0.05 | |||||
| Quercetin | 0.17 ± 0.02 | |||||
| Flavones | Luteolin | 0.45 ± 0.04 | [30] | |||
| 6,8-di-C-Glu-Apigenin | 4.15–8 | [31] | ||||
| Flavanones | Hesperetin | 31 ± 2 | 3.51–55.2 | [29,31] | ||
| Naringenin | 11 ± 2 | [29] | ||||
| Cranberry (Vaccinium spp.) |
Flavonols | Myricetin | 23 | [29] | ||
| Quercetin | 16 | |||||
| Grapefruit (Citrus × paradisi) |
Flavonols | Kaempferol | 0.4 ± 0.1 | [29] | ||
| Quercetin | 0.5 ± 0.1 | 0.19 | [29,31] | |||
| Flavanones | Hesperetin | 1.5 ± 0.3 | 0.25–1.79 | [29,31] | ||
| Naringenin | 53 ± 6 | 0.98–8 | ||||
| Narirutin | 2.5–17 | [31] | ||||
| Lemon (Citrus limon) |
Flavanones | Hesperetin | 17 | 3.84–41 | [29,31] | |
| Naringenin | 0.5 | [29] | ||||
| Flavanones | 6,8-di-C-Glu-Apigenin | 1–1.45 | [31] | |||
| 6,8-di-C-Glu-Diosmetin | 4.05–5.8 | |||||
| 7-O-Rut-Luteolin | 1.5–6.5 | |||||
| Apricot (Prunus spp.) |
Flavonols | Kaempferol | 0.38 ± 0.05 5.44 ± 0.12 |
[32,33] | ||
| Kaempherol-3-rutinoside | 0.03 | [28] | ||||
| Myricetin | 0.69 ± 0.07 | [32] | ||||
| Quercetin | 4.31 ± 0.07 | [33] | ||||
| Quercetin-3-O-glucoside | 7.57 ± 2.87 | [32] | ||||
| Quercetin 3-rutinoside | 0.23 ± 0.01 | [28] | ||||
| Rutin | 3.77 ± 0.05 | 0.16–0.26 | [33,34] | |||
| Flavan-3-ols | Catechin | 3.14 | [35] | |||
| Proanthocyanidins | 3.04 ± 0.08 | [28] | ||||
| Flavones | Apigenin | 0.22 ± 0.01 | [33] | |||
| Apigenin 7-O-glucoside | 60.47 ± 1.08 | |||||
| Luteolin | 0.68 ± 0.42 | [32] | ||||
| Luteolin 7-xyloside | 4.60 ± 0.02 | [33] | ||||
| Anthocyanins | Cyanidin 3-(4″-acetylrutinoside) | 56.71 ± 1.13 | [33] | |||
| Cyanidin 3-(6″-acetylglucoside) | 11.34 ± 0.16 | |||||
| Cyanidin 3-O-galactoside | 4.13 ± 0.05 | |||||
| Cyanidin 3-rutinoside | 4.47 ± 0.09 | |||||
| Petunidin 3-galactoside | 6.61 ± 0.05 | |||||
| Petunidin 3-rutinoside | 2.80 ± 0.05 |
| Source | Subclass | Major Compounds | Conc. mg/100 g FW |
Conc. mg/100 g DW | Refs. |
|---|---|---|---|---|---|
| Onion (Allium cepa) |
Flavonols | Isorhamnetin-4′-glucoside | 5.398 ± 0.042 | [48] | |
| Kaempferol | 4.13 ± 0.24 | [30] | |||
| Quercetin | 1.42 ± 0.06 | ||||
| Quercetin-3,4′-diglucoside | 29.646 ± 0.005 | 171.34 ± 0.13 | [48,49] | ||
| Flavones | Apigenin | 2.62 ± 0.12 | [30] | ||
| Anthocyanins | Cyanidin-3-(6″-malonylglucoside) | 1.718 ± 0.075 | [48] | ||
| Peonidin-3′-glucoside | 0.19 | [49] | |||
| Kale (Brassica oleracea var.) |
Flavonols | Isorhamnetin | 5.98 ± 0.41 | [30] | |
| Kaempferol | 2.4 ± 0.23 | ||||
| Quercetin | 0.48 ± 0.03 | ||||
| Flavones | Apigenin | 0.28 ± 0.02 | [30] | ||
| Luteolin | 2.39 ± 0.2 | ||||
| Celery (Apium graveolens) |
Flavones | Apigenin | 13.93 ± 0.52 0.461 |
79.42 ± 0.77 | [30,50,51] |
| Apigenin-7-O-glucoside | 156 ± 7 | [52] | |||
| Luteolin | 2.31 ± 0.11 0.088 |
62.43 ± 0.59 | [30,50,51] | ||
