Dosing & Uses
Dosage Forms & Strengths
tablet
- 100mg
- 200mg
- 400mg (Rx)
- 600mg (Rx)
- 800mg (Rx)
capsule
- 200mg
tablet, chewable
- 50mg
- 100mg
oral suspension
- 100mg/5mL
- 40mg/mL
Pain/Fever/Dysmenorrhea
OTC: 200-400 mg PO q4-6hr; not to exceed 1200 mg unless directed by physician
Prescription: 400-800 mg PO q6hr; not to exceed 3200 mg/day
Inflammatory Disease
400-800 mg PO q6-8hr; not to exceed 3200 mg/day
Osteoarthritis
300 mg, 400 mg, 600 mg, or 800 mg PO q6-8hr; not to exceed 3200 mg/day
Monitor for gastrointestinal (GI) risks
Rheumatoid Arthritis
300 mg, 400 mg, 600 mg, or 800 mg PO q6-8hr; not to exceed 3200 mg/day
Monitor for GI risks
Dosage Modifications
Significantly impaired renal function: Monitor closely; consider reduced dosage if warranted
Severe hepatic impairment: Avoid use
Dosage Forms & Strengths
tablet
- 100mg
- 200mg
- 400mg (Rx)
- 600mg (Rx)
- 800mg (Rx)
capsule
- 200mg
tablet, chewable
- 50mg
- 100mg
oral suspension
- 100mg/5mL
- 40mg/mL
Fever
<6 months
- Safety and efficacy not established
≥6 months
Pain
4-10 mg/kg/dose PO q6-8hr; not to exceed 40 mg/kg/day
Juvenile Idiopathic Arthritis
30-50 mg/kg/24hr PO divided q8hr; not to exceed 2.4 g/day
Patent Ductus Arteriosus
See ibuprofen IV drug monograph
Cystic Fibrosis (Off-label)
<4 years: Safety and efficacy not established
≥4 years: PO administration q12hr, adjusted to maintain serum levels of 50-100 mcg/mL; may slow disease progression in younger patients with mild lung disease
Dosing Considerations
Interactions
Interaction Checker
No Results
Contraindicated
Serious - Use Alternative
Significant - Monitor Closely
Minor
Contraindicated (0)
Serious - Use Alternative (28)
- aminolevulinic acid oral
aminolevulinic acid oral, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Avoid administering other phototoxic drugs with aminolevulinic acid oral for 24 hr during perioperative period.
- aminolevulinic acid topical
ibuprofen, aminolevulinic acid topical. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Each drug may increase the photosensitizing effect of the other.
- apixaban
ibuprofen and apixaban both increase anticoagulation. Avoid or Use Alternate Drug.
- aspirin
ibuprofen decreases effects of aspirin by Other (see comment). Avoid or Use Alternate Drug. Comment: Ibuprofen decreases the antiplatelet effects of low-dose aspirin by blocking the active site of platelet cyclooxygenase. Administer ibuprofen 8 h before aspirin or at least 2-4 h after aspirin. The effect of other NSAIDs on aspirin is not established.
ibuprofen increases toxicity of aspirin by anticoagulation. Avoid or Use Alternate Drug. increases risk of bleeding. - aspirin rectal
ibuprofen decreases effects of aspirin rectal by Other (see comment). Avoid or Use Alternate Drug. Comment: Ibuprofen decreases the antiplatelet effects of aspirin by blocking the active site of platelet cyclooxygenase. The effect of other NSAIDs on aspirin is not established.
- aspirin/citric acid/sodium bicarbonate
ibuprofen decreases effects of aspirin/citric acid/sodium bicarbonate by Other (see comment). Avoid or Use Alternate Drug. Comment: Ibuprofen decreases the antiplatelet effects of aspirin by blocking the active site of platelet cyclooxygenase. The effect of other NSAIDs on aspirin is not established.
- benazepril
ibuprofen, benazepril. pharmacodynamic antagonism. Avoid or Use Alternate Drug. Coadministration may result in a significant decrease in renal function. NSAIDs may diminish the antihypertensive effect of ACE inhibitors. The mechanism of these interactions is likely related to the ability of NSAIDs to reduce the synthesis of vasodilating renal prostaglandins.
- captopril
ibuprofen, captopril. pharmacodynamic antagonism. Avoid or Use Alternate Drug. Coadministration may result in a significant decrease in renal function. NSAIDs may diminish the antihypertensive effect of ACE inhibitors. The mechanism of these interactions is likely related to the ability of NSAIDs to reduce the synthesis of vasodilating renal prostaglandins.
- enalapril
ibuprofen, enalapril. pharmacodynamic antagonism. Avoid or Use Alternate Drug. Coadministration may result in a significant decrease in renal function. NSAIDs may diminish the antihypertensive effect of ACE inhibitors. The mechanism of these interactions is likely related to the ability of NSAIDs to reduce the synthesis of vasodilating renal prostaglandins.
- erdafitinib
ibuprofen will increase the level or effect of erdafitinib by affecting hepatic enzyme CYP2C9/10 metabolism. Avoid or Use Alternate Drug. If unable to avoid coadministration with strong CYP2C9 inhibitors, monitor closely for adverse reactions and consider decreasing dose accordingly. If strong CYP2C9 inhibitor is discontinued, consider increasing erdafitinib dose in the absence of any drug-related toxicities.
- fosinopril
ibuprofen, fosinopril. pharmacodynamic antagonism. Avoid or Use Alternate Drug. Coadministration may result in a significant decrease in renal function. NSAIDs may diminish the antihypertensive effect of ACE inhibitors. The mechanism of these interactions is likely related to the ability of NSAIDs to reduce the synthesis of vasodilating renal prostaglandins.
- ketorolac
ibuprofen, ketorolac. Either increases toxicity of the other by pharmacodynamic synergism. Contraindicated.
- ketorolac intranasal
ibuprofen, ketorolac intranasal. Either increases toxicity of the other by pharmacodynamic synergism. Contraindicated.
- lisinopril
ibuprofen, lisinopril. pharmacodynamic antagonism. Avoid or Use Alternate Drug. Coadministration may result in a significant decrease in renal function. NSAIDs may diminish the antihypertensive effect of ACE inhibitors. The mechanism of these interactions is likely related to the ability of NSAIDs to reduce the synthesis of vasodilating renal prostaglandins.
- methotrexate
ibuprofen increases levels of methotrexate by decreasing renal clearance. Avoid or Use Alternate Drug. Concomitant administration of NSAIDs with high dose methotrexate has been reported to elevate and prolong serum methotrexate levels, resulting in deaths from severe hematologic and GI toxicity. NSAIDs may reduce tubular secretion of methotrexate and enhance toxicity. .
- methyl aminolevulinate
ibuprofen, methyl aminolevulinate. Either increases toxicity of the other by pharmacodynamic synergism. Avoid or Use Alternate Drug. Each drug may increase the photosensitizing effect of the other.
- moexipril
ibuprofen, moexipril. pharmacodynamic antagonism. Avoid or Use Alternate Drug. Coadministration may result in a significant decrease in renal function. NSAIDs may diminish the antihypertensive effect of ACE inhibitors. The mechanism of these interactions is likely related to the ability of NSAIDs to reduce the synthesis of vasodilating renal prostaglandins.
- naproxen
ibuprofen will increase the level or effect of naproxen by acidic (anionic) drug competition for renal tubular clearance. Avoid or Use Alternate Drug. Therapeutic duplication
ibuprofen and naproxen both increase anticoagulation. Avoid or Use Alternate Drug. Therapeutic duplication
ibuprofen and naproxen both increase serum potassium. Avoid or Use Alternate Drug. Therapeutic duplication - oxaprozin
ibuprofen will increase the level or effect of oxaprozin by acidic (anionic) drug competition for renal tubular clearance. Avoid or Use Alternate Drug. Therapeutic duplication
ibuprofen and oxaprozin both increase anticoagulation. Avoid or Use Alternate Drug. Therapeutic duplication
ibuprofen and oxaprozin both increase serum potassium. Avoid or Use Alternate Drug. Therapeutic duplication - pemetrexed
ibuprofen increases levels of pemetrexed by unspecified interaction mechanism. Avoid or Use Alternate Drug. Especially in pts. w/mild moderate renal insufficiency. D/C NSAIDs 2 5 d before and 2 d after pemetrexed administration.
- perindopril
ibuprofen, perindopril. pharmacodynamic antagonism. Avoid or Use Alternate Drug. Coadministration may result in a significant decrease in renal function. NSAIDs may diminish the antihypertensive effect of ACE inhibitors. The mechanism of these interactions is likely related to the ability of NSAIDs to reduce the synthesis of vasodilating renal prostaglandins.
- pexidartinib
ibuprofen and pexidartinib both increase Other (see comment). Avoid or Use Alternate Drug. Pexidartinib can cause hepatotoxicity. Avoid coadministration of pexidartinib with other products know to cause hepatoxicity.
- pretomanid
ibuprofen, pretomanid. Either increases toxicity of the other by Other (see comment). Avoid or Use Alternate Drug. Comment: Pretomanid regimen associated with hepatotoxicity. Avoid alcohol and hepatotoxic agents, including herbal supplements and drugs other than bedaquiline and linezolid.
- quinapril
ibuprofen, quinapril. pharmacodynamic antagonism. Avoid or Use Alternate Drug. Coadministration may result in a significant decrease in renal function. NSAIDs may diminish the antihypertensive effect of ACE inhibitors. The mechanism of these interactions is likely related to the ability of NSAIDs to reduce the synthesis of vasodilating renal prostaglandins.
- ramipril
ibuprofen, ramipril. pharmacodynamic antagonism. Avoid or Use Alternate Drug. Coadministration may result in a significant decrease in renal function. NSAIDs may diminish the antihypertensive effect of ACE inhibitors. The mechanism of these interactions is likely related to the ability of NSAIDs to reduce the synthesis of vasodilating renal prostaglandins.
