Abstract
Purpose
SARS-COV-2 infection can develop into a multi-organ disease. Although pathophysiological mechanisms of COVID-19-associated myocardial injury have been studied throughout the pandemic course in 2019, its morphological characterisation is still unclear. With this study, we aimed to characterise echocardiographic patterns of ventricular function in patients with COVID-19-associated myocardial injury.
Methods
We prospectively assessed 32 patients hospitalised with COVID-19 and presence or absence of elevated high sensitive troponin T (hsTNT+ vs. hsTNT-) by comprehensive three-dimensional (3D) and strain echocardiography.
Results
A minority (34.3%) of patients had normal ventricular function, whereas 65.7% had left and/or right ventricular dysfunction defined by impaired left and/or right ventricular ejection fraction and strain measurements. Concomitant biventricular dysfunction was common in hsTNT+ patients. We observed impaired left ventricular (LV) global longitudinal strain (GLS) in patients with myocardial injury (-13.9% vs. -17.7% for hsTNT+ vs. hsTNT-, p = 0.005) but preserved LV ejection fraction (52% vs. 59%, p = 0.074). Further, in these patients, right ventricular (RV) systolic function was impaired with lower RV ejection fraction (40% vs. 49%, p = 0.001) and reduced RV free wall strain (-18.5% vs. -28.3%, p = 0.003). Myocardial dysfunction partially recovered in hsTNT + patients after 52 days of follow-up. In particular, LV-GLS and RV-FWS significantly improved from baseline to follow-up (LV-GLS: -13.9% to -16.5%, p = 0.013; RV-FWS: -18.5% to -22.3%, p = 0.037).
Conclusion
In patients with COVID-19-associated myocardial injury, comprehensive 3D and strain echocardiography revealed LV dysfunction by GLS and RV dysfunction, which partially resolved at 2-month follow-up.
Trial registration
COVID-19 Registry of the LMU University Hospital Munich (CORKUM), WHO trial ID DRKS00021225.
Similar content being viewed by others
Introduction
Since the outbreak of the SARS-CoV-2 global pandemic in December 2019, COVID-19 has been shown to represent a multi-organ disease with suspected impact on myocardial function. First reports of hospitalised COVID-19 patients from Wuhan, China, have found myocardial injury with elevated high-sensitive troponin (hsTNT) levels. COVID-19-related myocardial injury was associated with higher admission rate on intensive care unit (ICU) and mortality [1,2,3]. Although the prevalence of myocardial injury has been estimated as high as 30% in hospitalised COVID-19 patients, its aetiology is heterogeneous and not fully understood [4].
Previous echocardiographic reports of COVID-19 patients could not show impairment of left ventricular (LV) function by conventional ejection fraction (LVEF) [5]. Recent retrospective studies revealed that either left or right ventricular dysfunction was observed in longitudinal strain measurements, possibly predicting mortality in COVID-19 patients [6, 7]. So far, advanced three-dimensional and strain echocardiography (3DSE) has been lacking as part of the comprehensive clinical evaluation of COVID-19 patients.
In this prospective study, we evaluated left and right heart anatomy in COVID-19 patients with and without myocardial injury by comprehensive 3DSE to reveal different patterns of myocardial dysfunction. Additional follow-up 3DSE was used to clarify whether the observed myocardial dysfunction was transient in COVID-19 patients.
Methods
Study population
Patients with confirmed COVID-19 were included in this prospective study conducted at the Ludwig-Maximilians-University (LMU) Hospital in Munich, Germany. The following polymerase chain reaction (PCR) assays were used in the accredited routine diagnostics laboratory of the Department of Virology in order to confirm SARS-CoV-2 infection: the nucleocapsid (N1) reaction of the CDC protocol, the envelope amplification of the Charité protocol, the nucleocapsid amplification of the Seegene Allplex 2019-nCoV Assay, the Roche Cobas SARS-CoV-2 nucleocapsid reaction or the Xpert Xpress SARS-CoV-2 run on the GeneXpert System as previously described [8].