| Luteolin-7-O-glucoside | 654 ± 8 | [52] | |||
| Flavonols | Kaempferol | 0.46 ± 0.03 | 1.06 ± 0.03 | [30,51] | |
| Myricetin | 105.05 ± 4.46 | [53] | |||
| Rutin | 13.99 ± 0.58 | ||||
| Quercetin | 5.31 ± 0.21 | ||||
| Flavan-3-ols | Epicatechin | 8.90 ± 0.42 | [53] | ||
| Chili pepper (Capsicum var.) |
Flavonols | Isoquercetin | 1.742 ± 0.055 | [54] | |
| Kaempferol-3-glucoside | 3.479 ± 0.02 | ||||
| Myricetin | 2.388 ± 0.06 | ||||
| Quercetin | 0.16 ± 0.02 | [30] | |||
| Flavones | Apigenin | 0.5 | [30] | ||
| Luteolin | 2.54 ± 0.05 | ||||
| Radish (Raphanus raphanistrum subsp. sativus) |
Flavonols | Kaempferol | 3.23 ± 0.44 | [30] | |
| Quercetin | 0.52 ± 0.07 | ||||
| Flavones | Apigenin | 0.22 ± 0.03 | [30] | ||
| Luteolin | 1.95 ± 0.27 | ||||
| Soybean (Glycine max) |
Flavonols | Quercetin | 0.17 | [30] | |
| Flavones | Luteolin | 0.94 ± 0.12 | [30] | ||
| Spinach (Spinacia oleracea) |
Flavonols | Kaempferol | 0.89 ± 0.04 | [30] | |
| Cabbage (Brassica oleracea) |
Flavonols | Kaempferol | 3.12 ± 0.02 | 11.0 ± 0.8 | [30,55] |
| Quercetin | 0.49 | 16.1 ± 1.0 | |||
| Flavones | Luteolin | 3.27 ± 0.02 | [30] | ||
| Anthocyanins | Cyanidin-3,5-O-diglucoside | 3.2 | [56] | ||
| Cyanidin-3-(feruloyl)-diglucoside-5-glucoside | 7.3 | ||||
| Cyanidin-3-(sinapoyl)-O-diglucoside-5-O-glucoside | 2.7 | ||||
| Cyanidin-3-coumaroyl-dihexoside-5-hexoside | 9.4 | ||||
| Broccoli (Brassica oleracea var. italica) |
Flavonols | Kaempferol | 211 ± 6 | [30] | |
| Quercetin | 0.53 ± 0.03 |
| Source | Subclass | Major Compounds | Conc. mg/100 g FW | Conc. mg/100 g DW | Ref. |
|---|---|---|---|---|---|
| Celery (Apium graveolens) |
Flavones | Apigenin-7-O-glucoside | 156 ± 7 | [52] | |
| Luteolin-7-O-glucoside | 654 ± 8 | ||||
| Cumin (Cuminum cyminum) |
Flavones | Apigenin-7-O-glucoside | 146 ± 2 | [52] | |
| Luteolin-7-O-glucoside | 224 ± 7 | ||||
| Dill (Anethum graveolens) |
Flavonols | Isorhamnetin | 15–72 | [58] | |
| Kaempferol | 16–24 | ||||
| Quercetin | 48–110 | ||||
| Oregano (Origanum vulgare) | Flavones | Apigenin | 2–4 | [58] | |
| Apigenin-7-O-glucoside | 254 ± 1 | [52] | |||
| Luteolin | 0–3 | [58] | |||
| Luteolin-7-O-glucoside | 301 ± 1 | [52] | |||
| Fennel (Foeniculum vulgare) |
Flavones | Apigenin-7-O-glucoside | 43 ± 1 | [52] | |
| Luteolin-7-O-glucoside | 211 ± 4 | ||||
| Cress (Lepidium sativum) |
Flavonols | Isorhamnetin | 1 | [58] | |
| Kaempferol | 13 | ||||
| Basil (Ocimum basilicum) |
Flavones | Apigenin-7-O-glucoside | 18 | [52] | |
| Luteolin-7-O-glucoside | 127 ± 1 | ||||
| Marjoram (Origanum majorana) |
Flavones | Apigenin-7-O-glucoside | 83 ± 3 | [52] | |
| Luteolin-7-O-glucoside | 461 ± 7 | ||||
| Chives (Allium schoenoprasum) |
Flavonols | Isorhamnetin | 5 | [58] | |
| Kaempferol | 12 | ||||
