Abstract
The paper deals with molecular self-organization leading to formation of a protocell. Plausible steps towards a protocell include: polymerization of peptides and oligonucleotides on mineral surfaces; coevolution of peptides and oligonucleotides with formation of collectively autocatalytic sets; self-organization of short peptides into vesicles; entrapment of the peptide/oligonucleotide systems in mixed peptide and simple amphiphile membranes; and formation of functioning protocells with metabolism and cell division. The established propensity of short peptides to self-ordering and to formation of vesicles makes this sequence plausible. We further suggest that evolution of a protocell produced cellular ancestors of viruses as well as ancestors of cellular organisms.
Similar content being viewed by others
Introduction
Self-ordering of short peptides with formation of fibrils, microtubes and vesicles has recently become an area of intensive research (Santoso et al. 2002; Dobson 2003; Lu et al. 2003; Takahashi and Mihara 2004; Reches and Gazit 2004, 2006). One reason for increased interest is the major role of amyloid fibrils in a number of diseases, such as Alzeimer’s and Parkinson’s diseases and type II diabetes, affecting large numbers of people with devastating consequences. Another reason is a possibility of using the self-ordering property of short peptides to build ordered nanostructures for applications in nanotechnology.
This paper is an attempt to explore the role of self-organization of short peptides in the context of plausible steps leading to the origin of a protocell capable of biological evolution. The plausibility is based on three major considerations: evolutionary continuity, ubiquity of required environmental conditions, and robustness of the model (Deamer and Fleischaker 1993; Carny and Gazit 2005). Special attention is given to the role of short self-organizing peptides in the formation of a protocell. In follow-up work, we intend to incorporate the steps presented here in a computer simulation model that will serve as an adaptive framework capable of changing with new experimental findings.
The key event in the origin of life as we know it is formation of a system capable of both replication and translation with the properties of catalysis, energy use and growth. Such a system signifies transition from chemical evolution to biological evolution (Pattee 1965; Mora 1965). Here we will present our hypothesis on the origin of such a system leading to formation of a protocell.
A very general definition of a protocell involves self-maintenance, self-reproduction and evolvability (Luisi et al. 2006).
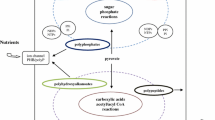
The following steps towards formation of a protocell will be discussed:
- 1.
Formation of collectively autocatalytic polynucleotide/polypeptide systems on a mineral surface.
- 2.
Self-ordering of short peptides and simple amphiphiles into vesicles and membranes covering the polynucleotide/polypeptide systems.
- 3.
Encapsulation of the polynucleotide/polypeptide assemblies by membranes and transfer of the encapsulated units (protocells) to water.
- 4.
Evolution of protocells; development of metabolism and of cell division.
Our approach to formation of protocells is based on several assumptions:
Coevolution of polypeptides and polynucleotides.
Initial polymerization of amino acids and nucleotides on mineral surfaces.
Fluctuating environmental conditions.
We will examine these assumptions in order and then will discuss plausible steps towards development of protocells. We will further suggest a possibility that the described protocells evolved to constitute cellular predecessors of contemporary viruses that later became parasitic and then devolved, as well as to constitute predecessors of the contemporary cellular life forms.
Since the Miller and Urey classical experiments conducted in a mixture of reduced gases (Miller 1953; Miller and Urey 1959) and follow-up studies conducted in a variety of atmospheres and with many energy sources (see review in Rode 1999), it seems reasonable to conclude that amino acids were present on the prebiotic Earth. This conclusion is further supported by recent theoretical models of Feng Tian and coworkers (Tian et al. 2005) indicating that hydrogen escaped from the atmosphere of the primordial Earth more slowly than previously thought, suggesting a more efficient production of prebiotic organic compounds by electric discharge.
A variety of prebiotic molecules including amino acids, hydrocarbons and even purines have been found in carbonaceous chondrites, comets and micrometeorites (Kvenvolden et al. 1970; Cronin and Pizzarello 1983; Chyba et al. 1990; Chyba and Sagan 1992; Pierazzo and Chyba 1999; Botta and Bada 2002; Meierhenrich et al. 2004). It is reasonable to assume that they may have arrived on the early Earth within fragments of these bodies. In the Murchison meteorite, dipeptides have been identified in addition to previously found amino acids (Meierhenrich et al. 2004).
Although the synthesis of nucleosides is still unresolved, several possible mechanisms of their formation have been suggested and reviewed (Joyce 1989; Ferris 1993; Orgel 2004). In the present work, we will assume that both amino acids and nucleosides were available on the early Earth and that metabolism of initial life forms was heterotropic.
A semipermeable membrane is a key feature of a protocell. Although presently phospholipid molecules and protein transport systems are major features of cell membranes, we consider it unlikely that they were available in the vicinity of polynucleotides and polypeptides on the early Earth. Instead, we suggest that the first membranes could have consisted of amphiphilic polypeptides, similar to the ones that have been found to self-organize into tubular or vesicular amyloid structures (Santoso et al. 2002; Vauthey et al. 2002; Takahashi and Mihara 2004; Reches and Gazit 2004), in combination with other prebiotically available amphiphilic molecules (Hargreaves and Deamer 1978; Lawless and Yuen 1979; Naraoka et al. 1999; Apel et al. 2002).
Coevolution of Polypeptides and Polynucleotides
Among origin of life scientists, a major divide exists between those who consider polypeptides as the first molecules exhibiting life-like organization (Dyson 1982; Kauffman 1986; Rode 1999) and those with the view that this role belongs to polynucleotides (Rich 1962; Gilbert 1986; Joyce 1989; Weiner and Maizels 1991; Maurel 1992). There are convincing arguments on both sides of the divide.
Proteins are universal catalysts of biochemical reactions. Known catalytic activity of short peptides (Shen et al. 1990; Lahav 1991; Li et al. 1992) makes these likely candidates for early involvement in prebiological molecular evolution. Peptides are also favored because amino acids are thought to have been easily available and ubiquitous on the early Earth. However, the problem with the proteins-first hypothesis is that it doesn’t indicate a clear path to replication and preservation of information, the functions necessary for biological evolution.
On the other hand, the proponents of the polynucleotide-first (or the RNA world) approach refer to the essential role of RNA molecules as information carriers (Rich 1962; Gilbert 1986; Joyce 1989; Weiner and Maizels 1991; Maurel 1992). This approach received increased support after the discovery that RNA molecules exhibit catalytic properties and therefore can be enzymes as well as replicators (Cech et al. 1981; Ban et al. 2000). The problem with the RNA world hypothesis is that it does not reveal how the initial RNA molecules might have been formed from the relatively short polynucleotides which, at best, had weak catalytic properties.
In all known life forms, protein synthesis requires RNA molecules and RNA synthesis requires proteins. Based on the continuity principle (Orgel 1968; Morowitz 1992; Weiner and Maizels 1991), the early collaboration of oligonucleotides and peptides seems to be a more plausible scenario. Various models that use this approach (Eigen 1971; Eigen and Schuster 1978; Brack and Barbier 1990; Lahav 1991, 1993) can be grouped together as coevolution hypotheses. According to these hypotheses, oligonucleotides served as templates for peptide synthesis as well as for their own replication. Peptides of certain compositions and, in some cases, oligonucleotides provided catalytic support of these reactions.