All patients are part of the COVID-19 Registry of the LMU University Hospital Munich (CORKUM, WHO trial ID DRKS00021225). Patients gave written informed consent for participation and analysis of their data. The study was approved by the local ethics committee (No: 20-245) and complies with the Declaration of Helsinki. Data collection contained patient characteristics, laboratory values, echocardiographic findings, and outcome which were recorded in the designated COVID-19 database.
We excluded patients from echocardiographic analysis, in which an elevation of hsTNT was biased by markedly elevated creatinine levels, previous or ongoing need for dialysis, or a high likelihood of pulmonary embolism as the cause of myocardial damage. Furthermore, patients with high suspicion of myocardial ischemia due to acute coronary syndromes, including ST segment alterations, were also excluded from the echocardiographic analysis. If patients had insufficient acoustic windows, e.g. due to prone position, they were also excluded.
Definitions
COVID-19 was confirmed by positive RT-PCR for SARS-CoV-2 from throat swabs, sputum or endotracheal suction. Presence of myocardial injury was defined by elevated hsTNT levels (Elecsys Troponin T hs by Roche Diagnostics GmbH, Vienna, Austria) above the 99th percentile upper reference limit (0.014 ng/ml). Acute respiratory distress syndrome (ARDS) was diagnosed according to the Berlin definition and Horovitz Index served for differentiation of severity as described before [5]. Pre-existing cardiovascular disease (CVD) comprised coronary artery disease, atrial fibrillation or known heart failure.
Systolic LV dysfunction (LVDsys) was indicated if 3D-LVEF was below 50% [9, 10], or if LV global longitudinal strain (LV-GLS) was above -16% [11]. Diastolic LV dysfunction (LVDdia) was present if three out of the following six parameters were found: Mitral peak E velocity ≤ 50 cm/s, E/A ratio ≤ 0.8, average E/e′ > 14, septal e′ velocity < 7 cm/s or lateral e′ velocity < 10 cm/s, tricuspid regurgitation (TR) velocity > 280 cm/s or left atrial volume (3D-LAV) index > 34 ml/m2 [12]. Systolic right ventricular (RV) dysfunction (RVDsys) was defined as 3D-RVEF below 45% [13] or impaired RV free wall strain (RV-FWS) above -20% [9].
Echocardiography
Initial comprehensive transthoracic echocardiography was performed on normal ward or intensive care unit (ICU) within 5–21 days after admission. Follow-up echocardiography took place in an outpatient setting. A X5-1 transducer on a EPIQ CVx cardiac ultrasound system (Philips Healthcare, Andover, MA) was used exclusively for COVID-19 patients during the study period. Data acquisition and analysis followed current recommendations for the assessment of native valve regurgitation and chamber quantification [14, 15]. LV linear dimensions (LV end-diastolic and end-systolic diameters) were measured in parasternal long axis. LV volumes (end-diastolic, end-systolic), 3D-LVEF, LV mass, left atrial end-systolic volume (3D-LAV) were measured with the semi-automatic 3D HeartModel tool (Philips Healthcare, Andover, MA). LV forward stroke volume was quantified with the Doppler velocity time integral method in the LV outflow tract. Transmitral pulse wave Doppler and tissue Doppler imaging were used to quantify diastolic function with E/A and average E/e′ ratio and septal as well as lateral wall movement. Myocardial contraction fraction (MCF) represents the ratio of stroke volume and myocardial volume (MV). MV was estimated by dividing LV mass by density (1.05 g/ml) [16]. LV global longitudinal strain (LV-GLS) analysis was performed with AutoStrain function (TOMTEC Imaging Systems, Unterschleissheim, Germany). Assessment of right ventricular (RV) dimension and function was performed with the 4D RV-FUNCTION tool (TOMTEC Imaging Systems, Unterschleissheim, Germany) [17], including 3D RV volumes (end-diastolic, end-systolic), 3D-RVEF, global RV stroke volume, tricuspid annular plane systolic excursion (TAPSE), RV mid-ventricular diameter and length, fractional area change (FAC) and RV-FWS. Right atrial (RA) end-systolic volume was calculated by single plane area-length method. We measured systolic tricuspid regurgitation (TR) peak gradient using the simplified Bernoulli equation. By adding the estimated RA pressure to the systolic TR peak gradient, we calculated the systolic pulmonary artery pressure (Echo-sPAP).