| Quercetin | 3 | ||||
| Parsley (Petroselinum crispum) |
Flavones | Apigenin | 0.44 ± 0.01 | [30] | |
| Apigenin-7-O-glucoside | 752 ± 17 | [52] | |||
| Luteolin | 1.42 ± 0.03 | [30] | |||
| Luteolin-7-O-glucoside | 125 ± 8 | [52] | |||
| Flavonols | Isorhamnetin | 1.12 ± 0.1 | [30] | ||
| Kaempferol | 1.85 ± 0.03 | ||||
| Myricetin | 151.03 ± 6.68 | [53] | |||
| Quercetin | 0–1 0.5 ± 0.01 71.33 ± 2.19 |
[30,53,58] | |||
| Rutin | 4.32 ± 0.23 | [53] | |||
| Flavan-3-ols | Epicatechin | 2.67 ± 0.11 | [53] | ||
| Thyme (Thymus vulgaris) |
Flavones | Apigenin | 5 | [58] | |
| Apigenin-7-O-glucoside | 16 | [52] | |||
| Luteolin | 51 | [58] | |||
| Luteolin-7-O-glucoside | 104 ± 2 | [52] | |||
| Lovage (Levisticum officinale) |
Flavonols | Kaempferol | 7 | [58] | |
| Quercetin | 170 | ||||
| Coriander (Coriandrum sativum) |
Flavonols | Quercetin | 5 | [58] | |
| Rosemary (Rosmarinus officinalis) | Flavones | Apigenin-7-O-glucoside | 50 ± 1 | [52] | |
| Luteolin | 4 | [58] | |||
| Luteolin-7-O-glucoside | 71 ± 2 | [52] | |||
| Mint (Mentha var.) |
Flavones | Apigenin | 18–99 | [58] | |
| Luteolin | 11–41 | ||||
| Sage (Salvia officinalis) |
Flavones | Apigenin-7-O-glucoside | 53 ± 1 | [52] | |
| Luteolin-7-O-glucoside | 495 ± 1 | ||||
| Watercress (Nasturtium officinale) |
Flavonols | Kaempferol | 1 | [58] | |
| Quercetin | 4 | [58] | |||
| Cinnamon (Cinnamomum var.) |
Flavan-3-ols | Proanthocyanins | 8960 | [28] | |
| Tarragon (Artemisia dranunculus) | Flavonols | Isorhamnetin | 5 | [58] | |
| Kaempferol | 11 | ||||
| Quercetin | 10 | ||||
| Flavones | Luteolin | 1 |
| Cancer Type | Cell Line | Compound | Conc. | Main Biological Effects | Ref. |
|---|---|---|---|---|---|
| Skin cancer | A431, SCC-13 | Fisetin | 0–80 µM | ↑ Apoptosis ↑ Cell cycle arrest at G2/M phase ↓ Cell viability ↓ Colony formation ↓ Δψm |
[88] |
| A375 | Luteolin | 0–80 µM | ↑ Apoptosis ↑ Cell cycle arrest at G0/G1 phase ↓ Colony formation ↓ Cell proliferation |
[89] | |
| B16F10 | Galangin | 0–100 µmol/L | ↑ Phosphor-p-38 MAPK ↑ Apoptosis ↓ Δψm ↓ Cell viability |
[90] | |
| SK-MEL-5, SK-MEL-28 | Silybin | 0–80 µM | ↑ Cell cycle arrest at G1 phase ↓ Cell viability ↓ Cell proliferation ↓ Kinase activity of MEK1/2 and RSK2 ↓ Expression of NF-κB, Ap-1 and STAT3 ↓ Phosphorylation of ERK1/2 and RSK2 |
[91] | |
| A375, RPMI-7951, Hs294T | Fisetin | 0–20 µM | ↓ Cell invasion ↓ Phosphorylation of MEK1/2 and ERK1/2 ↓ Activation of IKK ↓ Activation of the NF-κB signaling pathway |
[92] | |
| B16-F10 | Anthocyanins | 0–500 µg/mL | ↓ Cell proliferation | [93] | |
| 0–800 μg/mL | ↑ Cell cycle arrest at G0/G1 phase ↑ Apoptosis ↓ Cell viability ↓ Cell proliferation |
[94] | |||
| B16-F1 | Anthocyanins | 0–1 mg/mL | ↓ Cell growth ↓ Cell migration ↓ Tube formation ↓ Expression of MMP-2/-9 and VEGF ↓ Angiogenesis |