- siponimod
ibuprofen will increase the level or effect of siponimod by affecting hepatic enzyme CYP2C9/10 metabolism. Avoid or Use Alternate Drug. Coadministration of siponimod with drugs that cause moderate CYP2C9 AND a moderate or strong CYP3A4 inhibition is not recommended. Caution if siponimod coadministered with moderate CYP2C9 inhibitors alone.
- tacrolimus
ibuprofen, tacrolimus. Either increases toxicity of the other by Other (see comment). Avoid or Use Alternate Drug. Comment: Concomitant administration increases risk of nephrotoxicity.
- trandolapril
ibuprofen, trandolapril. pharmacodynamic antagonism. Avoid or Use Alternate Drug. Coadministration may result in a significant decrease in renal function. NSAIDs may diminish the antihypertensive effect of ACE inhibitors. The mechanism of these interactions is likely related to the ability of NSAIDs to reduce the synthesis of vasodilating renal prostaglandins.
Monitor Closely (248)
- acebutolol
acebutolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of acebutolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - aceclofenac
aceclofenac and ibuprofen both increase anticoagulation. Use Caution/Monitor.
aceclofenac and ibuprofen both increase serum potassium. Use Caution/Monitor. - acemetacin
acemetacin and ibuprofen both increase anticoagulation. Use Caution/Monitor.
acemetacin and ibuprofen both increase serum potassium. Use Caution/Monitor. - agrimony
ibuprofen and agrimony both increase anticoagulation. Use Caution/Monitor.
- albuterol
ibuprofen increases and albuterol decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- alfalfa
ibuprofen and alfalfa both increase anticoagulation. Use Caution/Monitor.
- alfuzosin
ibuprofen decreases effects of alfuzosin by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
- aliskiren
ibuprofen will decrease the level or effect of aliskiren by Other (see comment). Use Caution/Monitor. In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs with drugs that affect RAAS may increase the risk of renal impairment (including acute renal failure) and cause loss of antihypertensive effect. Monitor renal function periodically.
- alteplase
ibuprofen and alteplase both increase anticoagulation. Use Caution/Monitor. Potential for increased risk of bleeding, caution is advised.
- American ginseng
ibuprofen and American ginseng both increase anticoagulation. Use Caution/Monitor.
- amikacin
ibuprofen increases levels of amikacin by decreasing renal clearance. Use Caution/Monitor. Interaction mainly occurs in preterm infants.
- amiloride
amiloride and ibuprofen both increase serum potassium. Modify Therapy/Monitor Closely.
- antithrombin alfa
antithrombin alfa and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- antithrombin III
antithrombin III and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- arformoterol
ibuprofen increases and arformoterol decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- argatroban
argatroban and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- asenapine
ibuprofen decreases effects of asenapine by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
- aspirin
aspirin and ibuprofen both increase anticoagulation. Use Caution/Monitor.
aspirin and ibuprofen both increase serum potassium. Use Caution/Monitor. - aspirin rectal
aspirin rectal and ibuprofen both increase anticoagulation. Use Caution/Monitor.
aspirin rectal and ibuprofen both increase serum potassium. Use Caution/Monitor. - aspirin/citric acid/sodium bicarbonate
aspirin/citric acid/sodium bicarbonate and ibuprofen both increase anticoagulation. Use Caution/Monitor.
aspirin/citric acid/sodium bicarbonate and ibuprofen both increase serum potassium. Use Caution/Monitor. - atenolol
atenolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of atenolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - azficel-T
azficel-T, ibuprofen. Other (see comment). Use Caution/Monitor. Comment: Patients taking NSAIDS may experience increased bruising or bleeding at biopsy and/or injection sites. Concomitant use of NSAIDs is not recommended.
- azilsartan
ibuprofen, azilsartan. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
ibuprofen decreases effects of azilsartan by pharmacodynamic antagonism. Modify Therapy/Monitor Closely. NSAIDs decrease synthesis of vasodilating renal prostaglandins, and thus affect fluid homeostasis and may diminish antihypertensive effect. - bemiparin
bemiparin and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- benazepril
benazepril, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
- bendroflumethiazide
ibuprofen increases and bendroflumethiazide decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- betaxolol
betaxolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of betaxolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - betrixaban
ibuprofen, betrixaban. Either increases levels of the other by anticoagulation. Use Caution/Monitor.
- bimatoprost
bimatoprost, ibuprofen. unspecified interaction mechanism. Use Caution/Monitor. There are conflicting reports from studies of either increased or decreased IOP when ophthalmic prostaglandins are coadministered with NSAIDs (either systemic or ophthalmic).
- bisoprolol
bisoprolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of bisoprolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - bivalirudin
bivalirudin and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- budesonide
ibuprofen, budesonide. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of GI ulceration.
- bumetanide
ibuprofen increases and bumetanide decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
ibuprofen decreases effects of bumetanide by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis. - candesartan
candesartan and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of candesartan by pharmacodynamic antagonism. Modify Therapy/Monitor Closely. NSAIDs decrease synthesis of vasodilating renal prostaglandins, and thus affect fluid homeostasis and may diminish antihypertensive effect.
candesartan, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals. - captopril
captopril, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
- carbamazepine
ibuprofen will increase the level or effect of carbamazepine by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Use Caution/Monitor. Monitor plasma levels when used concomitantly
- carbenoxolone
ibuprofen increases and carbenoxolone decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- carvedilol
carvedilol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of carvedilol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - celecoxib
celecoxib and ibuprofen both increase anticoagulation. Use Caution/Monitor.
celecoxib and ibuprofen both increase serum potassium. Use Caution/Monitor. - celiprolol
celiprolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of celiprolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - chlorothiazide
ibuprofen increases and chlorothiazide decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- chlorpropamide
ibuprofen increases effects of chlorpropamide by unknown mechanism. Use Caution/Monitor. Risk of hypoglycemia.
- chlorthalidone
ibuprofen increases and chlorthalidone decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- choline magnesium trisalicylate
ibuprofen and choline magnesium trisalicylate both increase anticoagulation. Use Caution/Monitor.
ibuprofen and choline magnesium trisalicylate both increase serum potassium. Use Caution/Monitor. - cinnamon
ibuprofen and cinnamon both increase anticoagulation. Use Caution/Monitor.
- ciprofloxacin
ibuprofen, ciprofloxacin. Other (see comment). Modify Therapy/Monitor Closely. Comment: Mechanism: unknown. Increased risk of CNS stimulation and seizures with high doses of fluoroquinolones.
- citalopram
citalopram, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. If possible, avoid concurrent use.
- clomipramine
clomipramine, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. Clomipramine inhib. serotonin uptake by platelets.
- clopidogrel
clopidogrel, ibuprofen. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Clopidogrel and NSAIDs both inhibit platelet aggregation.
- cordyceps
ibuprofen and cordyceps both increase anticoagulation. Use Caution/Monitor.
- cortisone
ibuprofen, cortisone. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of GI ulceration.
- cyclopenthiazide
ibuprofen increases and cyclopenthiazide decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- cyclosporine
ibuprofen, cyclosporine. Either increases toxicity of the other by nephrotoxicity and/or ototoxicity. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis, increasing the risk of nephrotoxicity.
- dabigatran
dabigatran and ibuprofen both increase anticoagulation. Use Caution/Monitor. Caution is advised, both drugs have the potential to cause bleeding. Concomitant use may increase risk of bleeding.
- dalteparin
dalteparin and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- deferasirox
deferasirox, ibuprofen. Other (see comment). Use Caution/Monitor. Comment: Combination may increase GI bleeding, ulceration and irritation. Use with caution.
- defibrotide
defibrotide increases effects of ibuprofen by P-glycoprotein (MDR1) efflux transporter. Use Caution/Monitor. Defibrotide may enhance effects of platelet inhibitors.
- deflazacort
ibuprofen, deflazacort. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of GI ulceration.
- dexamethasone
ibuprofen, dexamethasone. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of GI ulceration.
- dichlorphenamide
dichlorphenamide, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Both drugs can cause metabolic acidosis.
- diclofenac
diclofenac and ibuprofen both increase anticoagulation. Use Caution/Monitor.
diclofenac and ibuprofen both increase serum potassium. Use Caution/Monitor. - diflunisal
diflunisal and ibuprofen both increase anticoagulation. Use Caution/Monitor.
diflunisal and ibuprofen both increase serum potassium. Use Caution/Monitor. - digoxin
ibuprofen and digoxin both increase serum potassium. Use Caution/Monitor.
- dobutamine
ibuprofen increases and dobutamine decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- dong quai
ibuprofen and dong quai both increase anticoagulation. Use Caution/Monitor.
- dopexamine
ibuprofen increases and dopexamine decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- doxazosin
ibuprofen decreases effects of doxazosin by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
- dronabinol
ibuprofen will increase the level or effect of dronabinol by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor. Dronabinol is a CYP2C9 substrate.
- drospirenone
drospirenone and ibuprofen both increase serum potassium. Modify Therapy/Monitor Closely.
- duloxetine
duloxetine, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. SSRIs inhib. serotonin uptake by platelets.
- edoxaban
edoxaban, ibuprofen. Either increases toxicity of the other by anticoagulation. Modify Therapy/Monitor Closely. Both drugs have the potential to cause bleeding, monitor closely. Promptly evaluate any signs or symptoms of blood loss.
- efavirenz
efavirenz will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor.
- eltrombopag
eltrombopag increases levels of ibuprofen by decreasing metabolism. Use Caution/Monitor. UGT inhibition; significance of interaction unclear.
- eluxadoline
ibuprofen increases levels of eluxadoline by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor. As a precautionary measure due to incomplete information on the metabolism of eluxadoline, use caution when coadministered with strong CYP2C9/10 inhibitors.