The coevolution scenario has been reinforced by studies of phosphoryl amino acids. Since volcanic activity on the early Earth was probably frequent, we can expect accumulation of pyrophosphate and polyphosphate on the Earth surface. Experimental studies by Li, Zhao, Cheng, and Zhou et al. (Li et al. 1992; Zhou et al. 1996; Cheng et al. 2004) have shown that reaction of these inorganic phosphates with amino acids produces N-phosphoryl amino acids having a high energy P–N bond. In preliminary model reactions of N-phosphoryl amino acids with amino acid and nucleoside mixtures, it appears that the high energy of the P–N bonds is transferred to form both peptide bonds and phosphodiester bonds. These results suggest the possibility of the simultaneous formation of peptides and oligonucleotides (Zhao and Cao 1994; Zhou et al. 1996; Cheng et al. 2004) at room temperature.
Initial Polymerization on Mineral Surfaces
There is experimental evidence that short peptides could be produced by polymerization of amino acids in a variety of prebiotic circumstances, such as by salt-induced reactions of amino acids (Saetia et al. 1993; Rode and Suwannachoti 1999) or by photoprocessing and thermal processing of amino acids in simulated interstellar ice (Ehrenfreund et al. 2001; Munoz et al. 2002; Meierhenrich et al. 2004).
Formation of short oligonucleotides could take place in frozen reaction conditions that concentrate reactants in eutectic regions between ice crystals (Sanchez et al. 1966; Stribling and Miller 1991; Gryaznov and Letsinger 1993). Kanavarioti et al. (2001) have found that in the presence of metal ions, freezing of dilute solutions of activated nucleotides produced short oligonucleotides, including notoriously difficult to polymerize oligouridilate. Cold temperatures causing freezing presumably prevailed on Earth in the early Archaean era due to lower Sun luminosity.
Further polymerization reactions in nature could take place on mineral surfaces in mud beds, on shores and in tidal pools with alternating wet and dry conditions, in pore space under the Earth’s surface (Colgate et al. 2003) or around hydrothermal vents at the sea bottom. Clays and other mineral surfaces were easily available in these locations.
Adsorption of amino acids, peptides, nucleotides or oligonucleotides on mineral surfaces promoted their polymerization by concentrating these molecules from the environment. There is also evidence that mineral surfaces could protect organic materials from ultraviolet radiation (Negron-Mendoza et al. 2006).
Formation of peptide bonds in polypeptides and phosphodiester bonds in polynucleotides requires removal of water; these reactions are not thermodynamically favored in an aqueous environment. For adsorbed molecules, access of water to the interfaces is structurally restricted and therefore hydrolysis is substantially diminished. Additional thermodynamic advantage of surface reactions over reactions in solution is related to the entropy of the reactions. If the reactants are attached to a mineral surface, the entropy of the polymerization reaction doesn’t have as great a negative value as it does for the same reaction in a solution, since it doesn’t involve a substantial decrease in mobility (Wachtershauser 1988).
Multiple experiments have shown that some common mineral surfaces, such as those of clay, catalyze polymerization reactions (Ferris 1993; Ertem and Ferris 1996; Ferris et al. 1996). For example, Ferris et al. (1996, 2005) found that, whereas polymerization of activated nucleotides in solution produced oligomers typically shorter than 10-mers, the presence of montmorillonite clay particles allowed their size to increase to 55 monomers. The results have been the same for purine and pyrimidine nucleotides (Kamura and Ferris 1999). The catalytic action of montmorillonite clays towards phosphodiester bond formation is thought to arise due to preferred orientation of adsorbed monomers on the clay surface and to the acidity of the clay surface (Ferris et al. 1989).
The presence of inorganic cations (Franchi et al. 2003; Lutay et al. 2006) and/or decrease in PH favors adsorption by increasing electrostatic interaction between the adsorbed molecules and the surface. The strength of adsorption was also observed to increase with the length of the polymer molecule (Lailach et al. 1968; Theng 1974, 1982).
Fluctuating Environmental Conditions
Heating and drying is a robust way to promote polymerization reactions of peptides and oligonucleotides. Several authors (Lahav and Chang 1976; Kuhn 1976; Lahav and White 1980; White and Erickson 1981) have studied the influence of fluctuations between hot/dry and cold/wet environmental conditions on condensation reactions in prebiotic times. Lahav and Chang (1976) reviewed the effect of a fluctuating environment on adsorption characteristics of amino acids and peptides on clay surfaces. They concluded that during dehydration these molecules become more strongly attached to clay surfaces. This happens due to increased ionization of water leading to protonation of basic functional groups in peptides with a resulting increase in attraction of peptides to anionic sites on clay. Dry heat drives loss of water that makes condensation reactions favorable. During a following rehydration, amino acids and short peptides are desorbed and redistributed in the aqueous solution.
Clay adsorption behavior of purine and pyrimidine bases and nucleotides follows a similar pattern as shown by Lailach et al. (1968) and Theng (1974): adsorption increases in a dry state and decreases in a wet state. Due to higher adsorption of longer molecules as compared to shorter ones, the oligomer to monomer ratio is higher on the clay surface than in solution (Lahav and Chang 1976; Lahav and White 1980). As the polymerization process continues, the longer polymer chains grow in preference to the formation of new short chains, since longer chains are less likely to be transferred into solution during hydration parts of the cycle. During dry/hot periods, hydrogen bonds of folded polynucleotide molecules melt making these molecules available to act as templates. Since longer oligomers are attached to the surface more strongly than shorter ones, they would be relatively fixed on the surface while short oligomers would diffuse across them and, over time, they would reach the long oligomer sites they can pair with. The preferable environment for base pairing is a dry part of the cycle with a not very high temperature.
With the assumptions stated above, we can outline a plausible sequence of steps towards formation of protocell:
Formation of Collectively Autocatalytic Sets of Polypeptide and Polynucleotide Molecules on Mineral Surfaces
Catalytic properties of proteins are well known. Although most of the prebiotic peptides were likely to be too short to form secondary or tertiary structures, Shen and coworkers have found experimentally that even very short peptides, for example the histidyl–histidine (His-His) dimer, exhibit catalytic properties (Shen et al. 1990). The authors concluded that the imidazole rings of His–His may act not only as a general base–acid catalyst, but will also create an activated intermediate for formation of phosphodiester or peptide bonds.
Even free amino acids have been shown to assemble around substrate molecules forming enzyme-like ‘active sites’ that exhibited specific catalytic activities towards the o-nitrophenol-β-D-galactopyranoside substrate (Bar-Nun et al. 1994). Later, Kochavi et al. (1997) polymerized a group of amino acids in the presence of a substrate acting as template and a prebiotic condensation agent, dicyandiamide, to form Cys2–Fe+2 polypeptides. These short oligopeptides were found to be highly specific catalysts for the substrate molecules that directed their formation. Carny and Gazit (2005) pointed to aromatic stacking interactions between nucleic bases and RNA binding proteins as a possible mechanism for specific binding of nucleotides to short amyloid-forming peptides (Blakaj et al. 2001). Polynucleotides (Orgel 2004) and metal ions (Lohrmann et al. 1980; Sawai 1976; Sawai et al. 1989, 1992; Ferris et al. 1989; Ferris 1993, 2006) constitute two additional classes of prebiotic catalysts.
There is experimental evidence that nucleotides and nucleotide bases deposited on mineral surfaces can be selective in binding amino acids from the solution (Soberby et al. 1996, 2000, 2002). This selectivity was shown to start as a bias in spatial arrangement of amino acids before formation of peptide bonds. Soberby and his coworkers (Soberby and Heckl 1998) studied the nature of this bias. They conducted molecular mechanics energy minimization computer experiments to model behavior of glycine molecules atop a uracil monolayer adsorbed on a MoS2 surface. Adsorption of amino acids on the proton accepting groups of the uracil monolayer positioned them favorably for peptide bond formation by placing the positively charged ammonium group of an amino acid molecule in close proximity to the negatively charged carboxylate group of the adjacent amino acid molecule. This proximity could not take place if the side chains of various amino acids having various lengths are oriented towards the surface. This leaves carboxylate and ammonium groups as the most likely candidates for direct interaction with the adsorbed bases.