[95] | |
| A431 | Resveratrol + ALA-PDT therapy | 0–120 mg/mL | ↑ Apoptosis ↑ MAPK pathway ↓ Cell proliferation |
[96] | |
| A375.S2 | Chrysin | 0–15 µM | ↑ Cell morphological changes ↓ Cell viability ↓ Cell migration and invasion ↓ Expression of MMP-2 ↓ Expression of NF-κB p65 |
[97] | |
| Breast cancer | MDA-MB-231, MCF-7 | Luteolin | 0–100 µM | ↓ Cell viability ↓ Cell migration ↓ Expression of Notch-1, Hes-1, Hey, VEGF, Cyclin D1 and MMP -Regulating miRNAs |
[98] |
| MCF-7, MDA-MB-231 | Epigallocatechin-3-gallate | 0–40 µM | ↑ TIMP -3 levels↓ Cell proliferation by restoring the MP/TIMP balance |
[99] | |
| MCF-7 | Hesperetin | 0–200 µM | ↑ ROS generation ↑ ASK1/JNK pathway ↑ Apoptosis ↓ Δψm |
[87] | |
| BT-474 | Apigenin | 0–100 µM | ↑ Apoptosis ↓ STAT3 signaling ↓ Cell proliferation ↓ Chlorogenic survival |
[100] | |
| MCF-7 | Kaempferol | 0–100 µM | ↑ Extracellular lactate levels ↓ Cell proliferation ↓ Glucose uptake |
[101] | |
| 0–100 mg/mL | ↑ Apoptosis ↓ Cell proliferation ↓ Δψm |
[102] | |||
| MDA-MB-231 | Isorhamnetin | 0–40 µM | ↓ Cell proliferation ↓ Cell migration ↓ Cell adhesion ↓ Expression of MMP-2 and MMP-9 |
[103] | |
| MDA-MB-231, MDA-MB-468 | Quercetin | 0–100 µM | ↓ Cell proliferation ↓ Cell viability ↓ β-Catenin |
[104] | |
| MDA-MB-231 (4175) LM2, MDA-MB-435 | Luteolin | 0–100 µM | ↑ Apoptosis ↓ Cell migration ↓ Cell viability ↓ VEGF secretion |
[105] | |
| MDA-MB-231 | Luteolin | 0–40 µM | ↑ Apoptosis ↓ Cell viability ↓ Expression of MMP-9 ↓ Cell migration ↓ Cell invasion |
[106] | |
| MDA-MB-453, MCF-7 | Luteolin | 10 µM | ↑ Apoptosis ↑ Expression of miR-203 ↓ Cell viability ↓ Ras/Raf/MEK/ERK signaling pathways |
[107] | |
| MDA-MB-231, MCF-7, MDA-MB-453 | Delphinidin | 40 μmol/L | ↓ Cell viability ↓ Cell proliferation ↓ Cell migration ↓ Wnt/β-catenin signaling pathway -Modulating miR-34a and HOTAIR |
[108] | |
| MCF-7 | Quercetin | 25 μmol/mL | ↑ Apoptosis ↑ ROS levels and MDA ↓ Cell viability ↓ Cell proliferation ↓ Antioxidant enzymes activity |
[109] | |
| Ovarian cancer | ES2 | Delphinidin | 0–100 µM | ↑ Apoptosis ↓ Cell proliferation ↓ Cell migration ↓ AKT, ERK1/2, and MAPK signaling pathways |
[110] |
| SK-OV-3 | Genistein | 0–90 µM | ↑ Apoptosis ↓ Cell proliferation ↓ Δψm |
[111] | |
| OVCAR-3, SKOV-3 | Kaempferol | 0–100 µM | ↑ Apoptosis ↑ Expression of DR4, DR5, CHOP, JNK, ERK1/2, p38 ↓ Cell proliferation -Modulates the expression of apoptotic pathway proteins |
[112] | |
| CAOV3 | Quercetin | 0–100 µM | ↑ Apoptosis ↓ Cell viability |
[113] | |
| A2780/CP70, OVCAR-3 | Kaempferol | 0–50 µM | ↑ Cell cycle arrest at G2/M phase via Chk2 ↑ Apoptosis via death receptors ↓ Cell viability |
[114] | |
| PA-1 | Quercetin | 0–200 µM | ↑ Apoptosis ↓ Cell viability ↓ Bcl-2, Bcl-xL |