- elvitegravir/cobicistat/emtricitabine/tenofovir DF
elvitegravir/cobicistat/emtricitabine/tenofovir DF, ibuprofen. Either increases toxicity of the other by decreasing renal clearance. Modify Therapy/Monitor Closely. Toxicity may result from coadministration of emtricitabine and tenofovir with other drugs that are also primarily excreted by glomerular filtration and/or active tubular secretion including high-dose or multiple-dose NSAIDs; alternatives to NSAIDs should be considered.
- emtricitabine
emtricitabine, ibuprofen. Either increases levels of the other by decreasing renal clearance. Modify Therapy/Monitor Closely. Toxicity may result from coadministration of emtricitabine with other drugs that are also primarily excreted by glomerular filtration and/or active tubular secretion including high-dose or multiple-dose NSAIDs; alternatives to NSAIDs should be considered.
- enalapril
enalapril, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
- enoxaparin
enoxaparin and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- ephedrine
ibuprofen increases and ephedrine decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- epinephrine
ibuprofen increases and epinephrine decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- epinephrine racemic
ibuprofen increases and epinephrine racemic decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- epoprostenol
ibuprofen and epoprostenol both increase anticoagulation. Use Caution/Monitor.
- eprosartan
eprosartan and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of eprosartan by pharmacodynamic antagonism. Modify Therapy/Monitor Closely. NSAIDs decrease synthesis of vasodilating renal prostaglandins, and thus affect fluid homeostasis and may diminish antihypertensive effect.
eprosartan, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals. - escitalopram
escitalopram, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. SSRIs inhib. serotonin uptake by platelets.
- esmolol
esmolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of esmolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - ethacrynic acid
ibuprofen increases and ethacrynic acid decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- etodolac
etodolac and ibuprofen both increase anticoagulation. Use Caution/Monitor.
etodolac and ibuprofen both increase serum potassium. Use Caution/Monitor. - fennel
ibuprofen and fennel both increase anticoagulation. Use Caution/Monitor.
- fenoprofen
fenoprofen and ibuprofen both increase anticoagulation. Use Caution/Monitor.
fenoprofen and ibuprofen both increase serum potassium. Use Caution/Monitor. - feverfew
ibuprofen and feverfew both increase anticoagulation. Use Caution/Monitor.
- fish oil triglycerides
fish oil triglycerides will increase the level or effect of ibuprofen by anticoagulation. Use Caution/Monitor. Prolonged bleeding reported in patients taking antiplatelet agents or anticoagulants and oral omega-3 fatty acids. Periodically monitor bleeding time in patients receiving fish oil triglycerides and concomitant antiplatelet agents or anticoagulants.
- fludrocortisone
ibuprofen, fludrocortisone. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of GI ulceration.
- fluoxetine
fluoxetine will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor.
fluoxetine, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. SSRIs inhib. serotonin uptake by platelets. - flurbiprofen
flurbiprofen and ibuprofen both increase anticoagulation. Use Caution/Monitor.
flurbiprofen and ibuprofen both increase serum potassium. Use Caution/Monitor. - fluvoxamine
fluvoxamine, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding; SSRIs inhib. srotonin uptake by platelets.
- fondaparinux
fondaparinux and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- formoterol
ibuprofen increases and formoterol decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- forskolin
ibuprofen and forskolin both increase anticoagulation. Use Caution/Monitor.
- fosinopril
fosinopril, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
- furosemide
ibuprofen increases and furosemide decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- garlic
ibuprofen and garlic both increase anticoagulation. Use Caution/Monitor.
- gemifloxacin
gemifloxacin, ibuprofen. Other (see comment). Modify Therapy/Monitor Closely. Comment: Increased risk of CNS stimulation and seizures with high doses of fluoroquinolones.
- gentamicin
ibuprofen increases and gentamicin decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- ginger
ibuprofen and ginger both increase anticoagulation. Use Caution/Monitor.
- ginkgo biloba
ibuprofen and ginkgo biloba both increase anticoagulation. Use Caution/Monitor.
- glimepiride
ibuprofen increases effects of glimepiride by unknown mechanism. Use Caution/Monitor. Risk of hypoglycemia.
- glipizide
ibuprofen increases effects of glipizide by unknown mechanism. Use Caution/Monitor. Risk of hypoglycemia.
- glyburide
ibuprofen increases effects of glyburide by unknown mechanism. Use Caution/Monitor. Risk of hypoglycemia.
ibuprofen increases levels of glyburide by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor. Strong CYP2C9 inhibitors may decrease glyburide metabolism. - green tea
green tea, ibuprofen. Other (see comment). Use Caution/Monitor. Comment: Combination may increase risk of bleeding.
- heparin
heparin and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- horse chestnut seed
ibuprofen and horse chestnut seed both increase anticoagulation. Use Caution/Monitor.
- hydralazine
ibuprofen decreases effects of hydralazine by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
- hydrochlorothiazide
ibuprofen increases and hydrochlorothiazide decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- hydrocortisone
ibuprofen, hydrocortisone. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of GI ulceration.
- ibrutinib
ibrutinib will increase the level or effect of ibuprofen by anticoagulation. Use Caution/Monitor. Ibrutinib may increase the risk of hemorrhage in patients receiving antiplatelet or anticoagulant therapies and monitor for signs of bleeding.
- icosapent
icosapent, ibuprofen. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Icosapent may prolong bleeding time. Periodically monitor if coadministered with other drugs that affect bleeding.
- imatinib
imatinib will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor.
imatinib, ibuprofen. Either increases toxicity of the other by Other (see comment). Modify Therapy/Monitor Closely. Comment: Imatinib may cause thrombocytopenia; bleeding risk increased when imatinib is coadministered with anticoagulants, NSAIDs, platelet inhibitors, and thrombolytic agents. - indapamide
ibuprofen increases and indapamide decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- indomethacin
ibuprofen and indomethacin both increase anticoagulation. Use Caution/Monitor.
ibuprofen and indomethacin both increase serum potassium. Use Caution/Monitor. - irbesartan
irbesartan and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of irbesartan by pharmacodynamic antagonism. Use Caution/Monitor. Antihypertensive effect of angiotensin receptor blockers may be attenuated by NSAIDs; monitor renal function and blood pressure periodically.
irbesartan, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals. - isoproterenol
ibuprofen increases and isoproterenol decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- ketoprofen
ibuprofen and ketoprofen both increase anticoagulation. Use Caution/Monitor.
ibuprofen and ketoprofen both increase serum potassium. Use Caution/Monitor. - ketorolac
ibuprofen and ketorolac both increase anticoagulation. Use Caution/Monitor.
ibuprofen and ketorolac both increase serum potassium. Use Caution/Monitor. - ketorolac intranasal
ibuprofen and ketorolac intranasal both increase anticoagulation. Use Caution/Monitor.
ibuprofen and ketorolac intranasal both increase serum potassium. Use Caution/Monitor. - labetalol
labetalol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of labetalol by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs diminish antihypertensive effects of beta-blockers. - lacosamide
ibuprofen increases levels of lacosamide by affecting hepatic enzyme CYP2C9/10 metabolism. Modify Therapy/Monitor Closely. Consider decreasing lacosamide dose when coadministered with strong CYP2C9 inhibitors.
- latanoprost
latanoprost, ibuprofen. unspecified interaction mechanism. Use Caution/Monitor. There are conflicting reports from studies of either increased or decreased IOP when ophthalmic prostaglandins are coadministered with NSAIDs (either systemic or ophthalmic).
- latanoprostene bunod ophthalmic
latanoprostene bunod ophthalmic, ibuprofen. unspecified interaction mechanism. Use Caution/Monitor. There are conflicting reports from studies of either increased or decreased IOP when ophthalmic prostaglandins are coadministered with NSAIDs (either systemic or ophthalmic).
- lesinurad (DSC)
ibuprofen will increase the level or effect of lesinurad (DSC) by affecting hepatic enzyme CYP2C9/10 metabolism. Modify Therapy/Monitor Closely.
- levalbuterol
ibuprofen increases and levalbuterol decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- levofloxacin
levofloxacin, ibuprofen. Other (see comment). Modify Therapy/Monitor Closely. Comment: Risk of CNS stimulation/seizure. Mechanism: Displacement of GABA from receptors in brain.
- levomilnacipran
levomilnacipran, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. SNRIs may further impair platelet activity in patients taking antiplatelet or anticoagulant drugs.
- lisinopril
lisinopril, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
- lithium
ibuprofen increases levels of lithium by decreasing renal clearance. Use Caution/Monitor.
- lornoxicam
ibuprofen and lornoxicam both increase anticoagulation. Use Caution/Monitor.
ibuprofen and lornoxicam both increase serum potassium. Use Caution/Monitor. - losartan
losartan and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of losartan by pharmacodynamic antagonism. Modify Therapy/Monitor Closely. NSAIDs decrease synthesis of vasodilating renal prostaglandins, and thus affect fluid homeostasis and may diminish antihypertensive effect.
losartan, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals. - lumacaftor/ivacaftor
lumacaftor/ivacaftor will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor. Ibuprofen it a substrate of CYP2C9. Lumacaftor has the potential to induce CYP2C9 substrates.
- meclofenamate
meclofenamate and ibuprofen both increase anticoagulation. Use Caution/Monitor.
meclofenamate and ibuprofen both increase serum potassium. Use Caution/Monitor. - mefenamic acid
ibuprofen and mefenamic acid both increase anticoagulation. Use Caution/Monitor.
ibuprofen and mefenamic acid both increase serum potassium. Use Caution/Monitor. - melatonin
melatonin increases effects of ibuprofen by anticoagulation. Use Caution/Monitor. Melatonin may decrease prothrombin time.
- meloxicam
ibuprofen and meloxicam both increase anticoagulation. Use Caution/Monitor.
ibuprofen and meloxicam both increase serum potassium. Use Caution/Monitor. - mesalamine
mesalamine, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Additive nephrotoxicity.