The energy minimized structures suggested that a linear hydrogen bond was formed between the protonated ammonium of glycine and the keto group of uracil. The authors found that the geometric patterns of proton acceptors in the adsorbed nucleotides are consistent with the distances between adjacent amino acid monomers in beta-sheet peptides. They explained the adsorption selectivity by differences in hydrogen bonding of various amino acids to the proton acceptors; these caused by a long range effect of amino acid R groups on the chemical properties of ammonium and carboxylate groups (Soberby et al. 2002).
The selectivity of bonding the polynucleotide bases towards amino acids is equivalent to the emergence of a sort of template. Since the selectivity is not very high, we will call peptides formed on such a template ‘statistical peptides’ (White 1980). Statistical peptides are non-random peptides related to each other by their sequences due to being inaccurately translated from the same polynucleotide template.
If, by chance, one of such rough templates produced a catalytic peptide, this could have started an autocatalytic process. One of the prerequisites of autocatalytic behavior is adsorption of the catalytic peptide in close proximity to its formation, so that it will promote replication of the polynucleotide that created it. In our model, adsorption on a mineral surface will provide the proximity and, as a result, some selectivity in catalysis of polynucleotide replication necessary for the formation of an autocatalytic cycle.
The polynucleotide that produced a catalytic peptide reproduced faster than the other polynucleotides. This, in turn, produced more catalytic peptides, etc. The autocatalytic reactions would further accelerate if, similar to the cases discussed above, a formed peptide was a specific catalyst for the replication of the polynucleotide that directed its formation.
In reality, however, due to the statistical character of polynucleotide templates, the peptide compositions as well as the replicated polynucleotide compositions had only statistical resemblance to their predecessors. Only some of them, which reproduced with high fidelity, participated in the autocatalytic cycle. This led to the slowing of replication rates and to ‘focusing’ the composition of the autocatalytic set.
For the autocatalytic set to ‘survive,’ the rate of replication had to be higher than the rate of degradation due to hydrolysis, high energy radiation or other causes plus the rate of loss from the immediate environment. The autocatalytic sets exhibiting higher growth rates and higher selectivities than their less efficient neighbors have been naturally selected. This resulted in further composition ‘focusing’ towards formation of a smaller subset of more efficient autocatalytic systems.
Continuation of natural selection required mutability of the base sequence. This mutability was provided by low accuracy of replication. Since the system was open to new molecules, the number and variety of molecules involved in the mutually catalytic activity grew and the set developed into a mutually catalytic network that could sustain itself (Kauffman 1993, 1995, 2000).
Self Organization of Short Peptides and Simple Amphiphiles into Membranes
Amphiphilic peptides forming the first membranes
Contemporary cell membranes have phospholipids as their major components. It seems not highly probable that phospholipids had been available for the protocells on the early Earth at the same location as peptides and oligonucleotides. Another consideration against phospholipids being the first membrane materials is their low permeability to polar and ionic molecules (Deamer et al. 1994; Mouritsen et al. 1995; and Monnard and Deamer 2001). In contemporary cells, this lack of permeability is overcome by complex protein transport systems, which were not likely to be available at the time of protocell development. Instead, the first membranes had to rely on passive diffusion for uptake of nutrients and energy sources from the environment.
There are several types of amphiphilic peptides that are capable of forming membrane-like structures. Polypeptides with alternating hydrophobic and hydrophilic amino acids tend to form beta-sheets with one hydrophobic and one hydrophilic surface (Brack and Orgel 1975; Brack 1993). These sheets aggregate into membrane bilayers with hydrophobic interior and hydrophilic exterior. Hydrophilic surfaces consist of charged ionic side chains of amino acids, while hydrophobic ones are formed from hydrophobic side chains. In prebiotic conditions, a tendency to form beta-sheet polypeptide structures was enhanced by preference of more prevalent short peptides to create beta-sheet rather than helical structures. These beta-sheet bilayers exhibit higher thermal and chemical stability as compared with alpha helices and therefore they accumulated preferentially (Brack 1993).
An interesting feature of polypeptide organization is that only homochiral polypeptides can form beta-sheets whereas the racemic ones stay mostly unstructured (Brack and Spach 1979). Molecules containing a mixture of homochiral and racemic segments form aggregates of stable optically pure beta-sheets surrounded by less stable racemic disordered segments. When these molecules are subjected to mild hydrolysis, the residual fraction is enriched in the more stable homochiral beta-sheet component (Brack 1993).
A variety of very short peptides have been found to self-assemble in-vitro into amyloid-like fibril structures, microtubular structures, or vesicular structures (Maji et al. 2001; Gazit 2002; Santoso et al. 2002; Reches et al. 2002; Vauthey et al. 2002; Reches and Gazit 2003, 2004, 2006; Lu et al. 2003; Dobson 2003; Carny and Gazit 2005). A large number of disorders including Alzeimer’s and Parkinson’s diseases and type II diabetes are associated with amyloid fibrils. The ability of polypeptide chains to form amyloid structures is recognized to be a generic feature of polypeptides, not limited to the specific ones associated with the particular clinical disorders.
Transmission electron microscopy, X-ray diffraction, and other biophysical techniques (Dobson 1999; Sipe and Cohen 2000; Maji et al. 2001; Reches et al. 2002) revealed that fibrils formed by polypeptides of various compositions are characterized by core structures composed of beta-sheets whose strands are perpendicular to the fibril axis. This structure is thought to be stabilized by interactions involving the polypeptide main chain, in particular by hydrogen bonds. Since the main chain is common to all polypeptides, this can explain the observation that fibrils formed from polypeptides with different sequences seem to be so similar (Dobson 2003).
Self-organization in a group of surfactant-like short peptides has been studied by Vauthey et al. (2002). These peptides contain seven to eight residues and are about 2 nm long and consist of a hydrophilic negatively charged aspartic acid head group and a hydrophobic tail made of alanine, valine, or leucine. In water, these peptides tend to self-assemble into bilayers that form vesicles and microtubes with diameters ranging from 30 to 50 nm. Molecular modeling results showed that 2 nm long V6D peptides are likely to participate in tail-to-tail packing and to be organized into a bilayer about 4 nm thick.
Among the amphiphilic peptides observed to self-assemble into membranous structures, the most relevant for the study of the origins of life are the ones that are composed of simple amino acids, those likely to be more abundant in prebiotic times. Glycine and aspartic acid are such simple amino acids that have been found in carbonaceous chondrites (Pizzarello 2004; Meierhenrich et al. 2004). Santoso et al. (2002) observed the self-organization of vesicles and microtubes by peptides consisting of two aspartic acids and a string of glycines, from two to ten residues long in water at neutral PH. These peptides could have been produced in prebiotic conditions by repeated hydration–dehydration cycling (Yanagawa et al. 1988) or by heating on dry clay surfaces (White et al. 1984).
Recently, Reches and Gazit (2004) demonstrated formation of well-ordered microtubes and closed-cage nanostructures between 50 and 100 nm in diameter by aromatic dipeptides, diphenylglycine molecules, as building blocks. These self-assembled structures demonstrated remarkable stability under extreme temperature, pressure and chemical conditions (Carny and Gazit 2005). The authors also found that similar structures are formed from diphenylalanine peptides in the presence of thiol. The diphenylalanine peptide constitutes the core recognition motif of Alzeimer’s beta-amyloid polypeptide. Carny and Gazit (2005) suggested that stacking interactions between aromatic moieties of the peptides provided energy as well as order and directionality for the initial formation of a pleated sheet structure. In addition to aromatic stacking interactions, the sheet was stabilized by hydrogen bonds. The closure of the extended sheet along one axis led to formation of tubular structures, similar to Alzeimer’s beta-amyloids, while polypeptide closure along two axes led to spherical vesicular structures.