[115] | |
| A2780, OVCAR-3, SKOV-3 | Apigenin Luteolin Myricetin |
0–100 µM | ↑ ROS levels ↑ MDA levels ↑ Apoptosis ↑ Cell cycle arrest at G0/G1 and G2/M phase ↓ Cell viability |
[116] | |
| Cervical cancer | HeLa | Quercetin | 0–100 µM | ↑ Apoptosis ↑ Cell cycle arrest at G2/M phase ↑ ROS levels ↓ Cell proliferation ↓ Δψm |
[117] |
| HeLa | Kaempferol | 0–100 mg/mL | ↓ Cell proliferation | [102] | |
| 2.5–100 µM | ↑ Bax ↓ Expression of Cyclin B1 ↓ Expression of CDK1 ↓ NF-κB nuclear translocation ↓ Bcl-2 |
[6] | |||
| 0–100 µM | ↑ Apoptosis ↓ Cell viability ↓ PI3K/AKT and hTERT pathways |
[118] | |||
| SiHa | Kaempferol | 0–100 µg/mL | ↑ Apoptosis ↑ Intracellular free Ca2+ ↓ Cell proliferation ↓ Δψm |
[119] | |
| Lung cancer | H446 | Genistein | 0–100 µM | ↑ Apoptosis ↑ Cell cycle arrest at G2/M phase ↓ Cell proliferation ↓ Cell migration |
[120] |
| NCI-H1299, -H460 | Luteolin | 0–50 µM | ↑ Apoptosis ↓ Cell viability |
[121] | |
| A549 | Kaempferol | 0–50 µM | ↓ Cell proliferation ↓ Cell migration ↓ TGF-β1-induced EMT |
[122] | |
| 0–100 mg/mL | ↓ Cell proliferation | [102] | |||
| RAW 264.7 | Luteolin | 0–30 µM | ↓ Cell proliferation ↓ Cell migration ↓ STAT6 phosphorylation and the TAM phenotype ↓ Expression of CCL2 and migration of monocytes |
[123] | |
| A549 | Genistein | 0–200 µM | ↑ Apoptosis ↑ Bax mRNA level ↑ Expression of miR-27a ↓ Cell proliferation ↓ Cell viability ↓ Bcl-2 mRNA level ↓ Expression of MET protein |
[124,125] | |
| A549 | Apigenin | 0–100 µM | ↓ Cell proliferation ↓ Cell migration and invasion by targeting the PI3K/Akt signaling pathway |
[126] | |
| A549, H1299 | Daidzein | 0–80 µmol/L | ↑ Apoptosis ↓ Cell proliferation |
[41] | |
| A549 | Delphinidin | 0–80 µM | ↓ Cell proliferation ↓ ERK, mTOR and p70S6K signaling pathways |
[127] | |
| A549 | Kaempferol | 0–50 µM | ↑ Apoptosis ↑ Expression of miR-340 ↓ Cell proliferation ↓ Cell viability ↓ Expression of Cyclin D1 ↓ p-PI3K and p-AKT levels |
[128] | |
| A549 | Fisetin | 0–40 µM | ↑ Apoptosis ↑ Cell cycle arrest at G2/M phase ↓ Cell viability ↓ Cell proliferation ↓ Cell adhesion ↓ Cell invasion ↓ Cell migration ↓ ERK signaling pathway via MEK1/2 |
[129] | |
| H1299, A549 | Epigallocatechin-3-gallate | 0–40 µM | ↑ Apoptosis ↓ Cell proliferation ↓ Expression of p-PI3K and p-Akt |
[130] | |
| A549 | Hesperetin | 0–100 µM | ↓ Cell proliferation | [9] | |
| A549 | Epigallocatechin-3 -gallate |
40 µM | ↑ miR-155 ↑ Cell cycle arrest at G0/G1 phase ↓ Cell proliferation ↓ miR-212 |
[131] | |
| Colon cancer | HT-29 | Kaempferol | 0–60 µmol/L | ↑ Apoptosis ↓ Δψm |
[132] |
| HT-29 | Epigallocatechin-3-gallate | 0–50 µM | ↑ MAPK and Akt signaling pathways ↓ p38 and ERK1/2 signaling pathways |
[133] | |
| HCT-116 | Resveratrol | 0–150 µM | ↑ Apoptosis ↑ DNA damage |