- metaproterenol
ibuprofen increases and metaproterenol decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- methyclothiazide
ibuprofen increases and methyclothiazide decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor. .
- methylprednisolone
ibuprofen, methylprednisolone. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of GI ulceration.
- metolazone
ibuprofen increases and metolazone decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- metoprolol
metoprolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of metoprolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - milnacipran
milnacipran, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. SSRIs inhib. serotonin uptake by platelets.
- mipomersen
mipomersen, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: Both drugs have potential to increase hepatic enzymes; monitor LFTs.
- mistletoe
ibuprofen increases and mistletoe decreases anticoagulation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- moexipril
moexipril, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
- moxifloxacin
moxifloxacin, ibuprofen. Other (see comment). Modify Therapy/Monitor Closely. Comment: Increased risk of CNS stimulation and seizures with high doses of fluoroquinolones.
- moxisylyte
ibuprofen decreases effects of moxisylyte by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
- mycophenolate
ibuprofen will increase the level or effect of mycophenolate by acidic (anionic) drug competition for renal tubular clearance. Use Caution/Monitor.
- nabumetone
ibuprofen and nabumetone both increase anticoagulation. Use Caution/Monitor.
ibuprofen and nabumetone both increase serum potassium. Use Caution/Monitor. - nadolol
nadolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of nadolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - nebivolol
nebivolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of nebivolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - nefazodone
nefazodone, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. SSRIs inhib. serotonin uptake by platelets.
- nettle
ibuprofen increases and nettle decreases anticoagulation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- norepinephrine
ibuprofen increases and norepinephrine decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- olmesartan
olmesartan and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of olmesartan by pharmacodynamic antagonism. Modify Therapy/Monitor Closely. NSAIDs decrease synthesis of vasodilating renal prostaglandins, and thus affect fluid homeostasis and may diminish antihypertensive effect.
olmesartan, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals. - ospemifene
ibuprofen increases levels of ospemifene by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor.
ibuprofen, ospemifene. Either increases levels of the other by plasma protein binding competition. Modify Therapy/Monitor Closely. - panax ginseng
ibuprofen and panax ginseng both increase anticoagulation. Use Caution/Monitor.
- parecoxib
ibuprofen and parecoxib both increase anticoagulation. Use Caution/Monitor.
ibuprofen and parecoxib both increase serum potassium. Use Caution/Monitor. - paroxetine
paroxetine, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. SSRIs inhib. serotonin uptake by platelets.
- pau d'arco
ibuprofen and pau d'arco both increase anticoagulation. Use Caution/Monitor.
- pegaspargase
pegaspargase increases effects of ibuprofen by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of bleeding events.
- peginterferon alfa 2b
peginterferon alfa 2b decreases levels of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor. When patients are administered peginterferon alpha-2b with CYP2C9 substrates, the therapeutic effect of these drugs may be altered.
- penbutolol
penbutolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of penbutolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - perindopril
perindopril, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
- phenindione
phenindione and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- phenoxybenzamine
ibuprofen decreases effects of phenoxybenzamine by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
- phentolamine
ibuprofen decreases effects of phentolamine by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
- phytoestrogens
ibuprofen and phytoestrogens both increase anticoagulation. Use Caution/Monitor.
- pindolol
pindolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of pindolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - pirbuterol
ibuprofen increases and pirbuterol decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- piroxicam
ibuprofen and piroxicam both increase anticoagulation. Use Caution/Monitor.
ibuprofen and piroxicam both increase serum potassium. Use Caution/Monitor. - pivmecillinam
pivmecillinam, ibuprofen. Either increases levels of the other by plasma protein binding competition. Use Caution/Monitor.
pivmecillinam, ibuprofen. Either increases levels of the other by decreasing renal clearance. Use Caution/Monitor. - potassium acid phosphate
ibuprofen and potassium acid phosphate both increase serum potassium. Modify Therapy/Monitor Closely.
- potassium chloride
ibuprofen and potassium chloride both increase serum potassium. Modify Therapy/Monitor Closely.
- potassium citrate
ibuprofen and potassium citrate both increase serum potassium. Modify Therapy/Monitor Closely.
- potassium iodide
potassium iodide and ibuprofen both increase serum potassium. Use Caution/Monitor.
- pralatrexate
ibuprofen increases levels of pralatrexate by decreasing renal clearance. Use Caution/Monitor. NSAIDs may delay pralatrexate clearance, increasing drug exposure. Adjust the pralatrexate dose as needed.
- prasugrel
ibuprofen, prasugrel. Either increases effects of the other by anticoagulation. Use Caution/Monitor. Chronic use of NSAIDs with prasugrel may increase bleeding risk.
- prazosin
ibuprofen decreases effects of prazosin by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
- prednisolone
ibuprofen, prednisolone. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of GI ulceration.
- prednisone
ibuprofen, prednisone. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of GI ulceration.
- probenecid
ibuprofen will increase the level or effect of probenecid by acidic (anionic) drug competition for renal tubular clearance. Use Caution/Monitor.
- propranolol
propranolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of propranolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - protamine
protamine and ibuprofen both increase anticoagulation. Modify Therapy/Monitor Closely.
- quinapril
quinapril, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
- ramipril
ramipril, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
- reishi
ibuprofen and reishi both increase anticoagulation. Use Caution/Monitor.
- reteplase
ibuprofen and reteplase both increase anticoagulation. Use Caution/Monitor. Potential for increased risk of bleeding, caution is advised.
- rivaroxaban
rivaroxaban, ibuprofen. Other (see comment). Use Caution/Monitor. Comment: NSAIDs are known to increase bleeding. Bleeding risk may be increased when NSAIDs are used concomitantly with rivaroxaban. Monitor for signs/symptoms of blood loss.
- rivastigmine
rivastigmine increases toxicity of ibuprofen by pharmacodynamic synergism. Use Caution/Monitor. Monitor patients for symptoms of active or occult gastrointestinal bleeding.
- sacubitril/valsartan
sacubitril/valsartan and ibuprofen both increase serum potassium. Use Caution/Monitor.
sacubitril/valsartan, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
ibuprofen decreases effects of sacubitril/valsartan by pharmacodynamic antagonism. Modify Therapy/Monitor Closely. NSAIDs decrease synthesis of vasodilating renal prostaglandins, and thus affect fluid homeostasis and may diminish antihypertensive effect. - salicylates (non-asa)
ibuprofen and salicylates (non-asa) both increase anticoagulation. Use Caution/Monitor.
ibuprofen and salicylates (non-asa) both increase serum potassium. Use Caution/Monitor. - salmeterol
ibuprofen increases and salmeterol decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- salsalate
ibuprofen and salsalate both increase anticoagulation. Use Caution/Monitor.
ibuprofen and salsalate both increase serum potassium. Use Caution/Monitor. - saw palmetto
saw palmetto increases toxicity of ibuprofen by unspecified interaction mechanism. Use Caution/Monitor. May increase risk of bleeding.
- sertraline
sertraline, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. SSRIs inhib. serotonin uptake by platelets.
- Siberian ginseng
ibuprofen and Siberian ginseng both increase anticoagulation. Use Caution/Monitor.
- silodosin
ibuprofen decreases effects of silodosin by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
- sodium picosulfate/magnesium oxide/anhydrous citric acid
ibuprofen, sodium picosulfate/magnesium oxide/anhydrous citric acid. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May be associated with fluid and electrolyte imbalances.
- sodium sulfate/?magnesium sulfate/potassium chloride
sodium sulfate/?magnesium sulfate/potassium chloride increases toxicity of ibuprofen by Other (see comment). Use Caution/Monitor. Comment: Coadministration with medications that cause fluid and electrolyte abnormalities may increase the risk of adverse events of seizure, arrhythmias, and renal impairment.
- sodium sulfate/potassium chloride/magnesium sulfate/polyethylene glycol
ibuprofen, sodium sulfate/potassium chloride/magnesium sulfate/polyethylene glycol. Other (see comment). Use Caution/Monitor. Comment: Caution when bowel preps are used with drugs that cause SIADH or NSAIDs; increased risk for water retention or electrolyte imbalance.
- sodium sulfate/potassium sulfate/magnesium sulfate
sodium sulfate/potassium sulfate/magnesium sulfate increases toxicity of ibuprofen by Other (see comment). Use Caution/Monitor. Comment: Coadministration with medications that cause fluid and electrolyte abnormalities may increase the risk of adverse events of seizure, arrhythmias, and renal impairment.
- sotalol
sotalol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of sotalol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - sparsentan
ibuprofen and sparsentan both increase nephrotoxicity and/or ototoxicity. Use Caution/Monitor. Coadministration of NSAIDS, including selective COX-2 inhibitors, may result in deterioration of kidney function (eg, possible kidney failure). Monitor for signs of worsening renal function with concomitant use with NSAIDs.
- spironolactone
spironolactone and ibuprofen both increase serum potassium. Modify Therapy/Monitor Closely.
- succinylcholine
ibuprofen and succinylcholine both increase serum potassium. Use Caution/Monitor.
- sulfasalazine
ibuprofen and sulfasalazine both increase anticoagulation. Use Caution/Monitor.
ibuprofen and sulfasalazine both increase serum potassium. Use Caution/Monitor. - sulindac
ibuprofen and sulindac both increase anticoagulation. Use Caution/Monitor.
ibuprofen and sulindac both increase serum potassium. Use Caution/Monitor. - tafluprost
tafluprost, ibuprofen. unspecified interaction mechanism. Use Caution/Monitor. There are conflicting reports from studies of either increased or decreased IOP when ophthalmic prostaglandins are coadministered with NSAIDs (either systemic or ophthalmic).