Additional Membrane-Forming Amphiphiles
In addition to polypeptides, a variety of other amphiphilic molecules including fatty acids and fatty alcohols could have been components of prebiotic membranes (Deamer 1997; Deamer et al. 2002). Some of these molecules are thought to have been synthesized abiotically in the early Solar System and delivered to Earth during late accretion as confirmed by finding these molecules in carbonaceous meteorites (Lawless and Yuen 1979; Naraoka et al. 1999; Monnard et al. 2002). They have been also produced on Earth under simulated prebiotic conditions (Deamer and Oro 1980; McCollom et al. 1999; Rushdi and Simoneit 2001).
Even short molecules of this type have been experimentally demonstrated to form vesicles (Hargreaves and Deamer 1978; Apel et al. 2002; Monnard and Deamer 2002). Generally, the permeability of these vesicles to ionic solutes increases with decrease in length and degree of unsaturation of hydrocarbon molecules. Minimal concentration necessary to produce a bilayer (critical bilayer concentration) also increases with decreasing length and the degree of unsaturation of these chains. This means that formation of vesicles from the short molecules can take place only at high concentration of these molecules. Such high concentration could have been obtained during hot-dry seasons on rock surfaces and on the surfaces of polypeptide–polynucleotide complexes.
It seems hardly plausible that primitive membranes have been composed of one pure amphiphile. We suggest that they contained a mixture of amphiphilic polypeptides with other prebiotically available amphiphiles such as mixed fatty acids and alcohols. The mixed fatty acids and alcohols have a lower critical bilayer concentration and exhibit higher stability than their pure components. For example, an aqueous solution of nonanoic acid required minimum concentration of 85 mM for vesicle formation, whereas addition of 2 mM of nonanol lowered this value to 20 mM while increasing the stability of the vesicles (Monnard and Deamer 2002).
Before phospholipid membranes became available, mixed membranes composed of peptides and other prebiotic amphiphiles could have served as early semipermeable membranes.
Encapsulation of Polynucleotide/Polypeptide Systems by Membranes
Computer simulations (Chipot and Pohorille 1996) have revealed a tendency of small amphiphilic peptides to accumulate and to organize at surfaces and interfaces. Therefore, we can expect amphiphilic peptides to form upon polypeptide–polynucleotide aggregates on mineral surfaces, in addition to forming vesicles in water. Since amphiphilic peptides exhibit lipid-like properties, they could have encapsulated these aggregates and performed the functions of first membranes.
The encapsulation could take place as a result of a dehydration–rehydration process, as described by Deamer and Barchfeld (1982), Shew and Deamer (1985), Chakrabarti et al. (1994), Monnard et al. (1997), and Monnard (2003). During the dehydration phase of the environmental cycle, multilamellar deposits were formed consisting of: the polypeptide–polynucleotide aggregates, surface layers of amphiphilic peptides and vesicles previously contained in water. Since a stable bilayer structure couldn’t be maintained around the flattened edges of vesicles during dehydration, fusion could have happened where the vesicles came into contact. In this process, the polypeptide–polynucleotide aggregates became sandwiches between the fused vesicles. During the rehydration phase of the cycle, these lamellar structures swelled and formed vesicles containing polypeptide–polynucleotide aggregates. A similar pattern of vesicle formation and encapsulation of large molecules within the vesicles has been observed with phospholipids as membrane-forming molecules (Deamer and Barchfeld 1982).
Protocell Organization and Function
Although definitions of protocells vary (Pohorille and Deamer 2002; Luisi 2002; Oberholzer and Luisi 2002), we use the most widely accepted one, that the protocell is a local molecular assembly that continuously regenerates itself, replicates itself, and is capable of evolving (Rasmussen et al. 2004; Luisi et al. 2006). We suggest that these characteristics could have been achieved by housing polypeptide–polynucleotide systems inside semipermeable membranes consisting of polypeptides mixed with other prebiotically available amphiphiles.
Experimental studies of encapsulation of NDP substrates and various polymeraze enzymes within a number of different amphiphilic vesicles (Chakrabarti et al. 1994; Walde et al. 1994; Yu et al. 2001; Treyer et al. 2002) demonstrated that all components of transcription–translation catalytic systems can be entrapped and remain active as a result of dehydration/rehydration encapsulation (Monnard 2003). RNA transcription and translation can proceed after encapsulation.
Permeability of membranes could have been provided by pores and channels or by transient defects due to thermal fluctuations. Permeability of short chain amphiphilic membranes increases with decrease in the length of the carbon chains (Paula et al. 1996) and the degree of unsaturation. Walde et al. (1994) have demonstrated that the oleic acid/oleate membranes provided enough permeability for even large ionic molecules like ADP, ADP and ATP to diffuse across the bilayers into vesicles while retaining nucleic acids and proteins. Mixed membranes composed of relatively short amphiphilic molecules were likely to have high passive permeability, sufficient for supporting the internal metabolic activities of protocells (Monnard and Deamer 2001).
There is a concern that the peptide membranes or the mixed membranes composed from peptides with other amphiphilic molecules couldn’t provide a permeability barrier to keep reactions in the confined space. Several studies have shown that vesicles formed from short peptides provide a good enough barrier to be used for drug delivery (Neumann et al. 1987; Kimura et al. 1999; Yan et al. 2007). However, additional experimental investigations of the permeability of peptide and mixed vesicles in the context of the origin of life are needed.
Studies of molecular self-assembly of surfactant-like peptides with variable glycine tails leading to formation of nanotubes and vesicles have demonstrated the dynamic character of the self-assembly and disassembly processes (Vauthey et al. 2002; Santoso et al. 2002). A more extensive experimental investigation of growth and division of protocells will help to answer the question of whether membranes composed of short peptides or the mixed peptide–simple amphiphiles membranes are flexible enough to accommodate the processes of growth and division.
In order to be ‘alive,’ the protocell needed a continuous flow of energy. One of the energy sources was the chemical energy of activated monomers. There are several hypotheses on the nature of the activated monomers in prebiotic conditions. We mentioned above that N-phosphoryl amino acids produced by reaction of inorganic phosphates with amino acids and having a high energy P–N bond have been found to promote formation of peptide and phosphodiester bonds. De Duve (1991) suggested that energy for the functioning of the first cells had been provided by thioesters of amino acids. Imidoesters of amino acids and nucleosides constitute another version of activated intermediates (Lohrman and Orgel 1973, 1976).
Important in the context of origins of life are prebiotic activating mechanisms that produce a continuous supply of chemical energy. Light energy is such a source. To capture the light energy, the light had to be absorbed by some photochemical process in a primitive pigment that released or took up protons resulting in creation of a proton gradient across a membrane. Polycyclic aromatic hydrocarbons (PAHs) have been identified as a sort of compound that might fulfill this role (Deamer 1997). PAH derivatives in the form of kerogen-like polymers constitute 90% of organic materials in carbonaceous chondrites and therefore were likely to be present on the early Earth. Since PAHs are relatively nonpolar, they might partition into membranes, where they could absorb light in the blue and near-UV parts of the spectrum and transformed the light energy into chemiosmotic potential.