Statistical analysis
Data are expressed as median with interquartile range (IQR) or number (n) with percentage of total. Continuous variables were checked for normal distribution with Kolmogorov–Smirnov-test. Student’s t test was applied for normally distributed, continuous variables; otherwise Mann–Whitney U test was used. Wilcoxon test was employed for pairwise comparison of variables between initial and follow-up measurements. Chi-square (χ2) test was employed for comparison of categorical variables. Pearsons’ r was used to assess bivariate correlation between different variables. Interobserver variability for exemplary measurements of LV end-diastolic diameter and TAPSE were obtained by analysis of 20 random patients by two independent echocardiographers. The results were analysed using intraclass correlation coefficient (ICC). Statistical significance was considered as of p value <0.05. All study variables were analysed using SPSS statistical software (IBM, USA, version 26) or graph pad prism software (GraphPad Software, USA, version 8).
Results
Patient characteristics of hospitalised COVID-19 patients with and without myocardial injury
Between February 28th and May 7th 2020, 116 patients were hospitalised for symptomatic COVID-19 disease. Patients were screened for suspected myocardial injury according to elevated hsTNT level. 76 patients were excluded due to elevated creatinine levels as sign of severe kidney failure or intermittent need for dialysis, markedly elevated D-Dimer with high suspicion for thromboembolism, electrocardiographic ST-segment changes as sign for (non-) ST-elevation myocardial infarction or missing patient consent. Echocardiography was performed in 40 patients, of whom eight patients were additionally excluded due to inadequate acoustic windows. Patients without elevated hsTNT were included to serve as control group. A total of 32 patients with comprehensive 3DSE were included in this analysis, 18 with suspected myocardial injury (hsTNT+) and 14 without myocardial injury (hsTNT-).
Patients characteristics and laboratory findings are depicted in Table 1. The majority of patients (88%) were male without significant difference between groups (94% in hsTNT+ vs. 79% in hsTNT-, p = 0.178). Patients with myocardial injury were older (68 years vs. 53 years, p = 0.001) and suffered from more comorbidities in comparison with hsTNT-patients. The study groups did not differ in terms of intake of medication. Newly started medication during the inpatient stay was comparable in both groups, although some patients were started on ACE-inhibitors, ARB/ARNI or betablockers as a response to echocardiographic findings on myocardial injury. Overall, 7 hsTNT+ patients but no hsTNT- patient received hydroxychloroquine at the discretion of the treating physician (p = 0.009).
Peak NT-proBNP levels significantly differed between both groups (744 vs. 117 ng/ml, p = 0.001) and serum creatinine levels were slightly higher in hsTNT+ patients (0.9 mg/dl (hsTNT+) vs. 0.8 mg/dl (hsTNT-), p = 0.026) but within normal reference intervals.[18] Levels of c-reactive protein and interleukin 6 of both groups were similar.
Clinical outcome of hospitalised COVID-19 patients with and without myocardial injury
Median duration of hospitalisation was 20 days, ranging from 5 days (minimum) to 73 (maximum) days with tendency of hsTNT+ patients to stay longer in hospital (Table 1). Admission to intensive care unit (61% vs. 29% patients, p = 0.031) did statistically differ, whereas need for intubation due to respiratory failure (10 vs. 4 patients, p = 0.133) did not statistically differ between groups. Median duration of ICU treatment was 15 (5–21) days. None of the 32 patients died during hospitalisation or time to follow-up.