- telmisartan
telmisartan and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of telmisartan by pharmacodynamic antagonism. Modify Therapy/Monitor Closely. NSAIDs decrease synthesis of vasodilating renal prostaglandins, and thus affect fluid homeostasis and may diminish antihypertensive effect.
telmisartan, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals. - temocillin
temocillin, ibuprofen. Either increases levels of the other by plasma protein binding competition. Use Caution/Monitor.
temocillin, ibuprofen. Either increases levels of the other by decreasing renal clearance. Use Caution/Monitor. - tenecteplase
ibuprofen and tenecteplase both increase anticoagulation. Use Caution/Monitor. Potential for increased risk of bleeding, caution is advised.
- tenofovir DF
tenofovir DF, ibuprofen. Either increases levels of the other by decreasing renal clearance. Modify Therapy/Monitor Closely. Toxicity may result from coadministration of tenofovir DF with other drugs that are also primarily excreted by glomerular filtration and/or active tubular secretion including high-dose or multiple-dose NSAIDs; alternatives to NSAIDs should be considered.
- terazosin
ibuprofen decreases effects of terazosin by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
- terbinafine
ibuprofen will increase the level or effect of terbinafine by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor.
- terbutaline
ibuprofen increases and terbutaline decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- ticagrelor
ticagrelor, ibuprofen. Either increases effects of the other by anticoagulation. Use Caution/Monitor. Increased risk of bleeding with use of ticagrelor and chronic NSAID use. .
- ticarcillin
ticarcillin, ibuprofen. Either increases levels of the other by plasma protein binding competition. Use Caution/Monitor.
ticarcillin, ibuprofen. Either increases levels of the other by decreasing renal clearance. Use Caution/Monitor. - ticlopidine
ticlopidine will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Use Caution/Monitor.
ticlopidine increases toxicity of ibuprofen by anticoagulation. Use Caution/Monitor. - timolol
timolol and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of timolol by pharmacodynamic antagonism. Use Caution/Monitor. Long term (>1 wk) NSAID use. NSAIDs decrease prostaglandin synthesis. - tobramycin inhaled
tobramycin inhaled and ibuprofen both increase nephrotoxicity and/or ototoxicity. Modify Therapy/Monitor Closely. Avoid concurrent or sequential use to decrease risk for ototoxicity
- tolazamide
ibuprofen increases effects of tolazamide by unknown mechanism. Use Caution/Monitor. Risk of hypoglycemia.
- tolbutamide
ibuprofen increases effects of tolbutamide by unknown mechanism. Use Caution/Monitor. Risk of hypoglycemia.
- tolfenamic acid
ibuprofen and tolfenamic acid both increase anticoagulation. Use Caution/Monitor.
ibuprofen and tolfenamic acid both increase serum potassium. Use Caution/Monitor. - tolmetin
ibuprofen and tolmetin both increase anticoagulation. Use Caution/Monitor.
ibuprofen and tolmetin both increase serum potassium. Use Caution/Monitor. - tolvaptan
ibuprofen and tolvaptan both increase serum potassium. Use Caution/Monitor.
- torsemide
ibuprofen increases and torsemide decreases serum potassium. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- trandolapril
trandolapril, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals.
- travoprost ophthalmic
travoprost ophthalmic, ibuprofen. unspecified interaction mechanism. Use Caution/Monitor. There are conflicting reports from studies of either increased or decreased IOP when ophthalmic prostaglandins are coadministered with NSAIDs (either systemic or ophthalmic).
- trazodone
trazodone, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. SSRIs inhib. serotonin uptake by platelets.
- triamcinolone acetonide injectable suspension
ibuprofen, triamcinolone acetonide injectable suspension. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Concomitant use of NSAIDS and corticosteroids increases the risk of gastrointestinal side effects. .
- triamterene
triamterene and ibuprofen both increase serum potassium. Modify Therapy/Monitor Closely.
- valoctocogene roxaparvovec
ibuprofen and valoctocogene roxaparvovec both increase Other (see comment). Use Caution/Monitor. Medications that may cause hepatotoxicity when combined with valoctogene roxaparvovec may potentiate the risk of elevated liver enzymes. Closely monitor these medications and consider alternative medications in case of potential drug interactions.
- valsartan
valsartan and ibuprofen both increase serum potassium. Use Caution/Monitor.
ibuprofen decreases effects of valsartan by pharmacodynamic antagonism. Modify Therapy/Monitor Closely. NSAIDs decrease synthesis of vasodilating renal prostaglandins, and thus affect fluid homeostasis and may diminish antihypertensive effect.
valsartan, ibuprofen. Either increases toxicity of the other by Other (see comment). Use Caution/Monitor. Comment: May result in renal function deterioration, particularly in elderly or volume depleted individuals. - venlafaxine
venlafaxine, ibuprofen. Either increases toxicity of the other by pharmacodynamic synergism. Use Caution/Monitor. Increased risk of upper GI bleeding. SSRIs inhib. serotonin uptake by platelets.
- vitamin K1 (phytonadione)
ibuprofen increases and vitamin K1 (phytonadione) decreases anticoagulation. Effect of interaction is not clear, use caution. Use Caution/Monitor.
- voclosporin
voclosporin, ibuprofen. Either increases toxicity of the other by nephrotoxicity and/or ototoxicity. Modify Therapy/Monitor Closely. Coadministration with drugs associated with nephrotoxicity may increase the risk for acute and/or chronic nephrotoxicity.
- vorapaxar
ibuprofen, vorapaxar. Either increases effects of the other by pharmacodynamic synergism. Use Caution/Monitor. Additive antiplatelet effect may occur.
- vortioxetine
ibuprofen, vortioxetine. Either increases effects of the other by anticoagulation. Use Caution/Monitor.
- warfarin
ibuprofen, warfarin. Either increases effects of the other by pharmacodynamic synergism. Modify Therapy/Monitor Closely. Drugs with antiplatelet properties may increase anticoagulation effect of warfarin.
- zanubrutinib
ibuprofen, zanubrutinib. Either increases effects of the other by anticoagulation. Modify Therapy/Monitor Closely. Zanubrutinib-induced cytopenias increases risk of hemorrhage. Coadministration of zanubritinib with antiplatelets or anticoagulants may further increase this risk.
- zotepine
ibuprofen decreases effects of zotepine by pharmacodynamic antagonism. Use Caution/Monitor. NSAIDs decrease prostaglandin synthesis.
Minor (102)
- aceclofenac
aceclofenac will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- acemetacin
acemetacin will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- acyclovir
ibuprofen will increase the level or effect of acyclovir by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- adefovir
ibuprofen increases levels of adefovir by enhancing GI absorption. Applies only to oral form of both agents. Minor/Significance Unknown.
- alendronate
ibuprofen, alendronate. Either increases toxicity of the other by pharmacodynamic synergism. Minor/Significance Unknown. Increased risk of GI ulceration.
- aminohippurate sodium
ibuprofen will increase the level or effect of aminohippurate sodium by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- amiodarone
amiodarone will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- amobarbital
amobarbital will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- anamu
ibuprofen and anamu both increase anticoagulation. Minor/Significance Unknown.
- aspirin
aspirin will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- aspirin rectal
aspirin rectal will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- aspirin/citric acid/sodium bicarbonate
aspirin/citric acid/sodium bicarbonate will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- balsalazide
ibuprofen will increase the level or effect of balsalazide by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- bendroflumethiazide
bendroflumethiazide will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- bosentan
bosentan will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- butabarbital
butabarbital will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- butalbital
butalbital will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- carbamazepine
carbamazepine will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- cefadroxil
cefadroxil will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- cefamandole
cefamandole will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- cefdinir
cefdinir will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- cefpirome
cefpirome will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- ceftibuten
ceftibuten will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- celecoxib
celecoxib will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- cephalexin
cephalexin will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- chlorothiazide
chlorothiazide will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- chlorpropamide
ibuprofen will increase the level or effect of chlorpropamide by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- chlorthalidone
chlorthalidone will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- choline magnesium trisalicylate
ibuprofen will increase the level or effect of choline magnesium trisalicylate by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- cimetidine
cimetidine will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- creatine
creatine, ibuprofen. Mechanism: pharmacodynamic synergism. Minor/Significance Unknown. (Theoretical interaction) Combination may have additive nephrotoxic effects.
- cyclopenthiazide
cyclopenthiazide will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- danshen
ibuprofen and danshen both increase anticoagulation. Minor/Significance Unknown.
- devil's claw
ibuprofen and devil's claw both increase anticoagulation. Minor/Significance Unknown.
- diclofenac
diclofenac will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- diclofenac topical
diclofenac topical, ibuprofen. Either increases effects of the other by pharmacodynamic synergism. Minor/Significance Unknown. Although low, there is systemic exposure to diclofenac topical; theoretically, concomitant administration with systemic NSAIDS or aspirin may result in increased NSAID adverse effects.
- diflunisal
diflunisal will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- digoxin
ibuprofen increases levels of digoxin by decreasing renal clearance. Minor/Significance Unknown.
- disulfiram
disulfiram will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- eplerenone
ibuprofen decreases effects of eplerenone by pharmacodynamic antagonism. Minor/Significance Unknown. NSAIDs decrease prostaglandin synthesis.
- etodolac
etodolac will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- etravirine
etravirine will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- felbamate
felbamate will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- fenoprofen
fenoprofen will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- feverfew
ibuprofen decreases effects of feverfew by pharmacodynamic antagonism. Minor/Significance Unknown.
- fluconazole
fluconazole will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- flurbiprofen
flurbiprofen will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- furosemide
ibuprofen decreases effects of furosemide by pharmacodynamic antagonism. Minor/Significance Unknown. NSAIDs decrease prostaglandin synthesis.
- ganciclovir
ibuprofen will increase the level or effect of ganciclovir by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- gentamicin
ibuprofen increases levels of gentamicin by decreasing renal clearance. Minor/Significance Unknown. Interaction mainly occurs in preterm infants.
- hydrochlorothiazide
hydrochlorothiazide will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- imidapril
ibuprofen decreases effects of imidapril by pharmacodynamic antagonism. Minor/Significance Unknown. NSAIDs decrease prostaglandin synthesis.