Activated amino acids and nucleotides that pass through the pores and channels of the membrane could serve as ‘food’ for mutually catalytic reactions leading to RNA polynucleotide replication and translation inside some of the protocells. In these protocells, polynucleotides and polypeptides accumulated. Some polypeptide molecules formed inside the protocell could have been incorporated into the membrane causing membrane growth.
The gradient of molecular concentrations across the protocell membrane created osmotic pressure that caused tension in the membrane. Small variations in tension could be accommodated if the bilayer tension created a better permeation path by disturbing the bilayer structure and creating transient defects or pores in the membrane (Polozov et al. 2001). Better permeation facilitated movement of small molecules and thereby decreased their concentration gradient across the membrane and decreased the osmotic pressure on the membrane.
With the growth of the protocell, the membrane tension increased. At some point, the membrane could no longer keep integrity and the protocell divided into two or more smaller ones, having in the aggregate smaller Gibbs free energy than the ‘parent’ protocell. At this time, a new criterion of fitness emerged: any protocells that divided to yield approximate copies of themselves accumulated in the environment and thus obtained selective advantage.
As Cheng et al. (2004) have found experimentally, fatty acid vesicles stressed by encapsulation of RNA oligomers tend to increase membrane area and to grow at the expense of unstressed vesicles. This observation indicates the emergence of competition among protocells.
Initial evolution of protocells might have been accelerated by the relative instability of RNA. As a result of the instability, some of the RNA molecules would have decomposed faster than they grew. Their decomposition provided building blocks for faster growing protocells. Among the latter, the protocells with more precise and efficient catalytic activities survived and gradually replaced less efficient ones.
Comparison of Protocells with Viruses
Studies of viruses can give us some insight into the formation and behavior of protein–nucleic acid systems. The simplest viruses are composed just of a single strand of RNA inside a protein shell (capsid). All the virus proteins studied so far have an antiparallel beta-barrel structure, which is sometimes called ‘jelly roll.’ The ‘jelly roll’ organization can be visualized as a formation of an hairpin structure, where beta strands are hydrogen bound to each other, followed by arrangement of these pairs side by side to form additional hydrogen bonds, and finally wrapping the pairs around an imaginary barrel (Silva and Rossman 1987; Prasad et al. 1994; Doan and Dokland 2003; Bowman et al. 2003).
Capsids are assembled from small repeating protein subunits; this has an effect of using smaller genome sequences to produce structural proteins. The protein subunits forming capsids are not symmetric. There is a limited number of ways they can be arranged symmetrically in order to achieve minimum free energy of the shell. One is a helical arrangement of units corresponding to the helical shape of nucleic acid (Holmes 1984; Kuznetsov et al. 2005); the other is the formation of triangular facets arranged with icosahedral symmetry (Doan and Dokland 2003; Tihova et al. 2004; Kuznetsov et al. 2005). Some icosahedral viruses have channels that traverse the entire thickness of the shell and provide an entrance and exit from the capsid (i.e. Bowman et al. 2003). The example is a 35-A in diameter channel along the fivefold symmetry axes found in the alfalfa mosaic virus (AMV) capsid (Kumar et al. 1997).
The assembly of nucleic acids and capsid proteins in-vitro to form viruses is a spontaneous process (Vriend et al. 1981; Sorger et al. 1986) which doesn’t require the help of external proteins. Since these molecules are held together by weak bonds such as hydrogen bonds or Van der Waals forces rather than covalent bonds, no enzymes are required for the virus assembly.
However, there are very significant differences between the described protocells and viruses. Contemporary viruses use a host cell’s reproduction system to reproduce themselves; they don’t reproduce outside of the host cell. In addition, they don’t metabolize on their own (Dimmock et al. 2001). Using host cell enzymes, viruses produce core nucleic acid and shell peptide molecules.
The ubiquity and stability of the protein-covered viruses (Kuznetsov et al. 2005; Freddolino et al. 2005) give support to the idea that the polynucleotide–polypeptide assemblies could have existed in a prebiotic environment before phospholipid membranes became available. For a very long time, viruses were considered to be by-products of cellular evolution rather than a part of the universal tree of life, because they don’t have ribosomes. However, this view is being challenged by new data, which established similarity between viruses infecting various life domains; this indicates that viruses had a common ancestor that predated the differentiation of host domains (Bell 2001; Forterre 2002, 2003, 2005, 2006; Hendrix 2004; Rice et al. 2004; Pascal et al. 2006).
The recent discovery and characterization of Mimivirus, the largest known virus, revealed the presence of all genes relevant to m-RNA translation with the exception of ribosome components. The presence of the translation-related apparatus in Mimivirus contradicts established views on viruses. The phylogenetic analysis of this virus supports the scenario that its translation-related genes are relics of a more complete ancestral protein-translation apparatus (Raoul et al. 2004). If this is the case, viruses are relics of old cellular organisms that have been out-competed by the ancestors of the Last Universal Common Ancestor (LUCA) in the [Darwinian] evolutionary process. Only those ancestors of present day viruses that infected LUCA ancestors and devolved have survived (Forterre 2003). In this scenario, protocells could be the common predecessors of viruses as well as the LUCA.
Conclusions
Plausible steps leading to the formation of a protocell have been suggested, based on the experimental and theoretical data available today.
The steps include the polymerization and coevolution of peptides and oligonucleotides on mineral surfaces leading to the formation of collectively autocatalytic sets; the self-ordering of short peptides and simple amphiphiles into vesicles; the entrapment of the peptide–oligonucleotide systems within these vesicles and the formation of protocells with semi-permeable membranes capable of primitive metabolism, growth and division.
Each of these steps can be experimentally tested. First, the formation of collectively autocatalytic sets of peptides and oligonucleotides when a variety of these molecules are polymerized on mineral surfaces in fluctuating environmental conditions should be experimentally verified.
Second, a range of conditions leading to self-organization of vesicles from short peptides or from mixtures of the short peptides with other amphiphilic molecules may be examined. This might be followed by a study of entrapment of the peptide–oligonucleotide systems within the vesicles.
The permeability of the vesicles to molecules of various sizes and polarities and to ions should be investigated. Finally, growth and division of the protocells might also be studied experimentally.