Echocardiographic analysis of left and right heart dimensions and function
Although LV dimensions did not differ between groups (Table 2), the RV was enlarged in hsTNT+ patients with RV end-systolic volume of 68 vs. 52 ml in hsTNT-patients (p = 0.019). Systolic LV function evaluated by 3D-LVEF was normal and comparable between groups with 52% (hsTNT+) vs. 59% (hsTNT-, p = 0.074). We observed a significant impairment of MCF (0.47 vs. 0.55, p = 0.016) and LV-GLS (-13.9% vs. -17.7%, p = 0.005) in hsTNT+ patients as indicator for systolic left ventricular dysfunction (LVDsys). In addition, LVDdia was more frequent in hsTNT+ compared to hsTNT- patients and concomitantly observed in about one half of hsTNT+ patients with systolic ventricular dysfunction.
Furthermore, systolic dysfunction of the right ventricle was observed more often in patients with myocardial injury than in patients without myocardial injury. In detail, FAC (37% vs. 43%, p = 0.014), 3D-RVEF (40% vs. 49%, p = 0.001) and RV-FWS (-18.5% vs. -28.3%, p = 0.003) were significantly reduced in hsTNT+ patients in contrast to normal values in hsTNT- patients. Troponin levels of patients with myocardial injury did not correlate with 3D-LVEF, LV-GLS, TAPSE, FAC, 3D-RVEF or RV-FWS.
Taken together, combined ventricular heart failure with either systolic biventricular dysfunction (LVDsys + RVDsys, LVDdia + RVDsys) or systolic and diastolic left ventricular dysfunction (LVDsys + LVDdia) or triple dysfunction (LVDsys + RVDsys + LVDdia) were present in about 56.3% of all patients and occurred more frequently in hsTNT + than in control (88.8% vs. 14.3%, p < 0.001; Fig. 1a).
Patterns of ventricular dysfunction in hospitalised patients with COVID-19. (a) Distribution of echocardiographic findings in all 32 patients hospitalised with COVID-19 and separated by evidence for myocardial injury according to elevated high-sensitive troponin T (hsTNT). hsTNT+ indicates myocardial injury, hsTNT- denotes no myocardial injury. (b) Distribution of echocardiographic findings on follow-up of 12 patients with previous history of COVID-19-associated myocardial injury. LVDsys, systolic left ventricular dysfunction; LVDdia, diastolic left ventricular dysfunction; RVDsys, systolic right ventricular dysfunction; n, number of patients
Echocardiographic follow-up
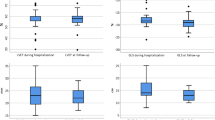
Twelve hsTNT+ patients underwent follow-up echocardiography within 52 (47–68) days after initial evaluation. Overall, six (33.3%) hsTNT + patients were lost to follow-up. The percentage of hsTNT + patients suffering from triple ventricular dysfunction (LVDsys + RVDsys + LVDdia) decreased from initially 44.4% to 8.3% at follow-up (Fig. 1b), whereas isolated ventricular dysfunction (LVDsys or LVDdia or RVDsys) occurred in three (25%) hsTNT+ patients at follow-up. In detail, 3DSE showed that LV-GLS (-13.9% vs. -16.5%, p = 0.013) and RV-FWS (-18.5% vs. -22.3%, p = 0.037) significantly improved in hsTNT+ patients (Fig. 2). Interestingly, RV diameters as well as global right ventricular stroke volume did increase in hsTNT+ patients upon follow-up in comparison with their initial measurements, but these did not significantly differ (Table S1).