- indapamide
indapamide will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- indomethacin
ibuprofen will increase the level or effect of indomethacin by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- ketoconazole
ketoconazole will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- ketoprofen
ibuprofen will increase the level or effect of ketoprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- ketorolac
ibuprofen will increase the level or effect of ketorolac by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- ketorolac intranasal
ibuprofen will increase the level or effect of ketorolac intranasal by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- leflunomide
leflunomide will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- levoketoconazole
levoketoconazole will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- lornoxicam
ibuprofen will increase the level or effect of lornoxicam by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- meclofenamate
meclofenamate will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- mefenamic acid
ibuprofen will increase the level or effect of mefenamic acid by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- meloxicam
ibuprofen will increase the level or effect of meloxicam by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- mesalamine
ibuprofen will increase the level or effect of mesalamine by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- methyclothiazide
methyclothiazide will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- metolazone
metolazone will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- metronidazole
metronidazole will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- miconazole vaginal
miconazole vaginal will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- nabumetone
ibuprofen will increase the level or effect of nabumetone by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- nateglinide
nateglinide will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- neomycin PO
ibuprofen increases levels of neomycin PO by decreasing renal clearance. Minor/Significance Unknown. Interaction mainly occurs in preterm infants.
- nilotinib
nilotinib will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- noni juice
ibuprofen and noni juice both increase serum potassium. Minor/Significance Unknown.
- ofloxacin
ofloxacin, ibuprofen. Other (see comment). Minor/Significance Unknown. Comment: Risk of CNS stimulation/seizure. Mechanism: Displacement of GABA from receptors in brain.
- parecoxib
ibuprofen will increase the level or effect of parecoxib by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- paromomycin
ibuprofen increases levels of paromomycin by decreasing renal clearance. Minor/Significance Unknown. Interaction mainly occurs in preterm infants.
- pentobarbital
pentobarbital will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- phenobarbital
phenobarbital will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- piroxicam
ibuprofen will increase the level or effect of piroxicam by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- primidone
primidone will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- rifampin
rifampin will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- rifapentine
rifapentine will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- rose hips
rose hips will increase the level or effect of ibuprofen by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- salicylates (non-asa)
ibuprofen will increase the level or effect of salicylates (non-asa) by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- salsalate
ibuprofen will increase the level or effect of salsalate by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- secobarbital
secobarbital will decrease the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- streptomycin
ibuprofen increases levels of streptomycin by decreasing renal clearance. Minor/Significance Unknown. Interaction mainly occurs in preterm infants.
- sulfamethoxazole
sulfamethoxazole will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- sulfasalazine
ibuprofen will increase the level or effect of sulfasalazine by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- sulindac
ibuprofen will increase the level or effect of sulindac by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- tobramycin
ibuprofen increases levels of tobramycin by decreasing renal clearance. Minor/Significance Unknown. Interaction mainly occurs in preterm infants.
- tolfenamic acid
ibuprofen will increase the level or effect of tolfenamic acid by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- tolmetin
ibuprofen will increase the level or effect of tolmetin by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- treosulfan
treosulfan decreases effects of ibuprofen by Mechanism: unspecified interaction mechanism. Minor/Significance Unknown.
- triamterene
triamterene, ibuprofen. Other (see comment). Minor/Significance Unknown. Comment: Risk of acute renal failure. Mechanism: NSAIDs decrease prostaglandin synthesis, which normally protect against nephrotoxicity.
ibuprofen increases toxicity of triamterene by pharmacodynamic antagonism. Minor/Significance Unknown. NSAIDs decrease prostaglandin synthesis, increasing the risk of nephrotoxicity. - valganciclovir
ibuprofen will increase the level or effect of valganciclovir by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- valproic acid
valproic acid will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- vancomycin
ibuprofen increases levels of vancomycin by decreasing renal clearance. Minor/Significance Unknown. Interaction mainly occurs in neonates.
- voriconazole
voriconazole will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
- willow bark
ibuprofen will increase the level or effect of willow bark by acidic (anionic) drug competition for renal tubular clearance. Minor/Significance Unknown.
- zafirlukast
zafirlukast will increase the level or effect of ibuprofen by affecting hepatic enzyme CYP2C9/10 metabolism. Minor/Significance Unknown.
Adverse Effects
1-10%
Dizziness (3-9%)
Epigastric pain (3-9%)
Heartburn (3-9%)
Constipation (1-3%)
Nausea (3-9%)
Rash (3-9%)
Tinnitus (3-9%)
Edema (1-3%)
Fluid retention (1-3%)
Headache (1-3%)
Vomiting (1-3%)
<1%
Acute renal failure (sometimes with acute tubular necrosis or hyperkalemia, polyuria, azotemia, cystitis, hematuria, decreased creatinine clearance, elevations in blood urea nitrogen (BUN) or creatinine without other manifestations of renal failure)
Agranulocytosis
Aplastic anemia
Erythema multiforme
Erythematous macular rashes
Exfoliative dermatitis
Hemolytic anemia (with or without positive direct antiglobulin test results)
Neutropenia
Thrombocytopenia (with or without purpura)
Toxic epidermal necrolysis (Lyell syndrome) and photosensitivity reactions
Warnings
Black Box Warnings
Cardiovascular risk
- Nonsteroidal anti-inflammatory drugs (NSAIDs) may increase risk of serious cardiovascular thrombotic events, myocardial infarction (MI), and stroke, which can be fatal
- Risk may increase with duration of use
- Patients with existing cardiovascular disease or risk factors for such disease may be at greater risk
- NSAIDs are contraindicated for perioperative pain in setting of coronary artery bypass graft (CABG) surgery
Gastrointestinal risk
- NSAIDs increase risk of serious GI adverse events, including bleeding, ulceration, and gastric or intestinal perforation, which can be fatal
- GI adverse events may occur at any time during use and without warning symptoms
- Elderly patients are at greater risk for serious GI events
Contraindications
Hypersensitivity to drug, other NSAIDs, aspirin, or excipients
Perioperative pain in setting of coronary artery bypass graft (CABG) surgery
Cautions
If pregnant or breastfeeding, ask a health professional before use; it is especially important not to use ibuprofen at 20 weeks or later in pregnancy unless definitely directed to do so by a doctor; it may cause problems in the unborn child or complications during delivery
Use caution in asthma (bronchial), cardiac disease, congestive heart failure (CHF), hepatic or renal impairment, hypertension. bleeding disorder, duodenal/gastric/peptic ulcer, stomatitis, systemic lupus erythematosus (SLE), ulcerative colitis, upper GI disease, late pregnancy (may cause premature closure of ductus arteriosus)
Long-term administration of NSAIDs may result in renal papillary necrosis and other renal injury; patients at greatest risk include elderly individuals; those with impaired renal function, hypovolemia, heart failure, liver dysfunction, or salt depletion; and those taking diuretics, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers
Junior Advil (100 mg): Doses higher than recommended may cause stomach bleeding
May cause serious adverse reactions, including exfoliative dermatitis, toxic epidermal necrolysis, Steven's Johnson syndrome reported
Children's and Junior Advil (50 mg, 100 mg): May cause severe and persistent sore throat
Fever, rash, abdominal pain, nausea, liver dysfunction, and meningitis have occurred in patients with collagen-vascular disease, especially SLE
Blurred vision, scotomata, and changes in color vision reported; discontinue therapy if symptoms occur
Platelet aggregation and adhesion may be decreased; monitor patients with coagulation disorders receiving the therapy
Risk of hyperkalemia may increase in patients with diabetes, the elderly, renal disease, or with concomitant use of agents that can induce hyperkalemia including ACE inhibitors; monitor potassium closely
May cause drowsiness and dizziness; may impair physical or mental abilities to operate heavy machinery or driving
NSAIDS, except aspirin, increase risk of heart attack, heart failure, and stroke; which can be fatal; the risk is higher if patients use more than it was directed or for longer than needed
Use caution in patients with high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, thyroid disease, diabetes, glaucoma, have trouble urinating due to an enlarged prostate gland, or had a stroke
Patients should inform healthcare professional if they have symptoms of heart problems or stroke, chest pain, trouble breathing weakness in one part or side of body, slurred speech, leg swelling
Not for use right before or after heart surgery
Keep out of reach of children; get medical help or contact poison control in case of overdose
Drug reaction with eosinophilia and systemic symptoms (DRESS)
- Drug Reaction reported in patients taking NSAIDs; some of these events have been fatal or life-threatening; DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling
- Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis; sometimes symptoms of DRESS may resemble an acute viral infection
- Eosinophilia is often present; because this disorder is variable in its presentation, other organ systems not noted here may be involved
- Early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident; if such signs or symptoms are present, discontinue therapy and evaluate the patient immediately
Heart Failure (HF) risk
- NSAIDS have the potential to trigger HF by prostaglandin inhibition that leads to sodium and water retention, increased systemic vascular resistance, and blunted response to diuretics
- NSAIDS should be avoided or withdrawn whenever possible
- AHA/ACC Heart Failure Guidelines; Circulation. 2016; 134
Pregnancy & Lactation
Pregnancy
There are no adequate and well-controlled studies in pregnant women; data from observational studies regarding potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive
Animal studies
- Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization
- In animal studies, administration of prostaglandin synthesis inhibitors (eg, ibuprofen), resulted in increased pre- and post-implantation loss
- Advise pregnant women of potential fetal risk
Clinical considerations
- There are no studies on effects during labor or delivery
- In animal studies, NSAIDs, including ibuprofen, inhibit prostaglandin synthesis, cause delayed parturition, and increase the incidence of stillbirth
Fetal toxicity
- Use of NSAIDs can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment
- Because of these risks, limit dose and duration of use between about 20 and 30 weeks of gestation and avoid use at about 30 weeks of gestation and later in pregnancy
- Use of NSAIDs at about 30 weeks gestation or later in pregnancy increases risk of premature closure of fetal ductus arteriosus
- Use of NSAIDs at about 20 weeks gestation or later in pregnancy has been associated with cases of fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment
- There are no available data with use in pregnant women to inform a drug-associated risk for major birth defects and miscarriage; however, there are published studies with each individual component of the drug combination
- Data from observational studies regarding potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive
- In animal reproduction studies, there were no clear developmental effects at doses up to 0.4-times the maximum recommended human dose (MRHD) in the rabbit and 0.5-times in the MRHD rat when dosed throughout gestation
- In contrast, an increase in membranous ventricular septal defects was reported in rats treated on gestation days 9 & 10 with 0.8-times the MRHD
- Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as ibuprofen, resulted in increased pre-and post-implantation loss
- Prostaglandins also have been shown to have an important role in fetal kidney development; in published animal studies, prostaglandin synthesis inhibitors have been reported to impair kidney development when administered at clinically relevant doses
- Unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery; if an NSAID is necessary at about 20 weeks gestation or later in pregnancy, limit use to lowest effective dose and shortest duration possible
- If treatment is needed for a pregnant woman, consider monitoring with ultrasound for oligohydramnios; if oligohydramnios occurs, discontinue therapy and follow up according to clinical practice
Labor or Delivery
- There are no studies on the effects of drug combination during labor or delivery; in animal studies, NSAIDs, including ibuprofen, inhibit prostaglandin synthesis, cause delayed parturition, and increase the incidence of stillbirth
Lactation
No lactation studies have been conducted; however, limited published literature reports that, following oral administration, ibuprofen is present in human milk at relative infant doses of 0.06-0.6% of the maternal weight-adjusted daily dose; no information is available on effects of ibuprofen on milk production or on a breastfed infant
Pregnancy Categories
A: Generally acceptable. Controlled studies in pregnant women show no evidence of fetal risk.