References
Apel CL, Mautner MN, Deamer DW (2002) Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for encapsulation of biopolymers. Biochim Biophys Acta 1559:1–9
Ban N, Nissen P, Hansen J, Moore PB, Seitz TA (2000) The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 289:905–920
Bar-Nun A, Kochavi E, Bar-Nun S (1994) Assemblies of free amino acids as possible prebiotic catalysts. J Mol Evol 39:116–122
Bell PJL (2001) Viral eukaryogenesis: was the ancestor of the nucleus a complex DNA virus? J Mol Evol 53:251–256
Blakaj DM, McConnell KJ, Beveridge GL, Baranger AM (2001) Molecular dynamics and thermodynamics of protein–RNA interactions: mutation of a conserved aromatic residue modifies stacking interactions and structural adaptation in U1A–stem loop 2 RNA complex. J Am Chem Soc 123:2548–2551
Botta O, Bada JL (2002) Extraterrestrial organic compounds in meteorites. Surv Geophys 23:411–467
Bowman BR, Baker ML, Rixon FJ, Chiu W, Quiocho FA (2003) Structure of the herpesvirus major capsid protein. EMBO J 22:757–765
Brack A (1993) From amino acids to prebiotic active peptides: a chemical reconstitution. Pure Appl Chem 65:1143–1151
Brack A, Orgel L (1975) Beta structures of alternating polypeptides and their possible prebiotic significance. Nature 256:383–388
Brack A, Spach G (1979) Beta-structures of polypeptides with L- and D-residues. J Mol Evol 13:35–46
Brack A, Barbier B (1990) Chemical activity of simple basic peptides. Orig Life Evol Biosph 20:139–144
Carny O, Gazit E (2005) A model for the role of short self-assembled peptides in the very early stages of the origin of life. FASEB J 19:1051–1055
Cech TR, Zaug AJ, Grabowsky PJ (1981) In vitro splicing of the ribosomal RNA precursor of tetrahymena: involvement of guanosine nucleotide in the excision of the intervening sequence. Cell 27:487–496
Chakrabarti AC, Breaker RR, Joyce G, Deamer DW (1994) Production of RNA by a polymerase protein encapsulated within phospholipids vesicles. J Mol Evol 39:555–559
Cheng CM, Liu XH, Li YM, Ma Y, Tan B, Wan R, Zhao YF (2004) N-phospholyl amino acids and biomolecular origins. Orig Life Evol Biosph 34:455–464
Chipot C, Pohorille A (1996) Structure and functions of simple peptides at membrane–water interfaces. Orig Life Evol Biosph 26:249–250
Chyba CF, Sagan C (1992) Endogenous production, exogenous delivery and impact–shock synthesis of organic molecules: an inventory for the origins of life. Nature 355:125–131
Chyba CF, Thomas PJ, Brookshaw L, Sagan C (1990) Cometary delivery of organic molecules to the early earth. Science 249:366–373
Colgate SA, Rasmussen S, Solem JC, Lackner K (2003) An astrophysical basis for a universal origin of life. Adv Complex Systems 6:487–505
Cronin JR, Pizzarello S (1983) Amino acids in meteorites. Adv Space Res 3:5–18
Deamer DW (1997) The first living systems: a bioenergetic perspective. Microbiol Molec Biol Rev 61:239–261
Deamer DW, Oro J (1980) Role of lipids in prebiotic structures. Biosystems 12:167–173
Deamer D, Barchfeld G (1982) Encapsulation of macromolecules by lipid vesicles under simulated prebiotic conditions. J Mol Evol 18:203–206
Deamer DW, Fleischaker G (1993) Origins of life; the central concepts. Jones and Barlett Publishers International, London
Deamer DW, Mahon EH, Bosco G (1994) Self-assembly and function of primitive membrane structures. In: Bengtson S et al (eds) Early life on earth, Columbia University Press, New York, pp 107–123
Deamer DW, Dworkin JP, Sandford SA, Bernstein MP, Allamandola LJ (2002) The first cell membranes. Astrobiology 2:371–380
De Duve C (1991) Blueprint for a cell: the nature and origin of life. Patterson, New York
Dimmock NJ, Easton AJ, Leppard KN (2001) Introduction to modern virology. Blackwell Science, London
Doan DNP, Dokland T (2003) Structure of the nucleocapsid protein of porcine reproductive and respiratory syndrome virus. Structure 11:1445–1451
Dobson C (1999) Protein misfolding, evolution and disease. Trends Biochem Sci 24:329–332
Dobson C (2003) Protein folding and misfolding. Nature 426:884–890
Dyson FJ (1982) A model for the origin of life. J Mol Evol 18:344–350
Ehrenfreund P, Glavin DP, Botta O, Cooper G, Bada J (2001) Extraterrestrial amino acids in Orgueil and Ivuna: tracing the parent body of CI type carbonaceous chondrites. Proc Natl Acad Sci USA 98:2138–2141
Eigen M (1971) Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 58:465–523
Eigen M, Schuster P (1978) The hypercycle. Naturwissenschaften 65:341–369
Ertem G, Ferris JP (1996) Synthesis of RNA oligomers on heterogeneous templates. Nature 379:238–240
Ferris JP (1993) Catalysis and prebiotic RNA. Orig Life Evol Biosph 23:307–315
Ferris JP (2005) Catalysis and prebiotic synthesis. Rev Mineral Geochem 59:187–210
Ferris JP (2006) Monmorillonite-catalysed formation of RNA oligomers: the possible role of catalysis in the origins of life. Philos Trans R Soc Lond B Biol Sci 361:1777–1786
Ferris JP, Ertem G, Agarwal V (1989) Mineral catalysis of the formation of dimers of 5′-AMP in aqueous solution: the possible role of monmorillonite clays in the prebiotic synthesis of RNA. Orig Life Evol Biosph 19:165–178
Ferris JP, Hill AR Jr, Liu R, Orgel LE (1996) Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381:59–61
Forterre P (2002) The origin of DNA genomes and DNA replication proteins. Curr Opin Microbiol 5:525–532
Forterre P (2003) The great virus comeback – from an evolutionary perspective. Res Microbiol 154:223–225
Forterre P (2005) The two ages of the RNA world, and the transition to the DNA world: a story of viruses and cells. Biochimie 87:793–803
Forterre P (2006) The origin of viruses and their possible roles in major evolutionary transitions. Virus Res 117:5–16
Franchi M, Ferris JP, Gallori E (2003) Cations as mediators of the adsorption of nucleic acids on clay surfaces in prebiotic environments. Orig Life Evol Biosph 33:1–16
Freddolino PL, Arkhipov AS, Larson SB, McPherson A (2005) Molecular dynamics simulations of the complete satellite tobacco mosaic virus. Structure 14:437–449
Gazit E (2002) The “correctly folded” state of proteins: is it a metastable state? Angew Chem Soc Int Ed Engl 41:257–259
Gilbert W (1986) Origin of life: the RNA world. Nature 319:618
Gryaznov SM, Letsinger RL (1993) Chemical ligation of oligonucleotides in the presence and absence of a template. J Am Chem Soc 115:3808–3809
Hargreaves WR, Deamer DW (1978) Liposomes from ionic, single-chain amphiphils. Biochemistry 17:3759–3768
Hendrix RW (2004) Hot new virus, deep connections. Proc Nat Acad Sci USA 101:7495–7496
Holmes KC (1984) The structure determination of tobacco mosaic virus. In: Jurnak FA, McPherson A (eds) Biological macromolecules and assemblies. Wiley, New York, pp 121–148
Joyce G (1989) RNA evolution and the origins of life. Nature 338:217–224
Kamura K, Ferris JP (1999) Clay catalysis of oligonucleotide formation: kinetics of the reaction of the 5′-phosphorimidazolides of nucleotides with the non-basic heterocyles uracil and hypoxantine. Orig Life Evol Biosph 29:563–591
Kanavarioti A, Monnard PA, Deamer DW (2001) Eutectic phases in ice facilitate nonenzymatic nucleic acid synthesis. Astrobiology 1:271–281