Trend of left ventricular global longitudinal strain and right ventricular free wall strain in patients with myocardial injury. Left ventricular global longitudinal strain (LV-GLS, a) and right ventricular free wall strain (RV-FWS, b) measurements in patients with COVID-19-associated myocardial injury at initial assessment and on follow-up after a median of 52 days. Red dots represent values below the reference value of LV-GLS of -16% (dotted black line, a) or RV-FWS of -20% (dotted black line, b), respectively. Green dots represent values within normal range. Dashed arrows represent improvement (green) or deterioration (red) of LV-GLS or RV-FWS. LV-GLS, left ventricular global longitudinal strain; RV-FWS, right ventricular free wall strain
Discussion
This is the first comprehensive echocardiographic study applying advanced 3D and strain imaging methods in hospitalised COVID-19 patients with and without myocardial injury. We demonstrated that COVID-19 patients frequently present with biventricular dysfunction, which partially resolves within two months after hospital discharge. In our study, patient characteristics, in particular age, hypertension and CVD, were comparable to larger hospitalised COVID-19 patient cohorts [1, 2, 19]. Our study shows that patients with myocardial injury were older, had more comorbidities and CVD, with higher rates of ICU admission and need for mechanical ventilation compared to patients without myocardial injury.
Importantly, conventional assessment of LV function by LVEF did not reveal differences between groups of myocardial injury. In line with the findings of a recent study of Janus et al. [7], we observed that rather LV-GLS than LVEF is the modality of choice to detect systolic left ventricular dysfunction (LVDsys) in patients with COVID-19-associated myocardial injury. LVDsys was mainly detected by impaired LV-GLS (12 out of 19 patients with LVDsys).
Furthermore, advanced 3DSE revealed that the majority of hospitalised COVID-19 patients had biventricular dysfunction. This pattern was highly prevalent in hsTNT+ patients (>80% of patients). Comparable to LVDsys, RVDsys was mainly diagnosed by impaired 3D-RVEF or RV-FWS. Of note, pathological patterns were also found in patients without cardiac preconditions. Severity of ventricular dysfunction or the aforementioned RV enlargement was not associated with the length of hospital or ICU stay or with duration of mechanical ventilation. Interestingly, RV diameters as well as global right ventricular stroke volume did increase in hsTNT+ patients upon follow-up in comparison with their initial measurements, but these did not significantly differ (Table S1). Concerning interobserver variability, ICCs were within acceptable range (LV end-diastolic diameter: 0.862, p < 0.001; TAPSE: 0.882, p < 0.001).
Importantly, troponin levels of patients with myocardial injury did not correlate with 3D-LVEF, LV-GLS, 3D-RVEF or RV-FWS. These findings support a diagnostic pathway of first identifying myocardial damage by biomarkers with subsequent characterisation by comprehensive 3DSE to reveal the extent and pattern of myocardial impairment that might be underestimated by point-of-care ultrasound or single laptop-based equipment as previously recommended [20]. Especially strain imaging was useful to evaluate biventricular myocardial contractility, revealing subclinical LV dysfunction in our cohort.
Recently, two reports have shown that COVID-19 patients can suffer from RVDsys and RV dilatation [6]. However, in the study of Arguilan et al., 30% of patients were intubated and mechanically ventilated at the time of examination and evidence of pulmonary artery embolism was present in about 50% [21], possibly contributing to RVDsys by increased RV afterload. In our study cohort with myocardial injury, increased afterload was scarce and only one patient had evidence of pulmonary embolism in computed tomography scan.
At follow-up, we detected an improvement of biventricular systolic function in patients with previous COVID-19-associated myocardial injury. In comparison with baseline measurements, LV-GLS and RV-FWS significantly improved. Although we cannot provide echocardiographic data before onset of COVID-19 in these patients, our findings implicate that biventricular dysfunction in patients with COVID-19-associated myocardial injury could be transient and partially or even completely resolve over time.
In clinical survey at follow-up, some patients reported on ongoing respiratory insufficiency, so evaluation of New York Heart Association (NYHA) functional classification was fairly limited in validity. However, NT-proBNP levels significantly improved (744 pg/nl (initial) vs. 130 pg/nl (follow-up), p = 0.026), encouraging our implications on transient myocardial dysfunction under COVID-19.