B: May be acceptable. Either animal studies show no risk but human studies not available or animal studies showed minor risks and human studies done and showed no risk. C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done. D: Use in LIFE-THREATENING emergencies when no safer drug available. Positive evidence of human fetal risk. X: Do not use in pregnancy. Risks involved outweigh potential benefits. Safer alternatives exist. NA: Information not available.Pharmacology
Mechanism of Action
Inhibits synthesis of prostaglandins in body tissues by inhibiting at least 2 cyclo-oxygenase (COX) isoenzymes, COX-1 and COX-2
May inhibit chemotaxis, alter lymphocyte activity, decrease proinflammatory cytokine activity, and inhibit neutrophil aggregation; these effects may contribute to anti-inflammatory activity
Absorption
Rapidly absorbed (85%)
Bioavailability: 80-100%
Onset: 30-60 min
Duration: 4-6 hr
Peak plasma time (adults)
- Conventional tablet: 120 min
- Chewable tablet: 62 min
- Oral suspension: 47 min
Peak plasma time (febrile children)
- Chewable tablet: 86 min
- Oral suspension: 58 min
Peak plasma concentration
- Conventional tablet: 20 mcg/mL
- Chewable tablet: 15 mcg/mL
- Oral suspension: 19 mcg/mL
Distribution
Protein bound: 90-99%; concentrations >20 mcg/mL
Vd: 0.12 L/kg (adults); 0.164 L/kg (children)
Metabolism
Rapidly metabolized in liver (primarily by CYP2C9; CYP2C19 substrate) via oxidation to inactive metabolites
Metabolites
- Metabolite A: (+)-2-[4'-(2-hydroxy-2-methylpropyl) phenyl] propionic acid
- Metabolite B: (+)-2-[4'-(2-carboxypropyl) phenyl] propionic acid
Elimination
Half-life: 2-4 hr (adults); 1.6 hr (children 3 mon to 1 year; 35-51 hr (day 3), 20-33 hr (day 5)
Excretion: Urine (50-60%; <10% unchanged); remainder in feces within 24 hr
Administration
Instructions
Take with food or 8-12 oz of water to avoid GI effects
Images
| BRAND | FORM. | UNIT PRICE | PILL IMAGE |
|---|---|---|---|
| Advil Migraine oral
-
|
200 mg capsule |  |
|
| IBU oral
-
|
800 mg tablet | 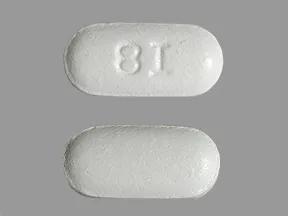 |
|
| IBU oral
-
|
600 mg tablet |  |
|
| IBU oral
-
|
400 mg tablet |  |
|
| Advil Liqui-Gels Minis oral
-
|
200 mg capsule |  |
|
| Ibuprofen IB oral
-
|
100 mg chewable tablet |  |
|
| Addaprin oral
-
|
200 mg tablet |  |
|
| IBU-200 oral
-
|
200 mg tablet |  |
|
| IBU-200 oral
-
|
200 mg tablet |  |
|
| I-Prin oral
-
|
200 mg tablet |  |
|
| I-Prin oral
-
|
200 mg tablet |  |
|
| Advil Liqui-Gel oral
-
|
200 mg capsule |  |
|
| Infant's Ibuprofen oral
-
|
50 mg/1.25 mL drops | 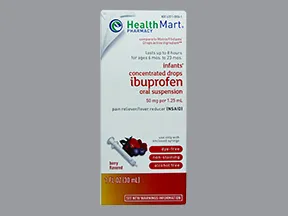 |
|
| Infant's Ibuprofen oral
-
|
50 mg/1.25 mL drops | 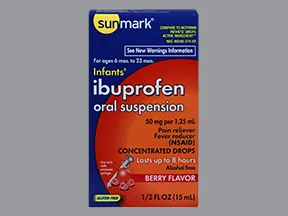 |
|
| Infant's Ibuprofen oral
-
|
50 mg/1.25 mL drops |  |
|
| Infant's Ibuprofen oral
-
|
50 mg/1.25 mL drops |  |
|
| Infant's Ibuprofen oral
-
|
50 mg/1.25 mL drops |  |
|
| Infant's Ibuprofen oral
-
|
50 mg/1.25 mL drops |  |
|
| Infant's Ibuprofen oral
-
|
50 mg/1.25 mL drops |  |
|
| Ibuprofen Jr Strength oral
-
|
100 mg chewable tablet |  |
|
| Ibuprofen Jr Strength oral
-
|
100 mg chewable tablet |  |
|
| Ibuprofen Jr Strength oral
-
|
100 mg chewable tablet |  |
|
| Wal-Profen oral
-
|
200 mg capsule |  |
|
| Wal-Profen oral
-
|
200 mg tablet |  |
|
| Wal-Profen oral
-
|
200 mg tablet |  |
|
| Wal-Profen oral
-
|
200 mg tablet |  |
|
| Infant's Advil oral
-
|
50 mg/1.25 mL drops |  |
|
| Children's Advil oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Advil oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Advil oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Advil oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Motrin oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Motrin oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Motrin oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Children's Ibuprofen oral
-
|
100 mg/5 mL suspension |  |
|
| Advil Junior Strength oral
-
|
100 mg chewable tablet |  |
|
| Caldolor intravenous
-
|
800 mg/8 mL (100 mg/mL) vial |  |
|
| Infant's Motrin oral
-
|
50 mg/1.25 mL drops |  |
|
| Infant's Motrin oral
-
|
50 mg/1.25 mL drops |  |
|
| Advil oral
-
|
200 mg tablet |  |
|
| Advil oral
-
|
200 mg tablet |  |
|
| Advil oral
-
|
200 mg tablet |  |
|
| Motrin IB oral
-
|
200 mg tablet |  |
|
| Motrin IB oral
-
|
200 mg capsule |  |
|
| Ibuprofen
-
|
200 mg capsule |  |
|
| Ibuprofen
-
|
200 mg tablet |  |
|
| Ibuprofen
-
|
600 mg tablet |  |
|
| Ibuprofen
-
|
800 mg tablet |  |
|
| Ibuprofen
-
|
200 mg tablet |  |
|
| Ibuprofen
-
|
800 mg tablet |  |
|
| Ibuprofen
-
|
600 mg tablet |  |
|
| Ibuprofen
-
|
800 mg tablet |  |
|
| Ibuprofen
-
|
400 mg tablet |  |
|
| Ibuprofen
-
|
600 mg tablet |  |
|
| Ibuprofen
-
|
400 mg tablet |  |
|
| Ibuprofen
-
|
800 mg tablet |  |
|
| Ibuprofen
-
|
400 mg tablet |  |
|
| Ibuprofen
-
|
600 mg tablet |  |
|
| Ibuprofen
-
|
400 mg tablet |  |
|
| Ibuprofen
-
|
800 mg tablet |  |
|
| Ibuprofen
-
|
600 mg tablet |  |
|
| Ibuprofen
-
|
200 mg capsule |  |
|
| Ibuprofen
-
|
100 mg chewable tablet |  |
|
| Ibuprofen
-
|
200 mg tablet |  |
|
| Ibuprofen
-
|
200 mg tablet |  |
|
| Ibuprofen
-
|
600 mg tablet |  |
|
| Ibuprofen
-
|
200 mg tablet |  |
|
| Ibuprofen
-
|
400 mg tablet |  |
|
| Ibuprofen
-
|
600 mg tablet |  |
|
| Ibuprofen
-
|
800 mg tablet |  |
|
| Ibuprofen
-
|
200 mg tablet |  |
|
| Ibuprofen
-
|
400 mg tablet | 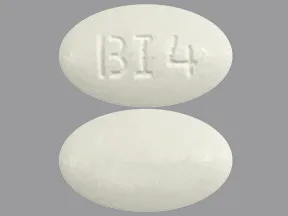 |
|
| Ibuprofen
-
|
100 mg/5 mL suspension |  |
|
| Ibuprofen
-
|
200 mg tablet |  |
|
| Ibuprofen
-
|
50 mg/1.25 mL drops |  |
|
| Ibuprofen
-
|
100 mg/5 mL suspension |  |
|
| Ibuprofen
-
|
100 mg/5 mL suspension |  |
|
| Ibuprofen
-
|
400 mg tablet |  |
|
| Ibuprofen
-
|
800 mg tablet |  |
|
| Ibuprofen
-
|
100 mg/5 mL suspension |  |
|
| Ibuprofen
-
|
100 mg chewable tablet |  |
|
| Ibuprofen
-
|
100 mg/5 mL suspension |  |
|
| Ibuprofen
-
|
200 mg tablet |  |
|
| Ibuprofen
-
|
200 mg tablet |  |
|
| Ibuprofen
-
|
100 mg/5 mL suspension |  |
|
| Ibuprofen
-
|
100 mg/5 mL suspension |  |
|
| Ibuprofen
-
|
100 mg/5 mL suspension |  |
|
| Ibuprofen
-
|
100 mg/5 mL suspension |  |
|
| Ibuprofen
-
|
100 mg/5 mL suspension |  |
Copyright © 2010 First DataBank, Inc.