Kauffman S (1986) Autocatalytic sets of proteins. J Theor Biol 119:1–24
Kauffman S (1993) The origins of order: self-organization and selection in evolution. Oxford University Press, New York, Oxford
Kauffman S (1995) At home in the universe: the seach for laws of self-organization and complexity. Oxford University Press, New York, Oxford
Kauffman S (2000) Investigations. Oxford University Press, New York, Oxford
Kimura S, Kim DH, Sugiyama J, Imanishi Y (1999) Vesicular self-assembly of a helical peptide in water. Langmuir 15:4461–4463
Kochavi E, Bar-Nun A, Fleminger G (1997) Substrate-directed formation of small biocatalysts under prebiotic conditions. J Mol Evol 45:342–351
Kuhn H (1976) Model consideration for the origin of life. Naturwissenschaften 63:68–80
Kumar A, Reddy V, Yusibov V, Chipman PR, Hata Y, Fita I, Fukuyama K, Rossman MG, Loesch-Fries LS, Baker TS, Johnson JE (1997) The structure of alfalfa mosaic virus capsid protein assembled as a T=1 icosahedral particle at 4.0 A resolution. J Virol 71:7911–7916
Kuznetsov YG, Daijogo S, Zhou J, Semler BL, McPherson A (2005) Atomic force microscopy analysis of icosahedral virus RNA. J Mol Biol 347:41–52
Kvenvolden KA, Lawless JG, Pering K, Peterson E, Flores J, Ponnamperuma C, Kaplan IR, Moore C (1970) Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 228:923–926
Lahav N (1991) Prebiotic co-evolution of self-replication and translation or RNA world? J Theor Biol 151:531–539
Lahav N (1993) The RNA-world and co-evolution hypotheses and the origin of life: implications, research strategies and perspectives. Orig Life Evol Biosph 23:329–344
Lahav N, Chang S (1976) The possible role of solid suface area in condensation reactions during chemical evolution: reevaluation. J Mol Evol 8:357–380
Lahav N, White DH (1980) A possible role of fluctuating clay–water systems in the production of ordered prebiotic oligomers. J Mol Evol 16:11–21
Lailach GE, Thompson TD, Brindley GW (1968) Absorption of pyrimidines, purines, and nucleosides by Li-, Na-, Mg-, and Ca-Montmorillonite. Clays Clay Miner 16:285–293
Lawless JG, Yuen GU (1979) Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 282:396–398
Li YM, Yin YW, Zhao YF (1992) Phosphoryl group participation leads to peptide formation from N-phosphoryl amino acids. Int J Pept Protein Res 39:375–381
Lohrman R, Orgel LE (1973) Prebiotic activation processes. Nature 244:418–420
Lohrman R, Orgel LE (1976) Template-directed synthesis of high molecular weight polynucleotide analogues. Nature 261:342–344
Lohrmann R, Bridson PK, Orgel LE (1980) Efficient metal-ion catalyzed template-directed oligonucleotide synthesis. Science 208:1464–1465
Lu K, Jacob J, Thiyagarajan P, Conticello VP, Lynn DG (2003) Exploiting Amyloid fibril lamination for nanotube self-assembly. J Am Chem Soc 125:6391–6393
Luisi PL (2002) Toward the engineering of minimal living cells. Anat Rec 268:208–214
Luisi PL, Ferri F, Stano P (2006) Approaches to semi-synthetic minimal cells: a review. Naturwissenschaften 93:1–13
Lutay AV, Chernolovskaya EL, Zenkova MA, Vlasov VV (2006) The nonenzymatic template-directed ligation of oligonucleotides. Biogeosciences Discus 3:243–249
Maji SK, Drew MGB, Banerjee A (2001) First crystallographic signature of amyloid-like fibril forming beta-sheet assemblage from tripeptide with non-coded amino acids. Chem Commun 19:1946–1947
Maurel MC (1992) RNA in evolution: a review. J Evol Biol 5:173–188
McCollom TM, Ritter G, Simoneit BRT (1999) Lipid synthesis under hydrothermal conditions by Fischer–Tropsch-type reactions. Orig Life Evol Biosph 29:153–166
Meierhenrich UJ, Munoz Caro G, Bredehoft JH, Jessberger EK, Thiemann WHP, van Dishoeck EF (2004) Identification of diamino acids in the Murchison meteorite. Proc Nat Acad Sci USA 101:9182–9186
Miller SL (1953) A production of amino acids under possible primitive earth conditions. Science 117:528–529
Miller SL, Urey HC (1959) Organic compound synthes on the primitive Earth: several questions about the origin of life have been answered, but much remains to be studied. Science 130:245–251
Monnard PA (2003) Liposome-entrapped polymerases as models for microscale/nanoscale bioreactors. J Membr Biol 191:87–97
Monnard PA, Deamer DW (2001) Nutrient uptake by protocells: a liposome model system. Orig Life Evol Biosph 31:147–155
Monnard PA, Deamer DW (2002) Membrane self-assembly processes: steps toward the first cellular life. Anat Rec 268:196–207
Monnard PA, Oberholzer T, Luisi PL (1997) Entrapment of nucleic acids in liposomes. Biochim Biophys Acta 1329:39–50
Monnard PA, Apel CL, Kanavarioti A, Deamer DW (2002) Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: implications for a prebiotic aqueous medium. Astrobiology 2:139–152
Mora PT (1965) In: Fox SW (ed) The origins of prebiological systems and of their molecular matrices. Academic, New York, p 39
Morowitz JH (1992) Beginning of cellular life. Yale University Press, New Haven
Mouritsen OG, Jorgenseb K, Honger T (1995) Permeability of lipid bilayers near the phase transition. In: Disalvo EA, Simon SA (eds) Permeability and stability of lipid bilayers. CRC Press, Boca Raton, pp 137–160
Munoz Caro G, Meierhenrich UJ, Schutte WA, Barbier B, Arcones Segovia A, Rosenbauer H, Thiemann WHP, Brack A, Greenberg JM (2002) Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 416:403–406
Naraoka H, Shimoyama A, Harada K (1999) Moleculer distribution of monocarboxylic acids in Asuka carbonaceous chondrites from Antarctica. Orig Life Evol Biosph 29:187–201
Negron-Mendoza A, Guzman A, Mosquiera FG, Ramos-Bernal R (2006) Role of clays as protecting agents in prebiotic chemistry. Orig Life Evol Biosph 36:260–261
Neumann R, Ringsdorf H, Patton EV, O’Brien DF (1987) Preparation and characterization of long chain amino acid and peptide vesicle membranes. Biochim Biophys Acta 898:338–348
Oberholzer T, Luisi PL (2002) The use of liposomes for constructing cell models. J Biol Phys 28:733–744
Orgel LE (1968) Evolution of the genetic apparatus. J Mol Biol 38:381–393
Orgel LE (2004) Prebiotic chemistry and the origin of the RNA world. Crit Rev Biochem Mol Biol 39:99–123
Pascal R, Boiteau L, Forterre P, Gargaud M, Lazcano A, Lopez-Garcia P, Maurel MC, Moreira D, Pereto J, Prieur D, Reisse J (2006) Prebiotic chemistry–biochemistry–emergence of life (4.4–2 Ga). Earth, Moon, and Planets 98:153–203
Pattee HH (1965) Experimental approaches to the origin of life problem. Adv Enzymol 27:381–415
Paula S, Volkov AG, Van Hoek AN, Haines TH, Deamer DW (1996) Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys J 70:339–348
Pierazzo E, Chyba CF (1999) Amino acid survival in large cometary impacts. Meteorit Planet Sci 34:909–918
Pizzarello S (2004) Chemical evolution and meteorites: an update. Orig Life Evol Biosph 34:25–34
Pohorille A, Deamer DW (2002) Artificial cells: prospects for biotechnology. Trends Biotechnol 20:123–128
Polozov IV, Anantharamaiah G, Segrest JP, Epand RM (2001) Osmotically induced membrane tension modulates membrane permeabilization by class L amphipathic helical peptides: nucleation model of defect formation. Biophys J 81:949–959