[134] | |
| HCT-116, SW480, LoVo, HT-29 | Naringenin | 0–200 µM | ↑ Apoptosis ↓ Cell viability |
[135] | |
| HCT-116, LoVo | Genistein | 0–100 µM | ↑ Apoptosis ↑ Bax mRNA level ↓ Cell proliferation ↓ Cell viability ↓ Phosphorylation of Akt |
[136] | |
| Liver cancer | HepG2, Huh-7, HA22T | Naringenin | 0–100 µM | ↓ Cell proliferation ↓ TPA-induced cancer cell proliferation |
[137] |
| Huh-7, HepG2, Hep3B, SK-Hep-1 | Xanthohumol | 0–15 µM | ↑ Apoptosis ↓ Cell viability ↓ Colony forming ↓ Notch1 signaling |
[138] | |
| HepG2 | Xanthohumol | 0–40 µM | ↓ cell proliferation ↑ Apoptosis -modulates NK-kB/p53 signaling pathways |
[139] | |
| Hepa1-6 | Genistein | 0–100 µM | ↑ Apoptosis ↓ Cell viability ↓ Cell proliferation |
[140] | |
| HepG2 | Kaempferol | 0–100 µM | ↑ Apoptosis ↓ Cell proliferation ↓ Cell migration ↓ Cell invasion ↓ Expression of miR-2I |
[141] | |
| Prostate cancer | PC-3 | Hesperetin | 0–120 µM | ↑ Apoptosis ↓ Cell proliferation ↓ NK-kB signaling pathway |
[142] |
| LNCaP | Kaempferol-3-O-rhamnoside | 0–926 µM | ↑ Apoptosis ↓ Cell proliferation |
[143] | |
| PC-3, DU145 | Resveratrol | 0–100 µM | ↑ Autophagy cell death | [144] | |
| Gastric cancer | SGC-7901, MKN28 | Kaempferol | 0–200 µM | ↑ Apoptosis ↑ Cell cycle arrest at G2/M phase ↓ Cell proliferation ↓ Cell viability |
[145] |
| HGC-27, SGC-7901 | Apigenin | 0–20 µg/mL | ↑ Apoptosis ↓ Cell proliferation ↓ Δψm |
[146] | |
| SGC-7901, MGC-803, HGC-27 | Hesperetin | 0–400 µM | ↑ Apoptosis ↓ Cell proliferation ↓ Δψm ↓ Cell viability ↓ ROS levels |
[147] | |
| HGC-27, SGC-7901 | Myricetin | 0–40 µM | ↑ Apoptosis ↑ Cell cycle arrest at G2/M phase ↓ Cell proliferation |
[148] | |
| SCG-7901 | Kaempferol | 0–100 mg/mL | ↓ Cell proliferation | [102] |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
|
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drețcanu, G.; Știrbu, I.; Leoplold, N.; Cruceriu, D.; Danciu, C.; Stănilă, A.; Fărcaș, A.; Borda, I.M.; Iuhas, C.; Diaconeasa, Z. Chemical Structure, Sources and Role of Bioactive Flavonoids in Cancer Prevention: A Review. Plants 2022, 11, 1117. https://doi.org/10.3390/plants11091117
Drețcanu G, Știrbu I, Leoplold N, Cruceriu D, Danciu C, Stănilă A, Fărcaș A, Borda IM, Iuhas C, Diaconeasa Z. Chemical Structure, Sources and Role of Bioactive Flavonoids in Cancer Prevention: A Review. Plants. 2022; 11(9):1117. https://doi.org/10.3390/plants11091117
Chicago/Turabian StyleDrețcanu, Georgiana, Ioana Știrbu, Nicolae Leoplold, Daniel Cruceriu, Corina Danciu, Andreea Stănilă, Anca Fărcaș, Ileana Monica Borda, Cristian Iuhas, and Zorița Diaconeasa. 2022. "Chemical Structure, Sources and Role of Bioactive Flavonoids in Cancer Prevention: A Review" Plants 11, no. 9: 1117. https://doi.org/10.3390/plants11091117