Patient Handout
ibuprofen intravenous
IBUPROFEN - INJECTION
(EYE-bue-PROE-fen)
COMMON BRAND NAME(S): Caldolor
WARNING: Nonsteroidal anti-inflammatory drugs (including ibuprofen) may rarely increase the risk for a heart attack or stroke. This effect can happen at any time while using this drug but is more likely if you use it for a long time. The risk may be greater in older adults or if you have heart disease or increased risk for heart disease (for example, due to smoking, family history of heart disease, or conditions such as high blood pressure or diabetes). Do not use this drug right before or after heart bypass surgery (CABG).Also, this drug may rarely cause serious (rarely fatal) bleeding from the stomach or intestines. This effect can occur without warning symptoms at any time while taking this drug. Older adults may be at higher risk for this effect.Stop using ibuprofen and get medical help right away if you notice any of these rare but serious side effects: stomach/abdominal pain that doesn't go away, black/tarry stools, vomit that looks like coffee grounds, chest/jaw/left arm pain, shortness of breath, unusual sweating, confusion, weakness on one side of the body, trouble speaking, sudden vision changes.
USES: Ibuprofen is used to help relieve mild to moderate pain. When used with an opioid (such as morphine), it may be used to relieve moderate to severe pain. It is also used to reduce fever.Ibuprofen is known as a nonsteroidal anti-inflammatory drug (NSAID). It works by blocking your body's production of certain natural substances that cause inflammation. This effect helps to decrease swelling, pain, or fever.
HOW TO USE: Read the Medication Guide provided by your pharmacist before you start using ibuprofen and each time you get a refill. If you have any questions, ask your doctor or pharmacist.If you are using this medication at home, read and learn all preparation and usage instructions from the manufacturer or from your health care professional. If you have any questions about using this medication properly, ask your doctor or pharmacist.Before using, check this product visually for particles or discoloration. If either is present, do not use the liquid.This medication is given by injection into a vein as directed by your doctor, usually over at least 30 minutes (adults) or over at least 10 minutes (children). When used to relieve pain, it is usually given every 6 hours as needed. When used to reduce fever, this drug may be given every 4 to 6 hours as needed. Infants 3 to 6 months of age should be given this medication as a single dose. Follow your doctor's directions carefully.Learn how to store and discard needles and medical supplies safely. Consult your pharmacist for more details.Drink plenty of fluids while using this medication unless your doctor tells you otherwise.The dosage is based on your medical condition, age, and response to treatment. In children, the dosage is also based on weight. To reduce your risk of stomach bleeding and other side effects, use this medication at the lowest effective dose for the shortest possible time. Do not increase your dose or use this drug more often or for longer than prescribed.If you are using this drug "as needed" (not on a regular schedule), remember that pain medications work best if they are used as the first signs of pain occur. If you wait until the pain has worsened, the medicine may not work as well.Tell your doctor if your pain or fever lasts or gets worse.
SIDE EFFECTS: See also Warning section.Nausea, dizziness, gas, headache, and upset stomach may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.This medication may raise your blood pressure. Check your blood pressure regularly and tell your doctor if the results are high.Tell your doctor right away if you have any serious side effects, including: hearing changes (such as ringing in the ears), mental/mood changes, vision changes, easy bruising/bleeding, unexplained stiff neck, signs of kidney problems (such as change in the amount of urine), symptoms of heart failure (such as swelling ankles/feet, unusual tiredness, unusual/sudden weight gain).This drug may rarely cause serious (possibly fatal) liver disease. Get medical help right away if you have any symptoms of liver damage, including: nausea/vomiting that doesn't stop, yellowing eyes/skin, dark urine, severe stomach/abdominal pain.A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: fever, swollen lymph nodes, rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.In the US -Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
PRECAUTIONS: Before using ibuprofen, tell your doctor or pharmacist if you are allergic to it; or to aspirin or other NSAIDs (such as naproxen, celecoxib); or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.Before using this medication, tell your doctor or pharmacist your medical history, especially of: liver disease, stomach/intestine/esophagus problems (such as bleeding, ulcers, recurring heartburn), heart disease (such as history of heart attack), high blood pressure, stroke, blood disorders (such as anemia, bleeding/clotting problems), asthma (including a history of worsening breathing after taking aspirin or other NSAIDs), growths in the nose (nasal polyps).Kidney problems can sometimes occur with the use of NSAID medications, including ibuprofen. Problems are more likely to occur if you are dehydrated, have heart failure or kidney disease, are an older adult, or if you take certain medications (see also Drug Interactions section). Drink plenty of fluids as directed by your doctor to prevent dehydration and tell your doctor right away if you have a change in the amount of urine.This drug may make you dizzy. Alcohol or marijuana (cannabis) can make you more dizzy. Do not drive, use machinery, or do anything that needs alertness until you can do it safely. Talk to your doctor if you are using marijuana (cannabis).This medicine may cause stomach bleeding. Daily use of alcohol and tobacco may increase your risk for stomach bleeding, especially when combined with this medicine. Limit alcohol and stop smoking. Consult your doctor or pharmacist for more information.Before having surgery, tell your doctor or dentist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products).This medication may make you more sensitive to the sun. Limit your time in the sun. Avoid tanning booths and sunlamps. Use sunscreen and wear protective clothing when outdoors. Tell your doctor right away if you get sunburned or have skin blisters/redness.Older adults may be at greater risk for stomach/intestinal bleeding, kidney problems, heart attack, and stroke while using this drug.Before using this medication, women of childbearing age should talk with their doctor(s) about the benefits and risks. Tell your doctor if you are pregnant or if you plan to become pregnant. This medication may harm an unborn baby and cause problems with normal labor/delivery. It is not recommended for use in pregnancy from 20 weeks until delivery. If your doctor decides that you need to use this medication between 20 and 30 weeks of pregnancy, you should use the lowest effective dose for the shortest possible time. You should not use this medication after 30 weeks of pregnancy.This medication passes into breast milk. While there have been no reports of harm to nursing infants, consult your doctor before breastfeeding.
DRUG INTERACTIONS: Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.Some of the products that may interact with this drug include: aliskiren, ACE inhibitors (such as captopril, lisinopril), angiotensin II receptor blockers (such as losartan, valsartan), other medications for arthritis (such as aspirin, methotrexate), cidofovir, corticosteroids (such as prednisone), lithium, "water pills" (diuretics such as furosemide).This medication may increase the risk of bleeding when used with other drugs that also may cause bleeding. Examples include anti-platelet drugs such as clopidogrel, "blood thinners" such as dabigatran/enoxaparin/warfarin, among others.Check all prescription and nonprescription medicine labels carefully for other pain/fever drugs (aspirin, NSAIDs such as celecoxib, ketorolac, or naproxen). These drugs are similar to ibuprofen, so taking one of these drugs while also using ibuprofen may increase your risk of side effects. However, if your doctor has directed you to take low-dose aspirin for heart attack or stroke prevention (usually 81-162 milligrams a day), you should continue taking the aspirin unless your doctor instructs you otherwise.Daily use of ibuprofen may decrease aspirin's ability to prevent heart attack/stroke. Talk to your doctor about using a different medication (such as acetaminophen) to treat pain/fever. If you must use ibuprofen, talk to your doctor about taking immediate-release aspirin (not enteric-coated/EC) while using ibuprofen. Use ibuprofen at least 8 hours before or at least 2 hours after your aspirin dose. Do not increase your daily dose of aspirin or change the way you take aspirin/other medications without your doctor's approval.
OVERDOSE: If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include: severe stomach pain, vomit that looks like coffee grounds, extreme drowsiness/dizziness.
NOTES: Do not share this medication with others.Lab and/or medical tests (such as complete blood count, liver/kidney function, blood pressure) may be done while you are using this medication. Keep all medical and lab appointments.This medication has been prescribed for your current condition only. Do not use it later for another condition unless told to do so by your doctor. A different medication may be necessary in those cases.
MISSED DOSE: Not applicable.
STORAGE: Store at room temperature away from light and moisture. Do not store in the bathroom. Keep all medications away from children and pets.Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Information last revised March 2024. Copyright(c) 2024 First Databank, Inc.
IMPORTANT: HOW TO USE THIS INFORMATION: This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you. This information is not individual medical advice and does not substitute for the advice of your health care professional. Always ask your health care professional for complete information about this product and your specific health needs.
Formulary
Adding plans allows you to compare formulary status to other drugs in the same class.
To view formulary information first create a list of plans. Your list will be saved and can be edited at any time.
Adding plans allows you to:
- View the formulary and any restrictions for each plan.
- Manage and view all your plans together – even plans in different states.
- Compare formulary status to other drugs in the same class.
- Access your plan list on any device – mobile or desktop.