Prasad BV, Rothnagel R, Jiang XI, Etes MK (1994) Three-dimensional structure of Baculovirus-expressed Norwalk virus capsids. J Virol 68:5117–5125
Raoul D, Audic S, Robert C, Abergel C, Renesto P, Ogata H, Scoba B, Suzan M, Claverie JM (2004) The 1.2-megabase genome sequence of mimivirus. Science 306:1344–1350
Rasmussen S, Chen L, Deamer D, Krakauer DC, Packard NH, Stadler PF, Bedau MA (2004) Transitions from nonliving to living matter. Science 303:963–965
Reches M, Gazit E (2003) Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 300:625–627
Reches M, Gazit E (2004) Formation of closed-cage nanostructures by self-assembly of aromatic peptides. Nano Lett 4:581–585
Reches M, Gazit E (2006) Designed aromatic homo-dipeptides: formation of ordered nanostructures and potential nanotechnological applications. Physical Biology 3:S10–S19
Reches M, Porat Y, Gazitt E (2002) Amyloid fibril formation by pentapeptide and tetrapeptide fragments of human calcitonin. J Biol Chem 277:35475–35480
Rice G, Tang L, Stedman K, Roberto F, Spuhler J, Gillitzer E, Johnson JE, Douglas T, Young M (2004) The structure of a thermophilic archaeal virus shows a double-stranded DNA viral capsid type that spans all domains of life. Proc Nat Acad Sci USA 101:7716–7720
Rich A (1962) On the problems of evolution and biochemical information transfer. In: Kasha M, Pullman (eds) Horizons in biochemistry. Academic, New York, pp 103–126
Rode BM (1999) Peptides and the origin of life. Peptides 20:773–786
Rode BM, Suwannachoti Y (1999) The possible role of Cu(II) for the origin of life. Coord Chem Rev 192:1085–1099
Rushdi AI, Simoneit BRT (2001) Lipid formation by aqueous Fischer–Tropsch-Type synthesis over a temperature range of 100 to 400 deg. C. Orig Life Evol Biosph 31:103–118
Saetia S, Liede KR, Eder AH, Rode BM 1993) Evaporation cycle experiments–a simulation of salt-induced peptide synthesis under possible prebiotic conditions. Orig Life Evol Biosph 23:167–176
Sanchez R, Ferris JP, Orgel LE (1966) Conditions for purine synthesis: Did prebiotic synthesis occur at low temperatures? Science 153:72–73
Santoso S, Hwang W, Hartman H, Zhang S (2002) Self-assembly of surfactant-like peptides with variable glycine tails to form nanotubes and nanovesicles. Nano Lett 2:687–691
Sawai H (1976) Catalysis of internucleotide bond formation by divalent metal ions. J Am Chem Soc 98:7037–7039
Sawai H, Kuroda K, Hojo T (1989) Uranyl ion as a highly effective catalyst for internucleotide bond formation. Bull Chem Soc Jpn 62:2018–2023
Sawai H, Higa K, Kuroda K (1992) Synthesis of cyclic and acyclic oligocytidylates by uranyl catalysis in aqueous solution. J Chem Soc Perkin Trans 1:505–508
Shen C, Lascano A, Oro J (1990) The enhancement activities of histidyl–histidine in some prebiotic reactions. J Mol Evol 31:445–452
Shew RL, Deamer DW (1985) A novel method for encapsulation of macromolecules in liposomes. Biochim Biophys Acta 816:1–8
Silva AM, Rossman MG (1987) Refined structure of southern bean mosaic virus at 2–9 A resolution. J Mol Biol 197:69–87
Sipe JD, Cohen AS (2000) Review: history of amyloid fibril. J Struct Biol 130:88–98
Soberby SL, Heckl WM (1998) The role of self-assembled monolayers of the purine and pyrimidine bases in the emergence of life. Orig Life Evol Biosph 28:283–310
Soberby SL, Heckl WM, Petersen GB (1996) Chiral symmetry breaking during the self-assembly of monolayers from achiral purine molecules. J Mol Evol 43:419–424
Soberby SL, Stockwell PA, Heckl WM, Petersen G (2000) Self-programmable, self-assembling two-dimensional genetic matter. Orig Life Evol Biosph 30:81–99
Soberby SJ, Petersen GB, Holm NG (2002) Primordial coding of amino acids by adsorbed purine bases. Orig Life Evol Biosph 32:35–46
Sorger PK, Stockley PG, Harrison SC (1986) Structure and assembly of turnip crinkle virus. J Mol Biol 191:639–658
Stribling R, Miller SL (1991) Attempted nonenzymatic template-directed oligomerization on polyadenylic acid template: implications for the nature of the first genetic material. J Mol Evol 32:282–288
Takahashi Y, Mihara H (2004) Construction of a chemically and conformationally self-replicating system of amyloid-like fibers. Bioorg Med Chem 12:693–699
Theng BKG (1974) The chemistry of clay–organic reactions. Wiley, New York
Theng BKG (1982) Clay–polymer interactions; summary and perspectives. Clays Clay Miner 30:1–10
Tian F, Toon OB, Pavlov AA, De Sterck H (2005) A hydrogen-rich early Earth atmosphere. Science 308:1014–1017
Tihova M, Dryden K, Le TL, Harvey SC, Johnson JE, Yeager M, Schneemann A (2004) Nodavirus coat protein imposes dodecahedral RNA structure independent of nucleotide sequence and length. J Virol 78:2897–2905
Treyer M, Walde P, Oberholzer T (2002) Permeability enhancement of lipid vesicles to nucleotides by use of sodium cholate: Basic studies and application to an enzyme-catalyzed reaction occurring inside the vesicles. Langmuir 18:1043–1050
Vauthey S, Santoso S, Gong H, Watson N, Zhang S (2002) Molecular self-assembly of surfactant-like peptides to form nanotubes and nanovesicles. Proc Nat Acad Sci USA 99:5355–5360
Vriend G, Hemminga MA, Verduin BJM, De Wit JL, Schaafsma TJ (1981) Segmental mobility involved in protein–RNA interaction in cowpea chlorotic mottle virus. FEBS Lett 134:167–171
Wachtershauser G (1988) Before enzymes and templates: theory of surface metabolism. Microbiol Rev 52:452–484
Walde P, Goto A, Monnard PA, Wessicken M, Luisi PL (1994) Oparin’s reactions revisited: enzymatic synthesis of poly(adenylic acid) in micelles and self-reproducing vesicles. J Am Chem Soc 116:7541–7547
Weiner AM, Maizels N (1991) Neutral evolution. In: Osawa S, Honjo T (eds) Evolution of life. Springer, Tokyo, pp 51–65
White DH (1980) A theory for the origin of a self-replicating chemical system. 1: Natural selection of the autogen from short, random oligomers. J Mol Evol 16:121–147
White DH, Erickson JC (1981) Enhancement of peptide bond formation by polyribonucleotides on clay surfaces in fluctuating environments. J Mol Evol 17:19–26
White DH, Kennedy RM, Macklin J (1984) Origins of life. D Reidel Publishing Company, New York
Yan X, He Q, Wang K, Duan L, Cui Y, Li J (2007) Transition of cationic dipeptide nanotubes into vesicles and oligonucleotide delivery. Angew Chem Int Ed 46:2431–2434
Yanagawa H, Ogawa Y, Kojima K, Ito M (1988) Construction of protocellular structures under simulated primitive earth conditions. Orig Life Evol Biosph 18:179–207
Yu W, Sato K, Wakabayashi M, Nakaishi T, Ko-Mitamura EP, Shima Y, Urabe I, Yomo T (2001) Synthesis of functional protein in liposome. J Biosci Bioeng 92:590–593
Zhao YF, Cao PS (1994) Phosphoryl amino acids: common origin for nucleic acids and proteins. J Biol Phys 20:283–287
Zhou WH, Ju Y, Zhao YF (1996) Simultaneous formation of peptides and nucleotides from n-phosphothreonine. Orig Life Evol Biosph 26:547–560
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fishkis, M. Steps Towards the Formation of A Protocell: The Possible Role of Short Peptides. Orig Life Evol Biosph 37, 537–553 (2007). https://doi.org/10.1007/s11084-007-9111-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-007-9111-4