Finally, we observed that with proper precautions including adequate personal protection equipment, comprehensive echocardiographic examinations can be safely performed during the COVID-19 pandemic.
Limitations
Our study cohort is relatively small but contains the largest comprehensive echocardiographic 3D and strain assessment of biventricular function in COVID-19 patients. Since no COVID-19 patient who underwent comprehensive echocardiography died, no predictors for mortality can be identified. Further, we cannot deduce from our data whether pathological findings in relatively old study cohort are associated with bad outcome in the sense of long-term heart failure. Since no previous comprehensive 3D and strain echocardiographic studies on patients with known cardiovascular disease were performed before the COVID-19 pandemic, we cannot exclude that our findings have existed before assessment.
The aetiology of myocardial injury in COVID-19 is not fully understood and beyond the scope of this study.
Conclusion
COVID-19 is associated with different patterns of systolic and diastolic biventricular dysfunction in hospitalised patients. The current prospective study shows that advanced 3D and strain echocardiography has superior diagnostic value over conventional 2D echocardiography, revealing LVDsys by LV-GLS and RVDsys by either 3D-RVEF or RV-FWS. The prognostic relevance of concomitant LVDsys, RVDsys and LVDdia will need to be further evaluated by endpoint analysis in the future in larger patient numbers. Taken together, comprehensive echocardiographic examinations can serve as a sensitive diagnostic tool to reveal pathologic patterns of myocardial function and their resolution after hospital discharge.
Data availability
The data that support the findings of this study are available from the corresponding authors upon request.
Code availability
Semi-automatic 3D HeartModel tool (Philips Healthcare, Andover, MA); AutoStrain function (TOMTEC Imaging Systems, Unterschleissheim, Germany); 4D RV-FUNCTION tool (TOMTEC Imaging Systems, Unterschleissheim, Germany).
References
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3.
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. China. JAMA Cardiol. 2020;5:802–810. https://doi.org/10.1001/jamacardio.2020.0950.
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. https://doi.org/10.1016/j.pcad.2020.03.001.
Akhmerov A, Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. https://doi.org/10.1161/CIRCRESAHA.120.317055.
Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, Cheng Y, Yan J, Ping H, Zhou Q. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan. China. Int J Cardiol. 2020;311:116–121. https://doi.org/10.1016/j.ijcard.2020.03.087.
Li Y, Li H, Zhu S, Xie Y, Wang B, He L, Zhang D, Zhang Y, Yuan H, Wu C, Sun W, Zhang Y, Li M, Cui L, Cai Y, Wang J, Yang Y, Lv Q, Zhang L, Xie M. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2287–2299. https://doi.org/10.1016/j.jcmg.2020.04.014.
Janus SE, Hajjari J, Karnib M, Tashtish N, Sadeer Gi A, Hoit B. Prognostic value of left ventricular global longitudinal strain in COVID-19. Am J Cardiol. 2020;131:134–136. https://doi.org/10.1016/j.amjcard.2020.06.053.
Muenchhoff M, Mairhofer H, Nitschko H, Grzimek-Koschewa N, Hoffmann D, Berger A, Rabenau H, Widera M, Ackermann N, Konrad R, Zange S, Graf A, Krebs S, Blum H, Sing A, Liebl B, Wolfel R, Ciesek S, Drosten C, Protzer U, Boehm S, Keppler OT. Multicentre comparison of quantitative PCR-based assays to detect SARS-CoV-2, Germany, March 2020. Euro Surveill. 2020;25:2001057. https://doi.org/10.2807/1560-7917.ES.2020.25.24.2001057.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39e14. https://doi.org/10.1016/j.echo.2014.10.003.
Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, Donal E, Sade LE, Ernande L, Garbi M, Grapsa J, Hagendorff A, Kamp O, Magne J, Santoro C, Stefanidis A, Lancellotti P, Popescu B, Habib G, Committee ESD, Committee ESD. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18:1301–10. https://doi.org/10.1093/ehjci/jex244.
D’Elia N, Caselli S, Kosmala W, Lancellotti P, Morris D, Muraru D, Takeuchi M, van den Bosch A, van Grootel RWJ, Villarraga H, Marwick TH. Normal global longitudinal strain: an individual patient meta-analysis. JACC Cardiovasc Imaging. 2020;13:167–9.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent LB, Cleveland O, Novara I, Rochester M, Bucharest R, St. Louis M. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–60. https://doi.org/10.1093/ehjci/jew082.
Muraru D, Badano LP, Nagata Y, Surkova E, Nabeshima Y, Genovese D, Otsuji Y, Guida V, Azzolina D, Palermo C, Takeuchi M. Development and prognostic validation of partition values to grade right ventricular dysfunction severity using 3D echocardiography. Eur Heart J Cardiovasc Imaging. 2020;21:10–21. https://doi.org/10.1093/ehjci/jez233.
Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr. 2017;30:303–71. https://doi.org/10.1016/j.echo.2017.01.007.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. https://doi.org/10.1093/ehjci/jev014.
King DL, El-Khoury Coffin L, Maurer MS. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40:325–9. https://doi.org/10.1016/s0735-1097(02)01944-7.
Orban M, Braun D, Deseive S, Stolz L, Stocker TJ, Stark K, Stremmel C, Hagl C, Massberg S, Hahn RT, Nabauer M, Hausleiter J. Transcatheter edge-to-edge repair for tricuspid regurgitation is associated with right ventricular reverse remodeling in patients with right-sided heart failure. JACC Cardiovasc Imaging. 2019;12:559–60. https://doi.org/10.1016/j.jcmg.2018.10.029.
Delanaye P, Cavalier E, Pottel H. Serum creatinine: not so simple! Nephron. 2017;136:302–8. https://doi.org/10.1159/000469669.
Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. https://doi.org/10.1001/jamacardio.2020.1286.
Dandel M. Cardiac manifestations of COVID-19 infection: the role of echocardiography in patient management. Infection. 2020;24:1–3. https://doi.org/10.1007/s15010-020-01507-3.
Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S, Lerakis S, Narula J. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. 2020;13:2459–2461. https://doi.org/10.1016/j.jcmg.2020.05.010.
Acknowledgements
We appreciate participation of all patients and their families in the CORKUM registry.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Deutsche Forschungsgemeinschaft (grant SFB 914, project B10 to L.T.W.) and Klinikum der Universitaet Muenchen.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Stéphanie Bieber, Ludwig T. Weckbach, Mathias Orban, Ines Schroeder, Michael Irlbeck, Clemens Scherer, Maximilian Muenchhoff and Johannes C. Hellmuth. Data analysis was performed by Stéphanie Bieber, Ludwig T. Weckbach and Mathias Orban. The first draft of the manuscript was written by Stéphanie Bieber and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
MO received speaker fees from Abbott Vascular and TOMTEC Imaging Systems. JH has received speaker honoraria from and serves as consultant for Abbott Vascular and Edwards Lifesciences. All other authors have no conflict of interest to declare that are relevant to the content of this article.
Ethics approval
Patient data were anonymized for analysis; the study was approved by the local ethics committee (No: 20-245) and complies with the Declaration of Helsinki of 1964.
Consent to participate and publication
Written informed consent for participation and publication was obtained from all patients prior to inclusion into CORKUM Registry and publication to this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bieber, S., Kraechan, A., Hellmuth, J.C. et al. Left and right ventricular dysfunction in patients with COVID-19-associated myocardial injury. Infection 49, 491–500 (2021). https://doi.org/10.1007/s15010-020-01572-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-020-01572-8